Introduction
Vascular calcification significantly affects the
health of the elderly. Increasing evidence has indicated that
vascular calcification is an actively regulated osteogenic process
(1). In addition, the
osteochondrocytic differentiation of bone marrow-derived
mesenchymal stem cells (MSCs) is a significant step in the
osteogenic processes (2). MSCs, a
main population of stem cells, which reside in the bone marrow,
adipose tissue, peripheral blood and synovium (3), are able to differentiate into
multiple cell lineages, including osteoblasts, adipocytes,
chondrocytes and cardiac myocytes (4). Additionally, they are able to
differentiate into functional regenerative units (5). Furthermore, previous studies have
revealed anti-inflammatory properties of MSCs, indicating their
usefulness in both organ transplantation and treatment of
autoimmune diseases (6).
Indeed, a number of preclinical and clinical studies
in diseases, including multiple sclerosis (7), diabetes (8), osteogenesis imperfecta (9) and cartilage defect (10), have already demonstrated that there
is therapeutic potential of MSCs. Successful exploitation of MSCs
has been reported in several preclinical models (11) and these models have demonstrated
that they enhanced tissue repair either by direct regeneration
(12) or by secreting paracrine
factors (8). The therapeutic
ability of MSCs has been augmented by genetic modifications
(5), which overexpress
prosurvival, or growth factor genes (6,9). In
clinical trials, MSCs have been able to repair injured myocardium
(13), bone (14) and soft tissue (11). Although MSC growth in injured
vascular tissue may regenerate normal vascular tissue, it can also
produce ectopic tissues (10,11),
including those observed in advanced atherosclerotic calcification.
MSCs are involved in the initiation and progression of various
vascular diseases (15). One
cannot rule out the possibility of severe or delayed side effects
that may have been observed in clinical trials (10,11).
However, the underlying mechanisms of the therapeutic effects of
MSCs remain elusive and controversial.
The Wnt pathways regulate endothelial dysfunction
and vascular smooth muscle cell (VSMC) proliferation and migration,
and thereby intimal thickening (16). Furthermore, they can regulate
inflammation (17) and foam cell
formation (16), pathological
angiogenesis (1) and calcification
(18), which are crucial processes
in plaque formation and stability (16). Of note, the Wnt pathways, which
have been identified as contributing to the regulation of
osteogenic mineralization during development and disease (18), control the fate of MSCs (19). It remains unknown whether these
MSCs during the vascular calcification differentiate into normal
VSMCs in vivo to treat damaged vascular tissue or to
calcified VSMCs to aggravate calcification correlated to the Wnt
pathways. At present, preventing MSC pathological differentiation
is a powerful and potential strategy to delay aging of individuals
and to promote application of cell therapy for treating
age-associated diseases (20).
Therefore, it is required to analyze mechanisms of MSC
differentiation in detail. In the present study the cell-cell
co-culturing system was used in order to observe in vitro
MSCs directly interact with normal or calcified VSMCs during
calcification and to investigate the gene expression of Wnt
pathways during the process.
Materials and methods
Rat bone-marrow-derived MSCs
The isolation and culturing of male MSCs were
performed as previously published (21). MSCs were cultured in Dulbecco’s
modified Eagle’s medium (DMEM), supplemented with 10%
heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin and
100 Ag/ml streptomycin (all Gibco Life Technologies, Carlsbad, CA,
USA) at 37°C in 5% CO2 and 95% air. MSCs were used at
passage three.
Rat aortic SMCs
Rat aortic VSMCs (A-10; ATCC, Manassas, VA, USA)
were grown in low-glucose DMEM. Osteosynthesis-inducing medium
(OS), which was used only in one osteoblastic differentiation
assay, contained DMEM with 0.1 μM dexamethasone, 10 mM sodium
β-glycerol-phosphate and 0.05 mM ascorbic acid-2-phosphate
(Sigma-Aldrich, St. Louis, MO, USA). The culture medium was removed
and replaced with fresh medium three times a week. VSMCs were
cultured for 21 days following the formation of calcified
nodules.
Co-culture conditions
Direct co-cultures were established by seeding two
different cell types: VSMCs or calcified VSMCs and MSCs together at
a ratio of 5,000:5,000 cells/1.7 cm2 onto either
gelatin-coated 1.7-cm2 chamber slides (Becton-Dickinson,
Oxford, UK) for immunohistochemical analysis, or gelatin-coated
75-cm2 tissue culture flasks (Becton-Dickinson) for
protein or RNA isolation. The cells were cultured in a 50:50 mix of
growth medium for the two cell types used at 37°C in a humidified
atmosphere of 5% CO2 in air, with the medium replaced
after 24 h. Next, the cells were cultured in a low-glucose DMEM
supplemented with 10% FBS at 37°C in a 5% CO2 incubator
for 14 days. The medium was changed every three days. Each
co-culture experiment was performed three times in order to
validate the results. The 8 groups were as follows: GS (calcified
SMCs); GS+M (calcified SMCs co-cultured with MSCs); GS+M+OS (MSCs
and calcified SMCs cultured in OS); S (SMCs); S+M (MSCs co-cultured
with SMCs); S+M+OS (SMCs and MSCs cultured in OS); M (MSCs); M+OS
(MSCs cultured in OS)
Osteoblastic differentiation was evaluated by the
cell morphology and activity of alkaline phosphatase (22) in cell lysates and alkaline
phosphatase (ALP) staining. Additionally, by investigating the mRNA
expression levels of genes encoding for proteins involved in Wnt
signaling, Wnt5a, LRP6, Ror2, c-Jun-N-terminal kinase (JNK) and
β-catenin in each group.
Flow cytometry
In analogy with a previous study (21), passage 3 MSCs were trypsinized,
washed with phosphate-buffered saline (PBS) and incubated with
fluorescein isothiocyanate phycoerythrin-conjugated monoclonal
antibodies (Biolegend, San Diego, CA, USA) specific against CD29,
CD90, CD45 or CD11b, or with PBS at 4°C for 30 min. The analysis
was performed by flow cytometry (Becton-Dickinson, Franklin Lakes,
NJ, USA) using Cell Quest software (Becton-Dickinson).
von Kossa staining
For von Kossa staining, cells were fixed at 4°C for
45 min with paraformaldehyde (Sigma-Aldrich), then the fixed cells
were incubated in 5% silver nitrate for 30 min under ultraviolet
light and air-dried until the development of a black color.
Calcification was observed under a CX31 light microscope (Olympus
Corporation, Tokyo, Japan).
ALP
ALP activity and expression were assessed as
previously described (21). The
cells were fixed with 4% paraformaldehyde (Sigma-Aldrich) and then
stained with the 5-bromo-4-chloro-3′-indolyphosphate and nitro-blue
tetrazolium (BCIP/NBT) phosphatase substrate system (KLP,
Gaithersburg, MD, USA) following the manufacturer’s instructions.
The ALP activity was determined in all the samples. The samples
were extracted with an assay buffer containing 50 mM Tris-HCl, 0.1%
Triton X-100 and 0.9% NaCl (pH 7.6), and the lysate was frozen.
Lysate samples were then thawed and the enzyme activity was
determined in duplicates using 0.1 M
4-p-nitrophenylphosphate as a substrate (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). The absorbance was
measured at 492 nm using a iMark Microplate Absorbance Reader
(Bio-Rad, Hercules, CA, USA). The total protein contents were
determined by the Bio-Rad protein assay (Bio-Rad).
Expression of genes encoding
Wnt-signaling proteins
The total RNA was isolated from the samples using
TRIzol reagent according to the manufacturer’s instructions
(Invitrogen, Carlsbad, CA, USA) and reverse-transcribed into cDNA
with a reverse transcription kit (Toyobo, Osaka, Japan).
Quantitative polymerase chain reaction (qPCR) was performed with an
ABI PRISM 7900 sequence detector system (Applied Biosystems)
according to the manufacturer’s instructions. Actin was used as an
endogenous control. The PCR reaction mixture contained SYBR green I
(Takara), cDNA and the primers. The following sequences of primers
for qPCR were used: Actin (110-bp), forward
5′-CGTTGACAT-CCGTAAAGACCTC-3′ and reverse
5′-TAGGAGCCAGGGCAGTAATCT-3′; β-catenin (190-bp), forward
5′-GGTGAAAATGCTTGGGTCG-3′ and reverse 5′-GATCTGAAGGCAGTCTGTCGTA-3′;
JNK (132-bp), forward 5′-AGCAGT-AGCCCCGCCAGTA-3′ and reverse
5′-TGTCAGTGTCCTTCCCACCTC-3′; Ror2 (154-bp), forward
5′-TGGGAACCGAACTATTTATGTG-3′ and reverse
5′-AGGAAAGACGAAGTGGCAGA-3′; Wnt5a (236-bp), forward
5′-CAACAG-CCGCTTCAACTCC-3′ and reverse 5′-TGACATAGCAGCACCAGTGA-3′;
and LRP6 (231-bp), forward 5′-TGATAACCAGTTCACGGATGC-3′ and reverse
5′-CCATTTGACAGGCGGAAAG-3′. All experiments were performed in
duplicate and normalized to actin as an invariant endogenous
control. The PCR conditions were 50°C for 2 min and 94°C for 2 min,
followed by 40 cycles of 94°C for 15 sec and 60°C for 30 sec. The
results were calculated using the ΔΔCT method and are presented as
the fold increase relative to actin expression.
Statistical analysis
Values are presented as the mean ± standard error of
the mean. The significance of differences was estimated by analysis
of variance followed by Student-Newmann-Keuls multiple comparison
tests. P<0.05 was considered to indicate a statistically
significant difference between values. All statistical analyses
were performed using SPSS software (version 11.0; SPSS Inc.
Chicago, IL, USA).
Results
Cultured passage three MSCs
Passage three rat MSCs were positive for CD29 and
CD90, while they were negative for CD45 and CD11b, and cells were
different from hemopoietic stem cells (Fig. 1).
Calcified VSMCs
VSMCs were cultured in OS for 21 days.
Representative images of von Kossa staining are shown in Fig. 2. Von Kossa staining revealed that
no calcification was present in VSMCs cultured without OS (Fig. 2A), and there were evidently
positively-stained, black, calcified nodules in VSMCs cultured with
OS (Fig. 2B, black arrows).
MSCs cultured in OS did not exhibit an
osteoblast phenotype when in direct contact with non-calcified
VSMCs
As previously published by our group (21), OS did not increase the ALP activity
when MSCs were cultured with non-calcified VSMCs. ALP staining was
negative (Fig. 3), and there were
no differences in ALP activity between the S and S+M groups;
however, ALP activity was slightly elevated in the S+M+OS group
(P<0.05).
MSCs exhibit an osteoblast phenotype when
in direct contact with calcified VSMCs
ALP staining was positive when MSCs were directly
co-cultured with calcified VSMCs, which was most significant in the
GS+M+OS group. However, ALP staining was negative when MSCs were
directly co-cultured with non-calcified VSMCs, and there was no
significant difference compared with that of VSMCs cultured alone
(Fig. 3). In addition, ALP
activity was more significant in the GS+M+OS and GS+M groups
compared with that in the GS group (***P<0.01;
Fig. 4A).
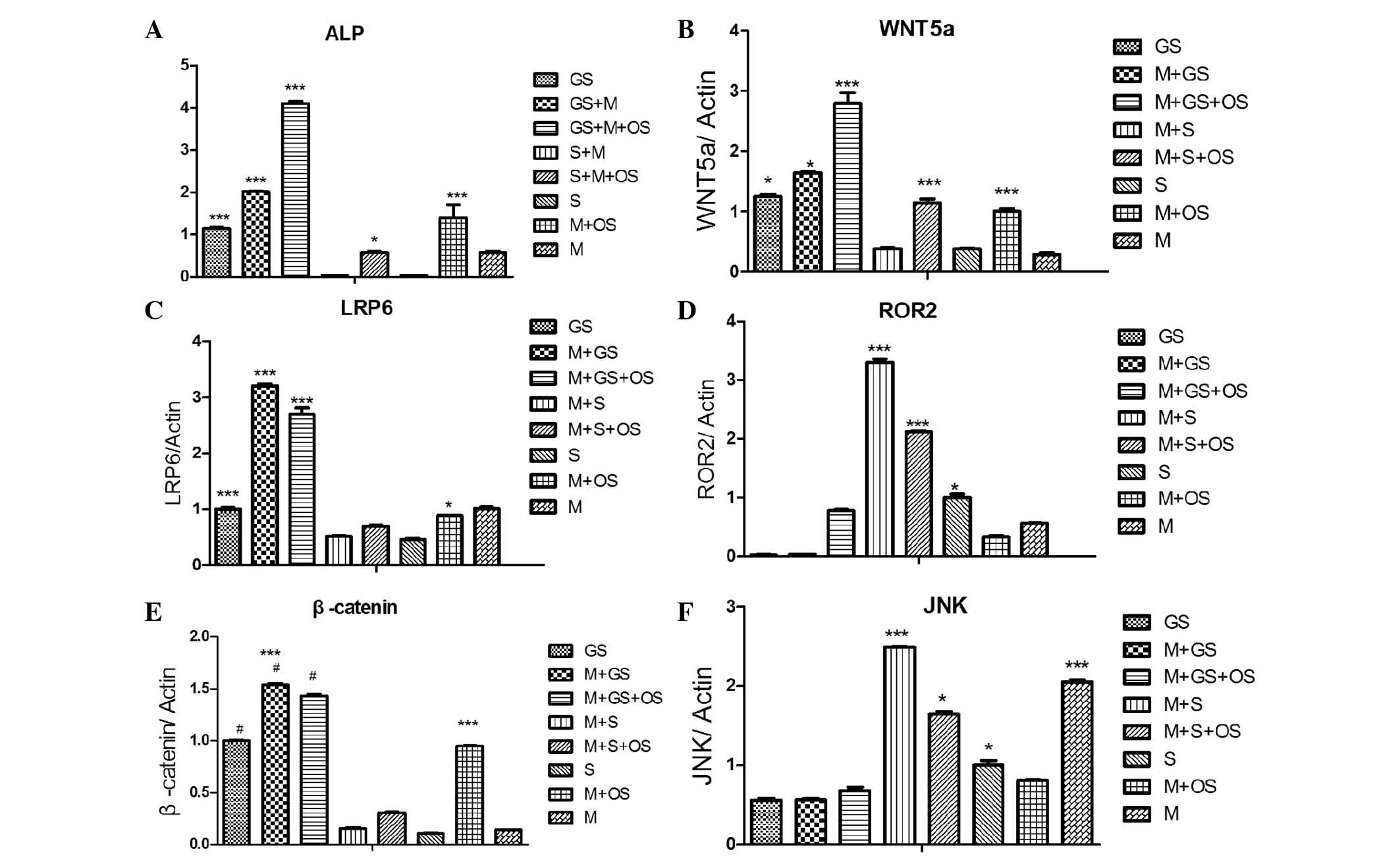 | Figure 4Direct co-cultures were established
by two different cell types, normal or calcified SMCs, being mixed
with MSCs at a ratio of 5,000:5,000. Next, cells were cultured for
14 days and the mRNA expression levels of the genes encoding
Wnt-signaling proteins, Wnt5a, LRP6, Ror2, JNK and β-catenin, were
investigated. (A) ALP activity of each groups. (B) mRNA levels of
wnt5a. (C) mRNA levels of LRP6. (D) mRNA levels of Ror2. (E) mRNA
levels of β-catenin. (F) mRNA levels of JNK.
***P<0.01, *P<0.05, compared with the
M+S group, the M+S+OS group, the S group. The 8 groups were as
follows: GS (calcified SMCs); GS+M (calcified SMCs co-cultured with
MSCs); GS+M+OS (MSCs and calcified SMCs cultured in OS); S (SMCs);
S+M (MSCs co-cultured with SMCs); S+M+OS (SMCs and MSCs cultured in
OS); M (MSCs); M+OS (MSCs cultured in OS) SMCs, smooth muscle
cells; MSCs, mesenchymal stem cells; JNK, c-Jun-N-terminal kinase;
ALP, alkaline phosphatase; OS, osteosynthesis-inducing medium. |
Different expression levels of genes
encoding for proteins involved in Wnt signaling may be associated
with the differentiation of MSCs cultured with normal or calcified
VSMCs
In order to directly investigate the changes in the
expression of proteins involved in the Wnt pathway, the gene
expression levels of Wnt5a, the Wnt co-receptors LRP6 and Ror2, as
well as the Wnt production of JNK and β-catenin were assessed in
each group. Wnt5a levels were highest in the M+GS, M+GS+OS, GS and
M+OS groups (***P<0.001); however, they were low in
the M+S and S groups (***P<0.001) (Fig. 4B). The gene expression levels of
LRP6 and β-catenin were significantly reduced in the M+S, M+S+OS
and S groups; whereas they were increased in the M+GS, M+GS+OS, GS
and M+OS groups (***P<0.001; Fig. 4C and E). Of note, the mRNA levels
of Ror2 and JNK were markedly reduced in the M+GS, M+GS+OS, GS and
M+OS groups only, while they were increased in the M+S, M+S+OS, S
and M groups (***P<0.01; Fig. 4D and F). Although decreases in Ror2
and JNK expression were also apparent in the M+GS, M+GS+OS, GS and
M+OS groups, they were not statistically significant (P>0.05).
No differences were identified between the mRNA expression levels
of the LRP6 and β-catenin genes investigated in the M+S, M+S+OS and
S groups (P>0.05).
Discussion
MSCs have a critical role in tissue regeneration and
homeostasis. However, it is noteworthy that the present study has
identified that circulating concentrations of stem cell-mobilizing
cytokines were associated with the levels of osteoprogenitor cells
and aortic calcification severity (23). The major factors, resident cellular
(15,23) and local environment (24), are likely to determine the fate of
MSCs. Additionally, previous studies indicated that direct
cell-to-cell contact between resident cells and MSCs was critical
in the differentiation of MSCs (25). The present study examined MSCs
co-cultured with non-calcified or calcified VSMCs in vitro by a
direct cell-cell co-culturing system. The data of the present study
indicated that direct cell-to-cell contact between resident cells,
VSMCs and MSCs, was decisional in the differentiation of MSCs into
non-calcified or calcified VSMCs.
Numerous cell surface receptors have been used to
create functional surfaces in order to enhance cell adhesion or to
alter cell morphology, including cell adhesion molecules (26), cadherins (27), etc. Wnt-signaling provides
instructive cues for the recruitment, maintenance and
differentiation of MSCs (28),
which is mediated through cell-cell interactions and is involved in
numerous developmental processes and cellular functions. Wnt
signaling has been implicated in the self-renewal and maintenance
of pluripotent stem cells (7,29).
Wnts are a family of secreted glycoproteins that bind to a class of
Frizzled (Frz) receptors (30).
The conserved Wnt cascade was composed by the canonical
Wnt/β-catenin and non-canonical Wnt/Ror2 pathways. It has been
reported that canonical Wnt-signaling can activate osteogenesis in
mineralization in certain cellular contexts, and promotes the
osteoblastogenesis of murine pluripotent mesenchymal and
osteoprogenitor cells by upregulation of runt-related transcription
factor 2 or osterix (31).
Additionally, β-catenin is a transcriptional co-adaptor that is
indispensable for osteogenic tissue mineralization and takes part
in osteochondrogenic differentiation of mural mesenchymal
progenitors (32). Stabilization
of cytoplasmic β-catenin is the hallmark of activated canonical Wnt
signaling (33,34). Mice with stabilized β-catenin
expressed in their cardiomyocytes also revealed a functional
decline following injury (33).
Therefore, numerous and diverse studies have converged on β-catenin
activation as a key component of arteriosclerotic physiology.
The low-density lipoprotein (LDL) receptor-related
protein (LRP) family is well known for its ability to bind multiple
ligands, including apolipoprotein E, very LDL remnants, lipoprotein
lipase, tissue-type and urokinase-type plasminogen activators and
thrombospondin I. To date, studies have only identified LRP5 and
LRP6 as coreceptors for the canonical Wnt signaling cascade. LRP6
is a single-pass transmembrane protein composed of four
extracellular epidermal growth factor type repeats and three LDLR
repeats (28). The important role
of the cytoplasmic region of LRP6 lies in promoting canonical Wnt
activation (35). In the present
study, the gene expression levels of LRP6 and β-catenin were
significantly reduced in the M+S, M+S+OS and S groups, whereas they
were increased in the M+GS, M+GS+OS, GS and M+OS groups.
In K562 cells, overexpression of Wnt5a through Ror2
is absorbed by the body to activate the nonclassical
Wnt5a/Ror2/Frz4 pathway and inhibits the canonical Wnt signaling
pathway. However, when Ror2 receptor expression is reduced or
absent, the Wnt5a/Frz4/LRP5 classical pathway is activated
(36). The objective of the
present study was to test the hypothesis that LRP6 and Ror2 have an
integral role in Wnt signaling in the differentiation of MSCs in
calcification. The observations of the present study support this
hypothesis and provide the first evidence of a well-defined pathway
linking an LDL receptor-associated protein to the differentiation
of MSCs in calcification. The Wnt pathways are capable of
regulating inflammation and foam cell formation, pathological
angiogenesis and calcification, which have critical roles in plaque
formation and stability. A greater understanding of the Wnt
pathways in the differentiation of MSCs in calcification may reveal
novel therapeutic targets for vascular disease. The contribution of
the Wnt signaling pathway in the differentiation of MSCs in
calcification was investigated, indicating that specific targeting
of this pathway may lead to treatment options for arteriosclerosis.
In the present study, the mRNA levels of Ror2 and JNK were markedly
reduced in the M+GS, M+GS+OS, GS and M+OS groups only, whereas they
were increased in the M+S, M+S+OS, S and M groups. Although a
decrease in the expression of Ror2 and JNK was also apparent in the
M+GS, M+GS+OS, GS and M+OS groups, it did not reach a statistically
significant difference.
However, a non-canonical Wnt-signaling pathway,
known as Wnt5a/Ror2, which in general transduces through the
JNK/planar cell polarity or the calcium-releasing pathways and
regulates cell movement, can inhibit canonical Wnt-signaling
(37). Wnt5a, a member of the Wnt
family that is indicated to have a role in hydrophobic cell-cell
interactions, is predominantly characterized as a non-canonical Wnt
ligand, which activates intracellular signaling via distinct
receptors or co-receptors (29).
Of note, the present study revealed that Wnt5a may be involved in
the pathogenesis of atherosclerosis rather than a protection from
it, and the expression of Wnt5a mRNA correlates with the severity
of atherosclerotic lesions (38).
The present study identified that Wn5a was highest in the M+GS,
M+GS+OS, GS and M+OS groups, but not in the M+S and S groups.
Ror2, an orphan tyrosine kinase possessing an
extracellular cysteine-rich Wnt binding domain (39), has been demonstrated to function as
a receptor for Wnt5a (30),
inducing a non-canonical cascade involving the activation of JNK
and the inhibition of canonical signaling (34). The interaction between Ror2 and
Wnt5a to mediate the non-canonical Wnt signaling pathway has
received great attention in recent years (34). In addition, developmental
phenotypes lacking Ror2 and Wnt5a lead to dwarfism, shortened
limbs, facial abnormalities, ventricular septal defects in the
heart and abnormalities in lung development (34). The absence of Ror2 leads to
enhanced Wnt/β-catenin signaling, specifically in cells that have
lost Ror2 expression (38). This
observation indicates that the intracellular domain of Ror2 is
required for Wnt5a/Ror2 signaling to be functional. Overall,
previous studies have indicated that Wnt5a/Ror2 signaling is
capable of inhibiting canonical Wnt signaling in vivo, and
point to Ror2 as being a potential therapeutic target for the
treatment of human diseases. It was revealed that the mRNA levels
of ROR2 and JNK were markedly reduced in the M+GS, M+GS+OS, GS and
M+OS groups only, whereas they were increased in the M+S, M+S+OS, S
and M groups.
The present study provided direct evidence for a
significant role of Wnt signaling mediating the differentiation of
MSCs in calcification. To date, further studies remain to be
undertaken in order to understand either the function of VSMCs or
the complexities of the signaling pathways activated as a
consequence of their stimulation or inhibition. It is expected that
further studies lead to an improved comprehension of processes and
substances involved in vascular remodeling and repair.
In conclusion, the present study demonstrated the
expression of Wnts in the progress of differentiation of MSCs in
calcification. MSCs are able to differentiate into different cell
phenotypes when in direct cell-cell contact with SMCs or calcified
SMCs, and the Wnt5a/Ror2 signaling pathway may be associated with
the determination of the differentiation of MSCs in this process.
This observation opens an avenue for the development of novel
strategies of MSC transplantation for treating
arteriosclerosis.
References
|
1
|
Boström KI, Rajamannan NM and Towler DA:
The regulation of valvular and vascular sclerosis by osteogenic
morphogens. Circ Res. 109:564–577. 2011.PubMed/NCBI
|
|
2
|
Olivares-Navarrete R, Hyzy SL, Park JH, et
al: Mediation of osteogenic differentiation of human mesenchymal
stem cells on titanium surfaces by a Wnt-integrin feedback loop.
Biomaterials. 32:6399–6411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abedin M, Tintut Y and Demer LL:
Mesenchymal stem cells and the artery wall. Circ Res. 95:671–676.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leroux L, Descamps B, Tojais NF, et al:
Hypoxia preconditioned mesenchymal stem cells improve vascular and
skeletal muscle fiber regeneration after ischemia through a
Wnt4-dependent pathway. Mol Ther. 18:1545–1552. 2010. View Article : Google Scholar
|
|
5
|
Alfaro MP, Pagni M, Vincent A, et al: The
Wnt modulator sFRP2 enhances mesenchymal stem cell
engraftment, granulation tissue formation and myocardial repair.
Proc Natl Acad Sci USA. 105:18366–18371. 2008.PubMed/NCBI
|
|
6
|
Moioli EK, Clark PA, Chen M, et al:
Synergistic actions of hematopoietic and mesenchymal
stem/progenitor cells in vascularizing bioengineered tissues. PLoS
One. 3:e39222008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qian H, Yang Y, Li J, et al: The role of
vascular stem cells in atherogenesis and post-angioplasty
restenosis. Ageing Res Rev. 6:109–127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bell GI, Meschino MT, Hughes-Large JM, et
al: Combinatorial human progenitor cell transplantation optimizes
islet regeneration through secretion of paracrine factors. Stem
Cells Dev. 21:1863–1876. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang Z, Ren PG, Ma T, Smith RL and
Goodman SB: Modulating osteogenesis of mesenchymal stem cells by
modifying growth factor availability. Cytokine. 51:305–310. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pelttari K, Winter A, Steck E, et al:
Premature induction of hypertrophy during in vitro
chondrogenesis of human mesenchymal stem cells correlates with
calcification and vascular invasion after ectopic transplantation
in SCID mice. Arthritis Rheum. 54:3254–3266. 2006.PubMed/NCBI
|
|
11
|
Kramann R, Kunter U, Brandenburg VM, et
al: Osteogenesis of heterotopically transplanted mesenchymal
stromal cells in rat models of chronic kidney disease. J Bone Miner
Res. 28:2523–2534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shabbir A, Zisa D, Suzuki G and Lee T:
Heart failure therapy mediated by the trophic activities of bone
marrow mesenchymal stem cells: a noninvasive therapeutic regimen.
Am J Physiol Heart Circ Physiol. 296:H1888–H1897. 2009. View Article : Google Scholar
|
|
13
|
Tran TC, Kimura K, Nagano M, et al:
Identification of human placenta-derived mesenchymal stem cells
involved in re-endothelialization. J Cell Physiol. 226:224–235.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chow K, Fessel JP, Kaoriihida S, et al:
Dysfunctional resident lung mesenchymal stem cells contribute to
pulmonary microvascular remodeling. Pulm Circ. 3:31–49. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsaousi A, Mill C and George SJ: The Wnt
pathways in vascular disease: lessons from vascular development.
Curr Opin Lipidol. 22:350–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marinou K, Christodoulides C, Antoniades C
and Koutsilieris M: Wnt signaling in cardiovascular physiology.
Trends Endocrinol Metab. 23:628–636. 2012. View Article : Google Scholar
|
|
17
|
Cheng CW, Yeh JC, Fan TP, et al:
Wnt5a-mediated non-canonical Wnt signalling regulates human
endothelial cell proliferation and migration. Biochem Biophy Res
Commun. 365:285–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ling L, Nurcombe V and Cool SM: Wnt
signaling controls the fate of mesenchymal stem cells. Gene.
433:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stolzing A, Jones E, McGonagle D and Scutt
A: Age-related changes in human bone marrow-derived mesenchymal
stem cells: consequences for cell therapies. Mech Ageing Dev.
129:163–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xin H, Xin F, Zhou S and Guan S: The
Wnt5a/Ror2 pathway is associated with determination of the
differentiation fate of bone marrow mesenchymal stem cells in
vascular calcification. Int J Mol Med. 31:583–588. 2013.PubMed/NCBI
|
|
21
|
Laumanns IP, Fink L, Wilhelm J, et al: The
noncanonical WNT pathway is operative in idiopathic pulmonary
arterial hypertension. Am J Respir Cell Mol Biol. 40:683–691. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pal SN, Clancy P and Golledge J:
Circulating concentrations of stem-cell-mobilizing cytokines are
associated with levels of osteoprogenitor cells and aortic
calcification severity. Circ J. 75:1227–1234. 2011. View Article : Google Scholar
|
|
23
|
Ball SG, Shuttleworth AC and Kielty CM:
Direct cell contact influences bone marrow mesenchymal stem cell
fate. Int J Biochem Cell Biol. 36:714–727. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yip CY and Simmons CA: The aortic valve
microenvironment and its role in calcific aortic valve disease.
Cardiovasc Pathol. 20:177–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang T, Xu Z, Jiang W and Ma A:
Cell-to-cell contact induces mesenchymal stem cell to differentiate
into cardiomyocyte and smooth muscle cell. Int J Cardiol.
109:74–81. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mangan SH, Van Campenhout A, Rush C and
Golledge J: Osteoprotegerin upregulates endothelial cell adhesion
molecule response to tumor necrosis factor-alpha associated with
induction of angiopoietin-2. Cardiovasc Res. 76:494–505. 2007.
View Article : Google Scholar
|
|
27
|
Menge T, Gerber M, Wataha K, et al: Human
mesenchymal stem cells inhibit endothelial proliferation and
angiogenesis via cell-cell contact through modulation of the
VE-Cadherin/β-catenin signaling pathway. Stem Cells Dev.
22:148–157. 2013.PubMed/NCBI
|
|
28
|
Towler DA and Demer LL: Thematic series on
the pathobiology of vascular calcification: an introduction. Circ
Res. 108:1378–1380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Johnson ML and Rajamannan N: Diseases of
Wnt signaling. Rev Endocr Metab Disord. 7:41–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ermakov S, Trofimov S, Malkin I and
Livshits G: A significant association exists between receptor
tyrosine kinase-like orphan receptor 2 gene variants and the
OPG/RANKL ratio in human plasma. Osteoporos Int. 23:1899–1907.
2012. View Article : Google Scholar
|
|
31
|
Qiu W, Chen L and Kassem M: Activation of
non-canonical Wnt/JNK pathway by Wnt3a is associated with
differentiation fate determination of human bone marrow stromal
(mesenchymal) stem cells. Biochem Biophys Res Commun. 413:98–104.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian Y, Cohen ED and Morrisey EE: The
importance of Wnt signaling in cardiovascular development. Pediatr
Cardiol. 31:342–348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takahashi N, Maeda K, Ishihara A, Uehara S
and Kobayashi Y: Regulatory mechanism of osteoclastogenesis by
RANKL and Wnt signaling. Front Biosci (Landmark Ed). 16:21–30.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grumolato L, Liu G, Mong P, et al:
Canonical and noncanonical Wnts use a common mechanism to activate
completely unrelated coreceptors. Genes Dev. 24:2517–2530. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yuan Y, Niu CC, Deng G, et al: The
Wnt5a/Ror2 noncanonical signaling pathway inhibits canonical Wnt
signaling in K562 cells. Int J Mol Med. 27:63–69. 2011.PubMed/NCBI
|
|
36
|
Laird DJ, Altshuler-Keylin S, Kissner MD,
et al: Ror2 enhances polarity and directional migration of
primordial germ cells. PLoS Genet. 7:e10024282011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Christman MA, Goetz DJ, Dicherson E, et
al: Wnt5a is expressed in murine and human atherosclerotic lesions.
Am J Physiol Heart Circ Physiol. 294:H2864–H2870. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mikels A, Minami Y and Nusse R: Ror2
receptor requires tyrosine kinase activity to mediate Wnt5A
signaling. J Biol Chem. 284:30167–30176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Y, Rubin B, Bodine PV and Billiard J:
Wnt5a induces homodimerization and activation of Ror2 receptor
tyrosine kinase. J Cell Biochem. 105:497–502. 2008. View Article : Google Scholar : PubMed/NCBI
|


















