Introduction
Lung cancer is the leading cause of
cancer-associated mortality and was responsible for ~1.38 million
deaths worldwide in 2008 (1). Lung
cancer is divided by histological classification into non-small
cell lung cancer and small cell lung cancer, the former of which
accounts for 80% of lung cancer cases. Despite the poor prognosis
of lung cancer, chemotherapy remains the primary strategy currently
available for the treatment of advanced stage disease. Platinum
drugs, including cisplatin and carboplatin, are the most frequently
used chemotherapeutic drugs for these diseases although tumor cell
resistance to platinum drugs is the main cause of clinical
treatment failure. Therefore, overcoming drug resistance is
urgently required to improve the clinical treatment efficacy for
lung cancer patients (2).
Platinum drugs bind to the nucleotide bases of DNA
to interfere with DNA synthesis, inhibit tumor cell proliferation
and induce apoptosis. The development of drug resistance in tumor
cells is a complex process. The mechanisms of tumor cell resistance
to platinum drugs include the following two aspects: (i)
Overexpression of multidrug resistance genes to reduce
intracellular accumulation of the drug; and (ii) the improved
anti-apoptotic ability of tumor cells. The in-depth investigation
of the signaling pathways involved in these two mechanisms and the
search for methods to overcome drug resistance are a primary focus
of cancer research. It has been established that elevated Src
kinase activity induces the overexpression of drug
resistance-associated genes, and the abnormally hyperactive AKT and
extracellular signal-regulated kinase (ERK) signaling pathways
cause metastasis and drug resistance in lung cancer (3–6).
Src is not only a tyrosine kinase with a molecular
weight of 60 kDa and a member of the membrane-associated Src family
kinases in cell protein tyrosine kinases, but also an important
role in regulating cell proliferation, migration, signal
transduction and other associated functions. Numerous studies have
demonstrated the abnormal activation of Src tyrosine kinase in a
variety of tumor tissues and cells, which has been confirmed to be
closely correlated with tumor growth, metastasis and angiogenesis,
and therefore provides a potential target for antitumor drugs.
Previously, sunitinib, a Src tyrosine kinase inhibitor, has
attracted notable attention for its close correlation with tumor
multidrug resistance and ability to reverse the multidrug
resistance of tumor cells through inhibition of Src tyrosine kinase
activity (7–9). However, the effects of sunitinib and
mechanisms of action in lung cancer multidrug resistance are yet to
be determined and require further study.
Materials and methods
Cells and cell culture
A549/DDP cisplatin-resistant human lung cancer cells
(Academy of Military Medical Science, Beijing, China) were cultured
in RPMI-1640 media (containing 10% fetal bovine serum) in an
incubator under 5% CO2, 37°C and saturated humidity
conditions, followed by digestion with 0.25% trypsin-EDTA for
sub-culturing. All of the experiments employed exponentially
growing cells.
Determination of the effect of sunitinib
on A549/DDP proliferation using the CellTiter-Glo luminescent
assay
The exponentially growing A549/DDP cells
(5×104 cells/ml) were seeded into 96-well microplates,
100 μl/well and cultured under 37°C, 5% CO2 and
saturated humidity conditions overnight to allow for cell adhesion.
Sunitinib (0, 1, 2, 5, 10, 20, 50 and 100 μM) was added to the
corresponding wells and cultured for a further 24 h. Next,
following the CellTiter-Glo kit (Promega Corporation, Madison, WI,
USA) instructions to detect cell viability the cells were lysed and
transferred to black 96-well plates. The ATP chemical reaction
induction reagent was added and mixed thoroughly and the
luminescence was determined with a microplate reader (BioTek,
Winnoski, VT, USA) after 10 min.
Determination of the cytotoxicity of
sunitinib on A549/DDP cells using the CellTiter-Glo luminescent
assay
The exponentially growing A549 or A549/DDP cells
(5×104 cells/ml) were seeded into 96-well microplates,
100 μl/well and cultured under 37°C, 5% CO2 and
saturated humidity conditions overnight to allow for cell adhesion.
Cisplatin (0, 1, 2, 5, 10, 20, 50 and 100 μM) was added to the
corresponding wells. Based on this, 0, 1 or 2 μM sunitinib was
additionally added and the culture was continued for 72 h. The
CellTiter-Glo kit instructions to detect cell viability were
followed as described above.
Determination of Src phosphorylation
level by western blot analysis
Sunitinib (0, 1 or 2 μM) was added to the
exponentially growing cells and cultured for 24 h. The cell lysate
was collected to extract the proteins and determine the protein
contents using the bicinchoninic acid (BCA) method. An equal
quantity of protein was obtained and separated in 12% sodium
dodecyl sulfate-polyacrylamide gel (SDS-PAGE). The protein was
transferred onto a polyvinylidene difluoride (PVDF; Millipore,
Billerica, MA, USA) membrane and incubated with rabbit anti-human
phosphorylated Src antibody (1:300; Biosource International, Inc.,
Camarillo, CA, USA) at 4°C overnight and then incubated with
specific horseradish peroxidase (HRP)-streptavidin conjugated
secondary antibody (Univ-bio, Shanghai, China) for 1 h. The
immunoreactive bands were then washed and developed using β-actin
as the internal control (1:5,000; Sigma, St. Louis, MO, USA).
Determination of the effect of sunitinib
on A549/DDP cell apoptosis using flow cytometry
Sunitinib (0, 1 or 2 μM) was added to the
exponentially growing A549/DDP cells and cultured for 24 h. A total
of 10 μM cisplatin was then also added and the culture was
continued for a further 24 h. The cells were harvested and
incubated with Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI) at room temperature away from light for 15
min, and then the apoptosis rate was detected with a flow cytometer
(Beckman Coulter, Brea, CA, USA).
Determination of the effect of sunitinib
on cell cycle of A549/DDP cells by flow cytometry
Sunitinib (0, 1 or 2 μM) was added to the
exponentially growing A549/DDP cells and cultured for 24 h. The
cells were then harvested and incubated with PI (with RNase A) at
room temperature away from light for 30 min, and the cell cycle
analysis was performed by a flow cytometer.
Determination of the effect of sunitinib
on drug excretion and accumulation in A549/DDP cells by flow
cytometry
Sunitinib (0, 1 or 2 μM) was added to the
exponentially growing A549/DDP cells and the cells were cultured
for 24 h. The cells were then harvested and incubated with Rh-123
at room temperature away from light for 60 min, or with
phycoerythrin-P-glycoprotein (P-gp) at room temperature away from
light for 30 min. The intracellular Rh-123 content and cellular
surface P-gp expression was then detected by a flow cytometer.
Determination of the effect of sunitinib
on glutathione (GSH) activity in A549/DDP cells by biochemical
assay
Sunitinib (0, 1 or 2 μM) was added to the
exponentially growing A549/DDP cells and the cells were cultured
for 24 h. The cells were harvested and lysed, following the kit
instructions to detect cellular GSH activity (Beyotime, Beijing,
China).
Determination of the effect of sunitinib
on multidrug resistance gene protein expression in A549/DDP cells
by western blot analysis
Sunitinib (0, 1 or 2 μM) was added to the
exponentially growing A549/DDP cells and culture was continued for
24 h. The cells were harvested and lysed to extract the proteins.
The protein contents in the cell lysate were then determined using
the BCA method. An equal quantity of proteins were obtained and
separated in 12% SDS-PAGE, and then transferred onto a PVDF
membrane and incubated with the respective monoclonal antibodies
[anti-multidrug resistance protein 1 (MDR1), 1:800; anti-multidrug
resistance-associated protein 1 (MRP1), 1:800; anti-lung resistance
protein (LRP), 1:800; anti-glutathione-S-transferase (GST), 1:800;
anti-ERCC1, 1:800; anti-survivin, 1:800; anti-Bcl-2, 1:800;
anti-p-AKT, 1:300; and anti-p-ERK, 1:300] at 4°C overnight. Then,
the cells were incubated with specific HRP-conjugated secondary
antibody for 1 h and the immunoreactive bands were washed and
developed using β-actin as the internal control (1:2,000;
Sigma).
Determination of the effect of sunitinib
on multidrug resistance-associated gene mRNA expression in A549/DDP
cells by quantitative polymerase chain reaction (PCR) assay
Sunitinib (0, 1 or 2 μM) was added to the
exponentially growing A549/DDP cells and cultured for 24. The cells
were then harvested and the total RNA was extracted using the
TRIzol method, obtaining the cDNA by reverse transcription using a
real-time PCR kit (Takara Bio, Inc., Dalian China). The primers
used were as follows: sense: 5′-AGGAAGACA TGACCAGGTATGC-3′ and
antisense: 5′-CCAACATCG TGCACATCAAAC-3′ for MDR1; sense:
5′-CTTCTGGAG GAATTGGTTGTATAGAAG-3′, and antisense: 5′-GGTAGA
CCCAGACAAGGATGTTAGA-3′ for MRP1; sense: 5′-CAG CTGGCCATCGAGATCA-3′
and antisense: 5′-TCCAGT CTCTGAGCCTCATGC-3′ for LRP; sense:
5′-TTCCTTACT GGTCCTCACATCTC-3′ and antisense: 5′-TCACCGGAT
CATGGCCAGCA-3′ for GST-π; sense: 5′-CCCTGGGAA TTTGGCGACGTAA-3′ and
antisense: 5′-CTCCAGGTA CCGCCCAGCTTCC-3′ for ERCC1; sense:
5′-GAATTC ATGGGTGCCCCGACGTTGCC-3′ and antisense: 5′-AGA
TCTTTCTTCTTATTGTTGGTTTCC-3′ for survivin; sense:
5′-TTGGCCCCCGTTGCTT-3′ and antisense: 5′-CGG TTGTCGTACCCCGTTCTC-3′
for Bcl-2; and sense: 5′-ATGGAAATCCCATCACCATCTT-3′ and antisense:
5′-CGCCCCACTTGATTTGG-3′ for glyceraldehyde 3-phosphate
dehydrogenase. Following denaturation at 94°C for 3 min, the
primers were amplified under the following conditions for 40
cycles: 95°C for 5 sec, 65°C for 35 sec, 72°C for 60 sec, and
extension at 72°C for 5 min after these cycles.
Determination of the effect of sunitinib
on the transcriptional activity of transcription factors in
A549/DDP cells using the dual luciferase reporter gene assay
A total of 3×104 cells were seeded in
24-well plates and cultured for 24 h to allow cell adhesion.
Following the method as described in the manufacturer’s
instructions; nuclear factor (NF)-κB, Twist, Snail and AP-1
luciferase reporter plasmids (Biominda, Tianjin, China) were added
to each well to transfect the cells and then the cells were
cultured for a further 6 h prior to washing off the plasmids that
were not transfected into the cells. The media was then changed and
0, 1 or 2 μM sunitinib was added to continue the culture in an
incubator for 24 h. Finally, the activity of the luciferases was
determined using a Dual-Glo™ Luciferase assay system (Promega
Corporation).
Statistical methods
The experimental data are represented as the mean ±
standard deviation and analyzed with SPSS 11.5 software (SPSS,
Inc., Chicago, IL, USA). Univariate analysis of variance was used
for comparison and P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of sunitinib on A549/DDP cell
proliferation
The CellTiter-Glo experimental results demonstrated
that the inhibitory effect of sunitinib on A549/DDP cell
proliferation was dose-dependent, it was identified that 1 μM
sunitinib did not have significant toxicity (inhibition rate
<5%) on A549/DDP cells and 2 μM sunitinib had low toxicity
(inhibition rate, 10–15%; Fig. 1).
Thus, the 1 and 2 μM sunitinib doses were selected for use in this
study.
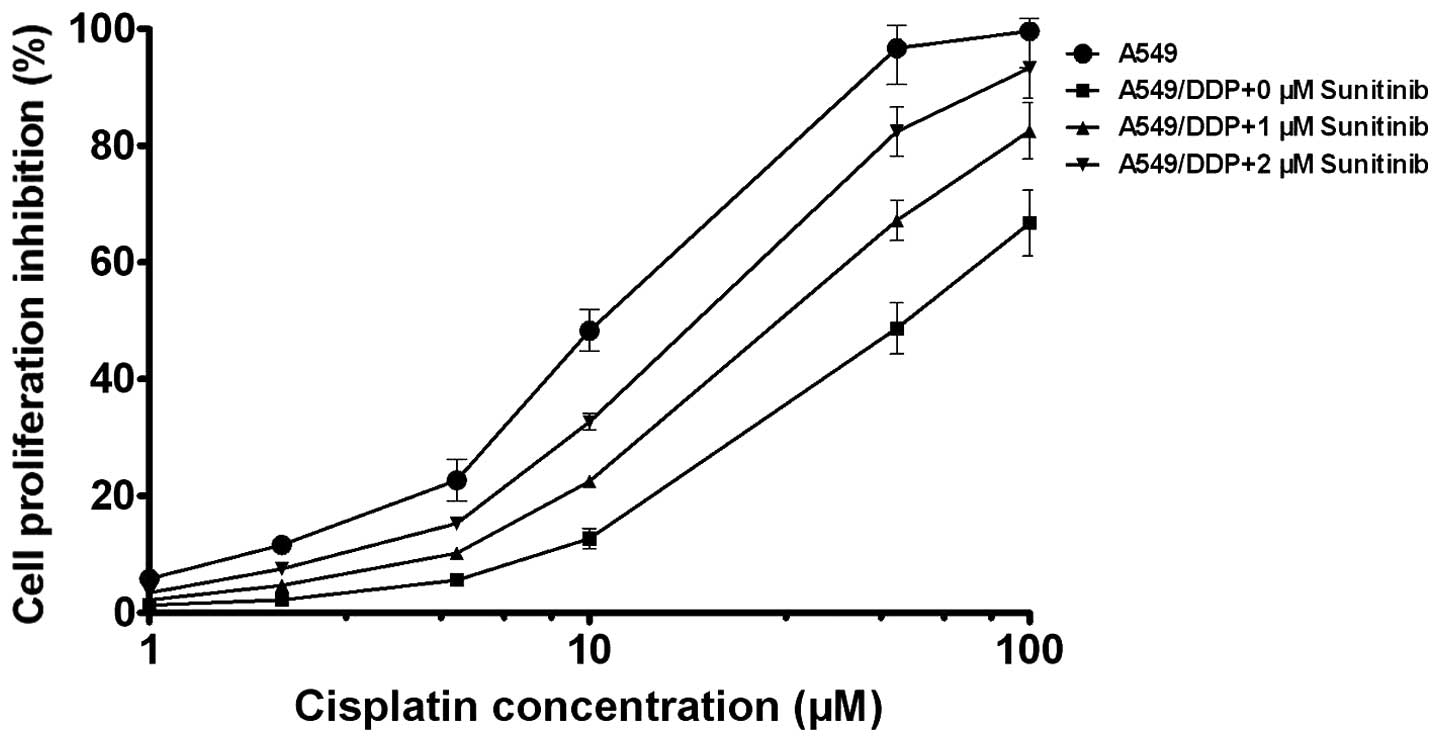
Sunitinib increases the sensitivity of
A549/DDP cells to cisplatin
The CellTiter-Glo experimental results demonstrated
that 1 and 2 μM sunitinib decreased the IC50 of
cisplatin for inhibiting A549/DDP cells; the IC50 of
cisplatin was 11.35 and 55.22 μM for inhibiting A549 cells and
A549/DDP cells, respectively. The IC50 of cisplatin was
38.53 and 21.72 μM for inhibiting A549/DDP cells following
treatment with 1 and 2 μM sunitinib, respectively; and the reversal
fold (RF) was 1.43 and 2.54, respectively (Fig. 2).
Inhibitory effect of sunitinib on Src
tyrosine kinase activity in A549/DDP cells
Western blot analysis demonstrated that the Src
tyrosine kinase inhibitor, sunitinib, reduced the phosphorylation
level of Src proteins in A549/DDP cells in a dose-dependent manner,
therefore demonstrating that sunitinib was able to inhibit Src
tyrosine kinase activity in tumor cells (Fig. 3).
Sunitinib inhibits P-gp expression in
A549/DDP cells, increases cellular Rh-123 content, enhances
apoptosis and arrests cell cycle
As demonstrated by the flow cytometry results,
sunitinib treatment resulted in a significant reduction in P-gp
expression in the A549/DDP cells. When compared with the control
group, the P-gp expression level was 29.5 and 16.6% in the 1 and 2
μM groups, respectively. The sunitinib treatment also resulted in a
significant elevation of Rh-123 content in A549/DDP cells, when
compared with the control group, the mean fluorescence intensity of
Rh-123 in tumor cells increased by 1.95- and 3.78-fold following
treatment with 1 and 2 μM of sunitinib, respectively (Fig. 4A and B).
 | Figure 4Effect of sunitinib on P-gp
expression, cellular Rh-123 content, apoptosis and cell cycle of
A549/DDP cells. The effect of sunitinib on (A) P-gp expression and
(B) cellular Rh-123 content was measured by a flow cytometry assay.
The data was presented as the mean ± SD, the error bars indicate
SD, n=3. *P<0.05, compared with the 0 μM sunitinib
group. SD, standard deviation; P-gp, P-glycoprotein. Effect of
sunitinib on (C) cell apoptosis and (D) the cell cycle of A549/DDP
cells was measured by a flow cytometry assay. The data was
presented as the mean ± SD, the error bars indicate SD, n=3.
*P<0.05, compared with the 0 μM sunitinib group. SD,
standard deviation. |
The flow cytometry results also demonstrated a
significantly higher apoptosis rate of A549/DDP cells following
sunitinib treatment. The apoptosis rate in the 1 and 2 μM group was
3.82- and 2.40-fold that of the 0 μM group, respectively. Sunitinib
treatment resulted in the arrest of A549/DDP cell cycle in G0/G1
phase; the ratio of tumor cells in G0/G1 phase was 47.86 and 52.19%
in the 1 and 2 μM groups, respectively, which was significantly
higher than the 41.97% in 0 μM group (Fig. 4C and D), further demonstrating the
ability of sunitinib to enhance apoptosis and arrest the cell cycle
of A549/DDP cells. The results demonstrate that sunitinib was able
to reduce drug excretion from tumor cells, increase drug content in
tumor cells and arrest the cell cycle, resulting in the enhanced
drug sensitivity and apoptosis of tumor cells.
Sunitinib downregulates the expression of
multidrug resistance-associated genes
Western blot analysis demonstrated that sunitinib
was able to reduce MDR1, MRP1, LRP, GST, ERCC1, survivin and Bcl-2
protein level in A549/DDP cells in a dose-dependent manner
(Fig. 5A), the results revealed
that sunitinib downregulated multidrug resistance-associated
protein expression in tumor cells, thereby enhancing the drug
sensitivity of tumor cells. The qPCR results revealed that
sunitinib downregulated MDR1, MRP1, LRP, GST, ERCC1, survivin and
Bcl-2 mRNA levels in the A549/DDP cells following treatment with 1
and 2 μM of sunitinib (Fig. 5B).
The results demonstrated that sunitinib was able to downregulate
MDR1, MRP1, LRP, GST, ERCC1, survivin and Bcl-2 gene transcription
in tumor cells, thereby enhancing the drug sensitivity of tumor
cells.
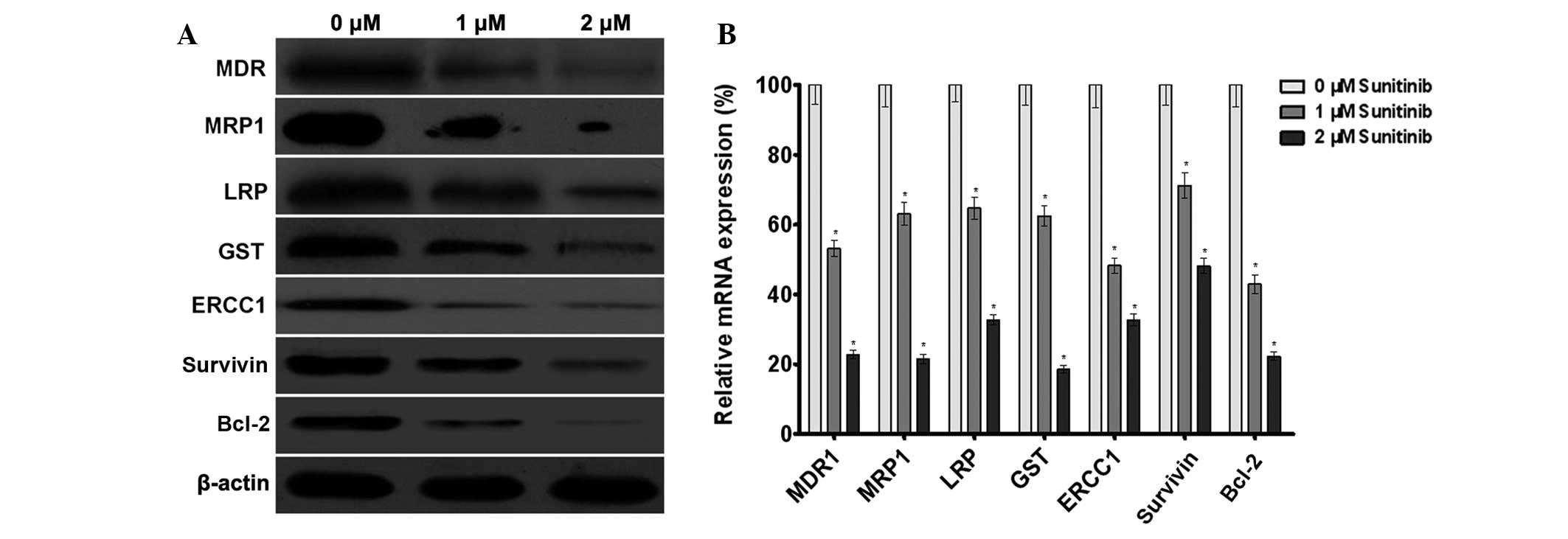 | Figure 5Effect of sunitinib on the expression
of the multidrug resistance-associated genes in A549/DDP cells. (A)
The effect of sunitinib on the protein expression of MDR1, MRP1,
LRP, GST, ERCC1, survivin and Bcl-2 in A549/DDP cells was measured
by western blot analysis. β-actin served as the internal control.
(B) The effect of sunitinib on the mRNA expression of MDR1, MRP1,
LRP, GST, ERCC1, survivin and Bcl-2 in A549/DDP cells was measured
by quantitative polymerase chain reaction assay. Glyceraldehyde
3-phosphate dehydrogenase was used as the internal control. The
data are presented as the mean ± SD, bars indicate SD, n=5;
*P<0.05, compared with the 0 μM sunitinib group.
MDR1, multidrug resistance protein 1; MRP1, multidrug
resistance-associated protein 1; LRP, lung resistance protein; GST,
glutathione-S-transferase; SD, standard deviation. |
Effect of sunitinib on multidrug
resistance-associated signal transduction pathways in tumor
cells
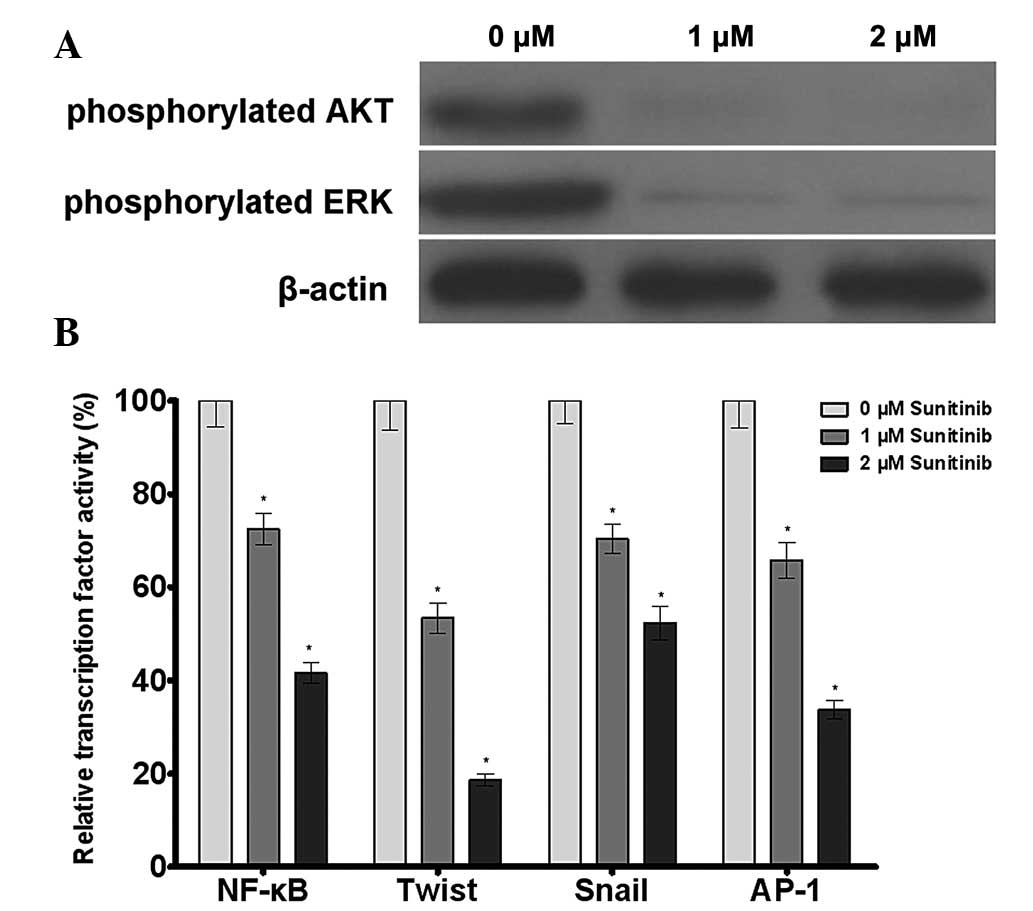
Western blot analysis revealed that sunitinib was
able to reduce the phosphorylation levels of AKT and ERK in
A549/DDP cells. The dual luciferase reporter gene assay results
demonstrated that sunitinib downregulated the transcriptional
activity of the transcription factors NF-κB, Twist, Snail and AP-1.
The results demonstrated that sunitinib regulated the
transcriptional activity of NF-κB, Twist, Snail and AP-1 by
inhibiting AKT and ERK phosphorylation, leading to the inhibition
of multidrug resistance gene expression and the reversal of tumor
cell drug resistance (Fig. 6).
Discussion
The development of tumor cell multidrug resistance
is the main cause of treatment failure of chemotherapeutic drugs;
thus, investigating the mechanisms of tumor multidrug resistance
and investigating potential solutions have important significance
in improving the clinical efficacy of chemotherapeutic drugs.
Studies have demonstrated that tumor cells may develop drug
resistance through the following mechanisms: (i) Overexpression of
ATP-binding cassette protein super family and other trans-membrane
protein genes to pump the drugs out of tumor cells and reduce the
intracellular drug concentration; (ii) overexpression of
detoxification and DNA repair enzymes to reduce the efficacy of
cytotoxic drugs; (iii) downregulation of the expression of drug
target molecules to reduce drug sensitivity; and (iv) activation of
tumor suppressor genes to inhibit drug-induced apoptosis. Among
them, the most extensively studied mechanism is drug resistance
induced by the overexpression of multidrug resistance genes. The
expression of these proteins reduces the drug concentration in
tumor cells or in the vicinity of the drug targets, rendering it
impossible for the administered drugs to reach the treatment sites
effectively and leading to the subsequent development of drug
resistance (10).
Multidrug resistance protein 1 (MDR1) as an
energy-dependent transmembrane glycoprotein is extensively
expressed in lung cancer tissues and expressed at moderate to low
level or not expressed at all in normal lung tissues. MDR1 protein
may function as a drug pump. When overexpressed in tumor cell
membranes, MDR1 protein binds to the antitumor drug and pumps the
intracellular drugs out of the cells actively using the energy
released by ATP hydrolysis, leading to a reduction of intracellular
drug concentration and drug resistance. MRP1 and LRP are important
drug resistance genes that have been identified in recent years and
act as major vault proteins (11).
They were first identified in lung cancer and have since been
demonstrated to be overexpressed in a variety of drug-resistant
cell lines (12). MRP1 affects the
intracellular transport and distribution of drugs, leading to drug
resistance (13).
GST is able to eliminate cisplatin-induced DNA
insertion. Simultaneously, the peroxidase activity of GST and GSH
reduces cisplatin-induced peroxide and covalent conjugates.
Therefore, GST, GSH and MDR1 exert synergistic effects; namely,
MDR1 pumps the cisplatin-glutathione conjugates, catalyzed by GST
and GSH, out of the cells and has an important role in cisplatin
resistance. Nucleotide excision repair is an important mechanism
responsible for cisplatin resistance. ERCC1 is a crucial pathway
for DNA repair and has an important role in the repair of
cisplatin-induced DNA damage; ERCC1 has been proven to be an
important drug resistance gene in tumors. As survivin and Bcl-2 are
two key genes in the regulation of apoptosis, that inhibit
apoptosis synergistically, the downregulation of survivin and Bcl-2
expression has emerged as an important pathway for reversing tumor
multidrug resistance (14–16).
The drug-resistant A549/DDP cells that are screened
from the A549 human lung cancer cell line by continuous exposure to
cisplatin are an excellent tool for investigating multidrug
resistance. The importance of Src tyrosine kinase in tumor
formation and development is well established, and it has been
demonstrated that the overexpression of Src leads to the
development of cancer. Furthermore, recent studies have
demonstrated that Src kinase is also involved in tumor cell
resistance to chemotherapeutic drugs, as the Src tyrosine kinase
inhibitor increases tumor cell sensitivity to chemotherapeutic
drugs and may even reverse drug resistance. Although the
association between Src tyrosine kinase and tumor drug resistance
remains unknown, the roles of Src tyrosine kinase inhibitors in
different types of drug-resistant tumor cells are worthy of further
study.
The present study demonstrated that the Src tyrosine
kinase inhibitor sunitinib was able to inhibit Src kinase activity
in A549/DDP cells, as evidenced by the reduced level of Src
phosphorylation. The CellTiter-Glo assay results revealed that this
inhibitory effect was further converted to enhance cell sensitivity
to cisplatin. One of the main mechanisms responsible for A549/DDP
cell drug resistance is the overexpression of MDR1 and MRP1, which
leads to the potentiation of the cell’s ability to excrete the drug
and the consequent reduction of drug concentration in the cells.
Therefore, the reversal of drug resistance may be associated with
the restoration of drug accumulation in these cells. As Rh-123, a
substrate of P-gp, indicates the expression of this protein in
cells and the ability of cancer cells to pump out the drug
(17,18), the effects of sunitinib on Rh-123
content in the cells was investigated using flow cytometry. The
results demonstrated that sunitinib treatment resulted in the
significant elevation of Rh-123 content in the cells, suggesting
that the inhibition of Src tyrosine kinase activity and the
reversal of drug resistance of A549/DDP cells are associated with
the inhibition of drug excretion from the cells, the increased
level of intracellular drug accumulation, and the consequent arrest
of the cell cycle and enhancement of apoptosis, which is consistent
with our hypothesis. Western blot analysis demonstrated that
sunitinib treatment resulted in downregulation of the expression
levels of ERCC1, survivin and Bcl-2 protein in the cells; while the
quantitative PCR results suggested that sunitinib-induced
downregulation of the expression of the aforementioned proteins was
likely achieved by downregulating the level of mRNA
transcription.
Furthermore, it was observed, through studies on
signal transduction pathways in tumor cells, that sunitinib was
able to inhibit the phosphorylation of AKT and ERK, leading to the
subsequent downregulation of the transcriptional activity of NF-κB,
Twist, Snail and AP-1. It was therefore hypothesized that sunitinib
is able to inhibit the expression of multidrug resistance genes and
reverse tumor drug resistance by inhibiting the aforementioned
signal transduction pathways. The present study demonstrated that
sunitinib was able to reverse the A549/DDP cell multidrug
resistance and that the mechanisms may be associated with the
downregulation of multidrug resistance gene expression in these
cells and the restoration of intracellular drug accumulation. The
results of this study have provided the experimental basis for the
application of drugs targeting Src tyrosine kinase and the clinical
reversal of lung cancer multidrug resistance.
References
|
1
|
Dearing KR, Sangal A and Weiss GJ:
Maintaining clarity: Review of maintenance therapy in non-small
cell lung cancer. World J Clin Oncol. 5:103–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hill C: Cancer prevention and screening.
Bull Cancer. 100:547–554. 2013.(In French).
|
|
3
|
Chen YT, Feng B and Chen LB: Update of
research on drug resistance in small cell lung cancer chemotherapy.
Asian Pac J Cancer Prev. 13:3577–3581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Curry NL, Mino-Kenudson M, Oliver TG, et
al: Pten-null tumors cohabiting the same lung display differential
AKT activation and sensitivity to dietary restriction. Cancer
Discov. 3:908–921. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park YH, Kim SU, Lee BK, et al: Prx I
suppresses K-ras-driven lung tumorigenesis by opposing
redox-sensitive ERK/cyclin D1 pathway. Antioxid Redox Signal.
19:482–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salminen A, Lehtonen M, Suuronen T, et al:
Terpenoids: natural inhibitors of NF-kappaB signaling with
anti-inflammatory and anticancer potential. Cell Mol Life Sci.
65:2979–2999. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peterson-Roth E, Brdlik CM and Glazer PM:
Src-induced cisplatin resistance mediated by cell-to-cell
communication. Cancer Res. 69:3619–3624. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reynolds C, Spira AI, Gluck L, et al:
Sunitinib malate in previously untreated, nonsquamous, non-small
cell lung cancer patients over the age of 70 years: results of a
Phase II trial. Invest New Drugs. 31:1330–1338. 2013.PubMed/NCBI
|
|
9
|
Wei L, Dai Q, Zhou Y, et al: Oroxylin A
sensitizes non-small cell lung cancer cells to anoikis via
glucose-deprivation-like mechanisms: c-Src and hexokinase II.
Biochim Biophys Acta. 1830:3835–3845. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Melguizo C, Prados J, Luque R, et al:
Modulation of multidrug resistance gene expression in peripheral
blood mononuclear cells of lung cancer patients and evaluation of
their clinical significance. Cancer Chemother Pharmacol.
71:537–541. 2013. View Article : Google Scholar
|
|
11
|
Zhang B, Liu M, Tang HK, et al: The
expression and significance of MRP1, LRP, TOPOIIβ, and BCL2 in
tongue squamous cell carcinoma. J Oral Pathol Med. 41:141–148.
2012.
|
|
12
|
Li XQ, Li J, Shi SB, et al: Expression of
MRP1, BCRP, LRP and ERCC1 as prognostic factors in non-small cell
lung cancer patients receiving postoperative cisplatin-based
chemotherapy. Int J Biol Markers. 24:230–237. 2009.
|
|
13
|
Wang J, Zhang J, Zhang L, et al:
Expression of P-gp, MRP, LRP, GST-π and TopoIIα and intrinsic
resistance in human lung cancer cell lines. Oncol Rep.
26:1081–1089. 2011.
|
|
14
|
Banerjee A, Qian P, Wu ZS, et al: Artemin
stimulates radio- and chemo-resistance by promoting
TWIST1-BCL-2-dependent cancer stem cell-like behavior in mammary
carcinoma cells. J Biol Chem. 287:42502–42515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han Y, Wang XB, Xiao N and Liu ZD: mRNA
expression and clinical significance of ERCC1, BRCA1, RRM1, TYMS
and TUBB3 in postoperative patients with non-small cell lung
cancer. Asian Pac J Cancer Prev. 14:2987–2990. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu JL, Wang Y, Jiang J, et al: Inhibition
of survivin expression and mechanisms of reversing drug-resistance
of human lung adenocarcinoma cells by siRNA. Chin Med J (Engl).
123:2901–2907. 2010.PubMed/NCBI
|
|
17
|
Munić V, Kelnerić Z, Mikac L and Eraković
Haber V: Differences in assessment of macrolide interaction with
human MDR1 (ABCB1, P-gp) using rhodamine-123 efflux, ATPase
activity and cellular accumulation assays. Eur J Pharm Sci.
41:86–95. 2010.PubMed/NCBI
|
|
18
|
Wei HB, Hu J, Shang LH, et al: A
meta-analytic review of ERCC1/MDR1 polymorphism and
chemosensitivity to platinum in patients with advanced non-small
cell lung cancer. Chin Med J (Engl). 125:2902–2907. 2012.PubMed/NCBI
|