Introduction
Myocardial ischemia-reperfusion (IR) injury is the
predominant injury resulting from revascularization treatments,
such as coronary thrombolysis, percutaneous coronary interventions,
coronary artery bypass grafts and cardiac transplants (1). During ischemia, mitochondria produce
reactive oxygen species (ROS) and during reperfusion an extra burst
of ROS generation occurs (2).
Furthermore, during reperfusion, multiple molecular inflammatory
cascades are activated; including those involving interleukin
(IL)-1 and tumor necrosis factor (TNF) α. Chemokine production is
induced within hours of reperfusion (3), with pro-inflammatory cytokines
sequentially inducing cell autophagy (4).
Autophagy is a type of programmed cell death, which
has been indicated to be significant in cell homeostasis, as well
as cell defense and adaptation to adverse environments (5–7).
Autophagy has an important role in the heart and activation of
autophagy has been observed in a variety of heart diseases,
including cardiac hypertrophy, heart failure and IR injury.
Autophagy performs two opposing functions in the heart (8); it has a protective role during
nutrient deprivation and other forms of cellular stress, however,
excessive autophagy may be used for self-destruction (9).
Ulinastatin is a multivalent enzyme inhibitor, which
is predominantly used for the treatment of pancreatitis, severe
infection-induced acute circulatory failure, as well as for the
prevention of multiple organ failure (10). Recently, ulinastatin has been found
to exhibit myocardial protective effects through inhibiting the
expression of TNF, reducing levels of oxygen free radicals and
increasing levels of endogenous nitric oxide (11,12).
During myocardial IR injury, ulinastatin has been reported to show
protective effects via the induction of an anti-inflammatory
response (1). However, whether the
protective effect of ulinastatin in cardiomyocytes is associated
with cardiomyocyte autophagy, is yet to be elucidated. The present
study aimed to investigate whether ulinastatin reduces
cardiomyocyte injury through regulating autophagy and its
associated mechanisms.
Material and methods
Animals
All animal experiments were approved by the Animal
Research Ethics Committee of the Second Military Medical University
(Shanghai, China). The experiments conformed with the Guide for the
Care and Use of Laboratory Animals published by the US National
Institutes of Health.
Cell culture and experimental
protocols
Neonatal cardiomyocytes were prepared from the
hearts of Sprague-Dawley rats (age, <3 days) (13). On day four, the cardiomyocytes were
randomly divided into the following three groups: Con, a control
group where the cells were cultured in Dulbecco’s modified Eagle’s
medium and incubated in an atmosphere of 5% CO2 for 24
h; HR, a hypoxia-reoxygenation group where the cells were incubated
with 1% O2, 5% CO2 and 94% N2 for
24 h, followed by 5% CO2 and 95% air for 6 h; and an
ulinastatin group, where the cells were treated with
1×104 U/l ulinastatin for 30 min prior to HR, then were
incubated under the same conditions as the HR group. In order to
investigate whether mammalian target of rapamycin (mTOR) was
involved in the protective effect of ulinastatin, the cells were
treated with the mTOR inhibitor, rapamycin (20 nM) 30 min prior to
ulinastatin treatment.
MTT assay
An MTT assay was used to assess cardiomyocyte
vitality. The cardiomyocytes were cultured in 96-well plates at a
density of 1×103 cells. MTT solution (10 μl; Sigma, St.
Louis, MO, USA) was added to the growing cells and incubated for 4
h. The crystals were then solubilized by adding 100 μl
solubilization solution. The absorbance of the purple solution was
determined at a wavelength of 450 nm using a microtiter plate
reader (Bio-Rad, Hercules, CA, USA).
Western blot analysis
The protein concentration was determined using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology, Shanghai, China) according the manufacturer’s
instructions. Equal quantities of protein (50 μg) from the
cardiomyocytes were subjected to western blot analysis using
anti-LC3-I, -LC3-II (Sigma-Aldrich, St. Louis, MO, USA),
anti-p-Akt, -p-mTOR and -p-P70S6K (Cell Signaling Technology, Inc.,
Beverly, MA, USA) primary antibodies to detect LC3, p-Akt
(Ser-473), p-mTOR (Ser-2448) and p-P70S6K (Thr-389) protein
expression, respectively. An enhanced chemiluminescence detection
kit (Amersham Biosciences, Picastaway, NJ, USA) was used to detect
the immunoreactive protein bands. The autophagy results were
presented as the LC3-II/LC3-I expression ratio.
In vivo rat model and experimental
protocols
Sprague-Dawley rats weighing between 250 and 300 g
were anesthetized using intraperitoneal injection of 10% chloral
hydrate (300 mg/kg body weight), prior to endotracheal intubation.
IR was induced by ligating the left anterior descending artery as
previously reported (14). Thirty
rats were randomly divided into three equal groups: Control group,
where the rats underwent thoracotomy without ligation; IR group,
where the rats were treated with ischemia for 30 min and
reperfusion for 6 h; and an ulinastatin group, where the rats were
treated with ulinastatin (1×104 U/kg body weight) by
intraperitoneal injection, 30 min prior to IR, followed by the same
conditions as the IR group.
Measuring infarct size
Myocardial infarct size was measured as previously
described (15). The total left
ventricular area, infarct area (INF) and area at risk (AAR) were
assessed. The percentage of the INF/AAR was calculated.
Lactate dehydrogenase (LDH) assay
Blood and culture serum were collected following
reperfusion from the rats and cultured cardiomyocytes,
respectively, in order to determine LDH levels.
Statistical analysis
Quantitative data are presented as the mean ±
standard error. Statistical significance was determined using
one-way analysis of variance and P<0.05 was considered to
indicate a statistically significant difference.
Results
Ulinastatin attenuates HR-induced
cardiomyocyte injury in vitro
Following HR, a decrease in cardiomyocyte vitality
and an increase in LDH levels in the culture serum were observed.
To assess the protective effect of ulinastatin, cardiomyocytes were
treated with ulinastatin prior to HR. Cell vitality was found to
increase and LDH levels in the culture serum were found to decrease
in the HR+ulinastatin group compared with the HR group (Fig. 1).
Ulinastatin attenuates HR-induced
cardiomyocyte autophagy in vitro
In order to analyze cardiomyocyte autophagy, LC3
protein expression was assessed using western blot analysis. The
LC3-II/LC3-I expression ratio was used to measure the relative
autophagy levels in cardiomyocytes. HR was found to induce
cardiomyocyte autophagy, which was attenuated by ulinastatin
treatment (Fig. 2).
Ulinastatin inhibits cardiomyocyte
autophagy through mTOR in vitro
In order to investigate the mechanism underlying the
anti-autophagic effect of ulinastatin in cardiomyocytes, the
protein expression of p-Akt (Ser-473), p-mTOR (Ser-2448) and
p-P70S6K (Thr-389) was analyzed. Treatment with ulinastatin prior
to HR significantly increased the protein expression of p-Akt,
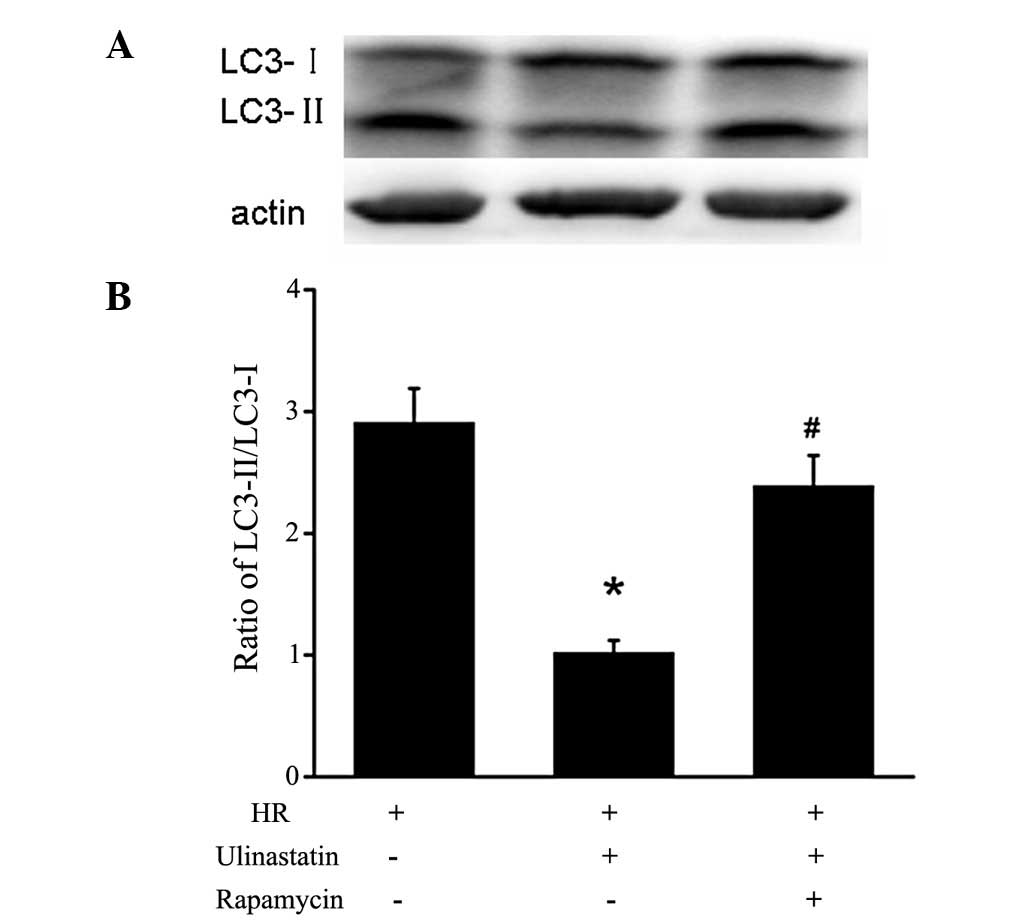
p-mTOR and p-P70S6K compared with the HR group (Fig. 3). Furthermore, the LC3-II/I
expression ratio was significantly increased in the cells treated
with rapamycin+ulinastatin compared with those treated with
ulinastatin, indicating that rapamycin attenutates the
ulinastatin-induced inhibition of autophagy (Fig. 4).
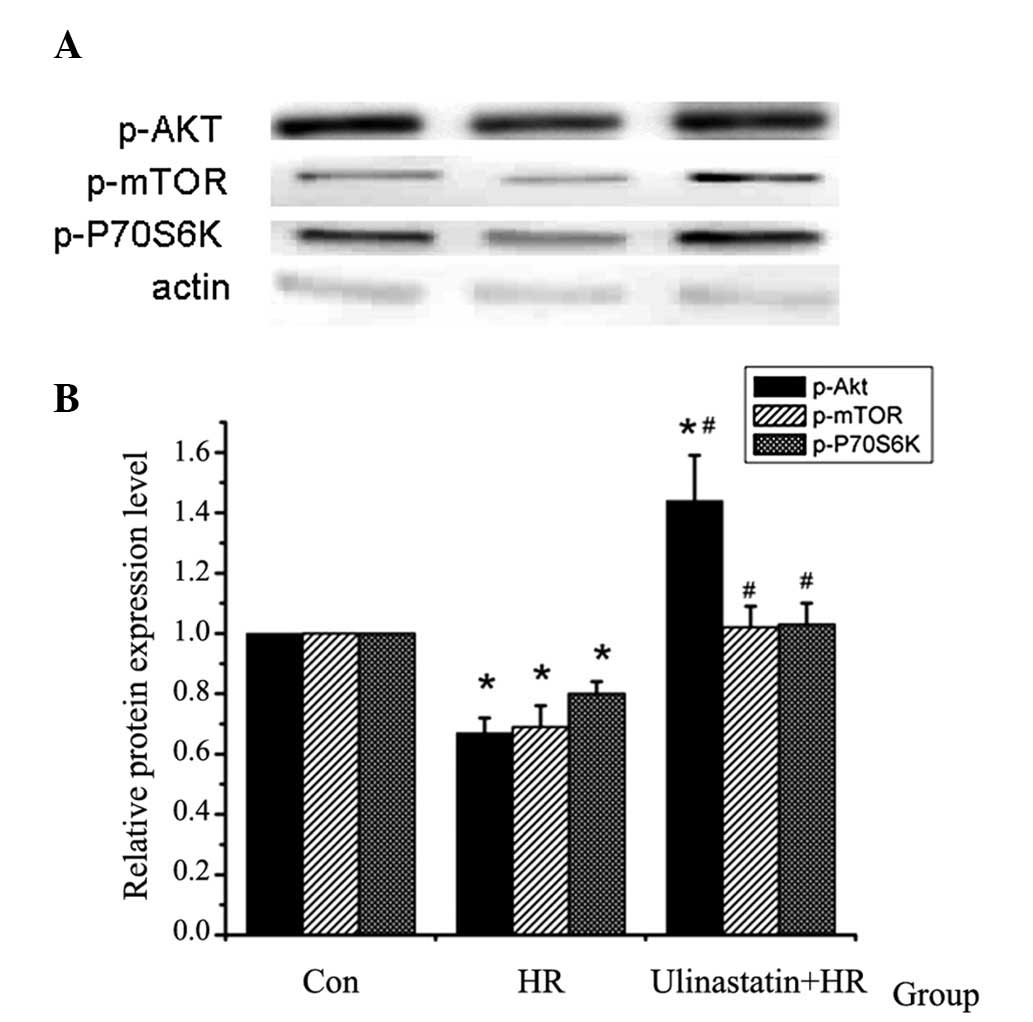 | Figure 3p-Akt, p-mTOR and p-P70S6K protein
expression detected using western blot analysis (n=5). (A) Western
blot analysis of p-Akt, p-mTOR and p-P70S6K protein expression in
the different groups. (B) Relative protein expression of p-Akt,
p-mTOR and p-P70S6K in the different groups. p-Akt, p-mTOR and
p-P70S6K expression were found to be downregulated by HR, and this
HR-induced downregulation was attenuated by ulinastatin.
(*P<0.001, P=0.017 vs. Con and #P=0.008
vs. HR). p-, phosphorylated; Akt, protein kinase B; mTOR, mammalian
target of rapamycin; P70S6K, P70S6 kinase; Con, control; HR,
hypoxia-reoxygenation. |
Ulinastatin attenuates myocardial IR
injury by inhibiting autophagy in vivo
To demonstrate the protective myocardial and
anti-autophagic effects of ulinastatin, the rats were treated with
ulinastatin (1×104 U/kg body weight) 30 min prior to IR.
Ulinastatin inhibited IR-induced cardiomyocyte autophagy and
reduced the relative area of the infarct, serum LDH levels and
inflammation (Fig. 5).
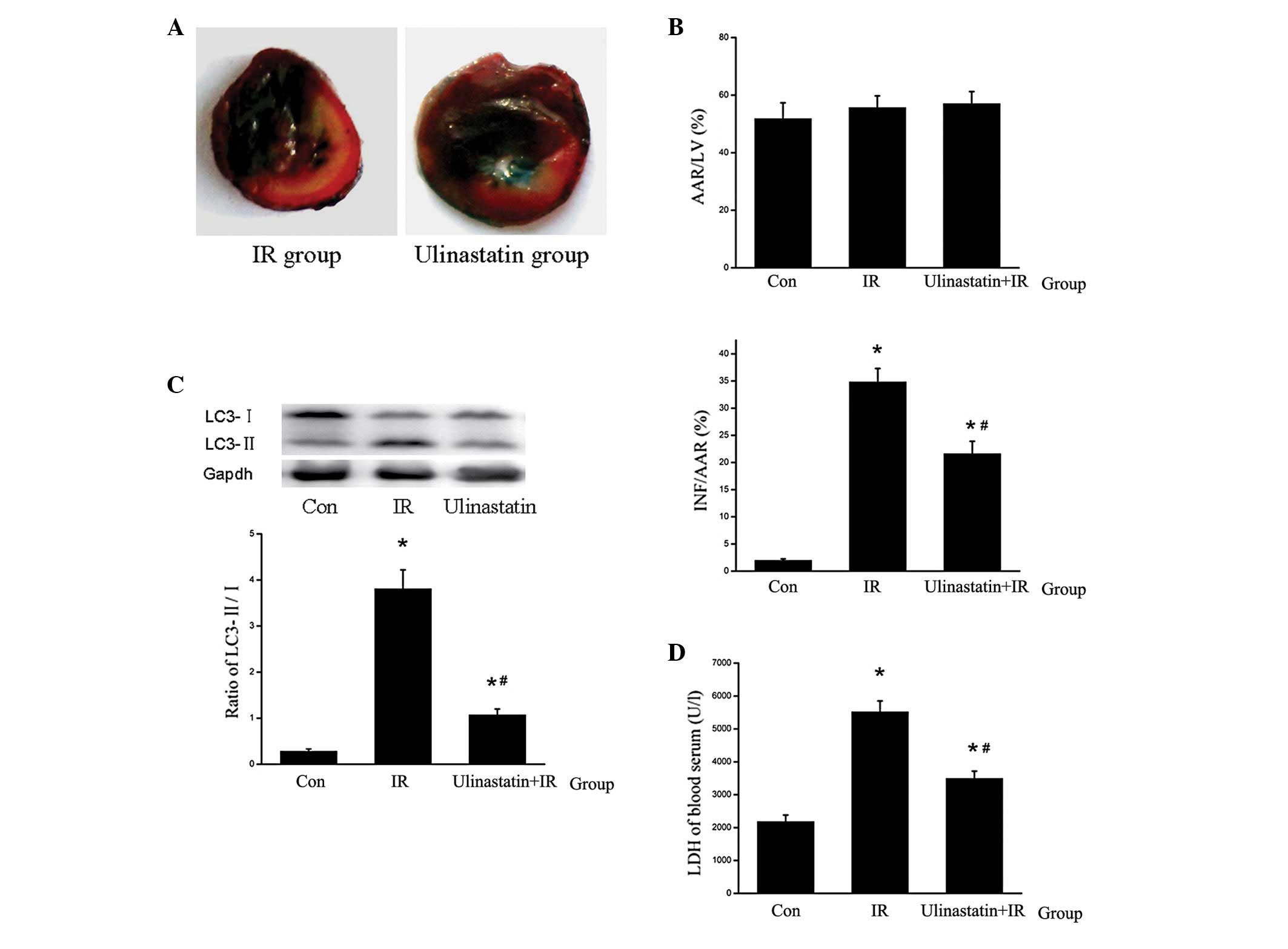 | Figure 5Ulinastatin inhibits cardiomyocyte
autophagy and attenuates IR-induced myocardial injury (n=6). (A)
Mid-myocardial cross sections of triphenyltetrazolium
chloride-stained hearts. Dark blue area, nonischemic zone; white
area, INF; red area, AAR (B) AAR/LV and INF/AAR ratios. No
significant difference was observed in AAR/LV among the Con, IR and
ulinastatin groups. However ulinastatin was found to significantly
attenuate the INF/AAR ratio in the ulinastatin+IR group compared
with the IR group. *P<0.001 vs. Con and
#P<0.001 vs. IR. (C) LC3-II/LC3-I ratio in the
different groups. LC3-II was upregulated by IR and downregulated by
ulinastatin in vivo. *P<0.001,
*P=0.047 vs. Con and #P<0.001 vs. IR. (D)
LDH levels in the blood serum. Ulinastatin reduced LDH levels in
the blood serum compared with the IR group. *P<0.001,
*P=0.002 vs. Con and #P<0.001 vs. IR. IR,
ischemia reperfusion; AAR, area at risk; LV, left ventricle; Con,
control; LC3, light chain 3; INF, infracted tissue. |
Discussion
Ulinastatin has been proposed as a myocardial
protective agent. Ulinastatin has been reported to improve
myocardial contractility, reduce myocardial infarct size and
decrease serum levels of creatine kinase and cardiac troponin I
following myocardial IR injury in vivo (1). In the present study, cultured primary
cardiomyocytes were treated with ulinastatin prior to HR in
vitro. Ulinastatin was found to improve cell vitality and
reduce LDH levels, thus ulinastatin may have a protective effect
against HR injury in cardiomyocytes in vitro. Furthermore,
the myocardial protective effect of ulinastatin was was found to be
associated with various mechanisms in an in vivo rat heart
model. It has previously been reported that ulinastatin may
contribute to the recovery of cardiac function following
reperfusion by reducing mitochondrial dysfunction and maintaining
energy production (16). Moreover,
the protective effect of ulinastatin may be associated with
anti-inflammatory effects. For example, ulinastatin has been
reported to reduce the expression of TNF and IL-6 and upregulate
that of IL-10 and -13 (12,17–21).
It has been reported that TNF stimulates autophagy,
while IL-13 suppresses autophagy through stimulating the
phosphoinositide 3-kinase/mTOR signal transduction pathway
(4,9,22).
Therefore, ulinastatin may inhibit autophagy via the downregulation
of TNF and the upregulation of IL-13. Autophagy is regulated by
autophagy-related genes (Atgs), among which beclin 1 is required
for the vesicle nucleation step of autophagy. Autophagosome
formation involves two complexes, Atg12-Atg5-Atg16 and
Atg4-Atg7-Atg3. These complexes are involved in the conversion of
the soluble form of LC3 (LC3-I) to the autophagic
vesicle-associated form (LC3-II), which is used as a marker of
autophagy (23). The LC3-II/LC3-I
ratio has been used for analyzing autophagy in numerous studies. In
a previous study on an IR model, autophagy was found to be markedly
enhanced and inhibition of autophagy, via the downregulation of
beclin 1, was observed to have a protective effect in vivo
(24).
In the present study, HR-induced LC3-II protein
expression was found to be downregulated by ulinastatin in
vitro. Furthermore, ulinastatin was observed to upregulate
p-Akt, p-mTOR and p-P70S6K protein expression. To assess whether
ulinastatin inhibits HR-induced cardiomyocyte autophagy through
activating mTOR, cells were treated with the mTOR-specific
inhibitor, rapamycin prior to HR and ulinastatin treatment.
Rapamycin treatment was found to upregulate LC3-II, indicating that
rapamycin attenuated the anti-autophagic effect of ulinastatin.
Therefore, ulinastatin may protect cardiomyocytes against HR injury
by inhibiting autophagy through activating Akt/mTOR. This may be
associated with ulinastatin-induced anti-inflammatory effects.
In order to investigate the anti-autophagic effect
of ulinastatin in vivo, a rat IR model was established.
Certain rats were treated with ulinastatin (1×104U/kg
body weight) 30 min prior to IR. Inflammation was found to be
reduced by ulinastatin. Furthermore, myocardium LC3-II protein
expression, myocardial infarct size and serum LDH levels were
observed to be reduced in the ulinastatin group compared with the
IR group.
In conclusion, the present study demonstrated that
ulinastatin has an important protective role against IR injury by
regulating autophagy via the mTOR signaling pathway. Furthermore,
the protective effect of ulinastatin may be associated with its
anti-inflammatory effect.
Acknowledgements
The present study was supported by the Tian Pu
Research Foundation, Nature Science Foundation of Science and
Technology Commission of Shanghai Municipality (grant no.
112ZR1454600) and the National Natural Science Foundation of China
(grant no. 81200181).
References
|
1
|
Shin IW, Jang IS, Lee SM, et al:
Myocardial protective effect by ulinastatin via an
anti-inflammatory response after regional ischemia/reperfusion
injury in an in vivo rat heart model. Korean J Anesthesiol.
61:499–505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vassalli G, Milano G and Moccetti T: Role
of mitogen-activated protein kinases in myocardial
ischemia-reperfusion injury during heart transplantation. J
Transplant. 2012:9289542012.
|
|
3
|
Ishii D, Schenk AD, Baba S and Fairchild
RL: Role of TNFalpha in early chemokine production and leukocyte
infiltration into heart allografts. Am J Transplant. 10:59–68.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li C, Capan E, Zhao Y, et al: Autophagy is
induced in CD4+ T cells and important for the growth
factor-withdrawal cell death. J Immunol. 177:5163–5168.
2006.PubMed/NCBI
|
|
5
|
Meléndez A, Tallóczy Z, Seaman M,
Eskelinen EL, Hall DH and Levine B: Autophagy genes are essential
for dauer development and life-span extension in C. elegans.
Science. 301:1387–1391. 2003.PubMed/NCBI
|
|
6
|
Stromhaug PE and Klionsky DJ: Approaching
the molecular mechanism of autophagy. Traffic. 2:524–531. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Otto GP, Wu MY, Kazgan N, Anderson OR and
Kessin RH: Macroautophagy is required for multicellular development
of the social amoeba Dictyostelium discoideum. J Biol Chem.
278:17636–17645. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gurusamy N and Das DK: Is autophagy a
double-edged sword for the heart? Acta Physiol Hung. 96:267–276.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Meyer GR and Martinet W: Autophagy in
the cardiovascular system. Biochim Biophys Acta. 1793:1485–1495.
2009.
|
|
10
|
Inoue K, Takano H, Shimada A, et al:
Urinary trypsin inhibitor protects against systemic inflammation
induced by lipopolysaccharide. Mol Pharmacol. 67:673–680. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu CE, Zhang MY, Zou CW and Guo L:
Evaluation of the pharmacological function of ulinastatin in
experimental animals. Molecules. 17:9070–9080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao YZ, Tu YY, Chen X, Wang BL, Zhong YX
and Liu MH: Protective effect of ulinastatin against murine models
of sepsis: inhibition of TNF-α and IL-6 and augmentation of IL-10
and IL-13. Exp Toxicol Pathol. 64:543–547. 2012.PubMed/NCBI
|
|
13
|
Jian X, Xiao-yan Z, Bin H, et al: MiR-204
regulate cardiomyocyte autophagy induced by hypoxia-reoxygenation
through LC3-II. Int J Cardiol. 148:110–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He B, Xiao J, Ren AJ, et al: Role of miR-1
and miR-133a in myocardial ischemic postconditioning. J Biomed Sci.
18:222011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhuo Y, Chen PF, Zhang AZ, Zhong H, Chen
CQ and Zhu YZ: Cardioprotective effect of hydrogen sulfide in
ischemic reperfusion experimental rats and its influence on
expression of survivin gene. Biol Pharm Bull. 32:1406–1410. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masuda T, Sato K, Noda C, et al:
Protective effect of urinary trypsin inhibitor on myocardial
mitochondria during hemorrhagic shock and reperfusion. Crit Care
Med. 31:1987–1992. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanaka R, Fujita M, Tsuruta R, et al:
Urinary trypsin inhibitor suppresses excessive generation of
superoxide anion radical, systemic inflammation, oxidative stress,
and endothelial injury in endotoxemic rats. Inflamm Res.
59:597–606. 2010. View Article : Google Scholar
|
|
18
|
Koga Y, Fujita M, Tsuruta R, et al:
Urinary trypsin inhibitor suppresses excessive superoxide anion
radical generation in blood, oxidative stress, early inflammation,
and endothelial injury in forebrain ischemia/reperfusion rats.
Neurol Res. 32:925–932. 2010. View Article : Google Scholar
|
|
19
|
Wu YJ, Ling Q, Zhou XH, et al: Urinary
trypsin inhibitor attenuates hepatic ischemia-reperfusion injury by
reducing nuclear factor-kappa B activation. Hepatobiliary Pancreat
Dis Int. 8:53–58. 2009.PubMed/NCBI
|
|
20
|
Chen CC, Liu ZM, Wang HH, He W, Wang Y and
Wu WD: Effects of ulinastatin on renal ischemia-reperfusion injury
in rats. Acta Pharmacol Sin. 25:1334–1340. 2004.PubMed/NCBI
|
|
21
|
Ren B, Wu H, Zhu J, et al: Ulinastatin
attenuates lung ischemia-reperfusion injury in rats by inhibiting
tumor necrosis factor alpha. Transplant Proc. 38:2777–2779. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deretic V: Autophagy as an immune defense
mechanism. Curr Opin Immunol. 18:375–382. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nishida K, Kyoi S, Yamaguchi O, Sadoshima
J and Otsu K: The role of autophagy in the heart. Cell Death
Differ. 16:31–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsui Y, Takagi H, Qu X, et al: Distinct
roles of autophagy in the heart during ischemia and reperfusion:
roles of AMP-activated protein kinase and Beclin 1 in mediating
autophagy. Circ Res. 100:914–922. 2007. View Article : Google Scholar : PubMed/NCBI
|