Introduction
Mesenchymal stem cells (MSCs) are progenitors of
mesoderm tissue, and can be induced to differentiate into
adipocytes, osteocytes, chondrocytes and myocytes under the
appropriate conditions (1,2). Studies investigating in vitro
tissue engineering have broadened the application of, and basic
research into, MSCs and their regulators (3–5).
Oxygen has been shown to act not only as a cellular substrate, but
also as a communication signal that can regulate stem cell
properties, including survival, multiplication, differentiation and
‘stemness’ (6–10). It is unknown whether the native
microenvironment of MSCs should be defined as hypoxic or primary
normoxic (11); however, it has
been established that oxygen concentration is a critical regulator
of MSCs, and may therefore control their differentiation fate.
MSCs have been considered the most important seed
cells, and are widely used in the experimental and clinical
treatment of musculoskeletal disorders and degenerative diseases
(12,13). A central issue in the treatment of
bone defects and necrosis, is how to promote the multiplication and
ensure the effective osteogenesis of MSCs at the delivery site.
Furthermore, the ultimate results of transplanting MSCs into a
hypoxic environment in vivo are poorly understood, and the
underlying mechanisms are unclear. Controversy remains regarding
the effects of hypoxia on the osteogenic differentiation of MSCs
(14,15). Salim et al (9) found that a short period of anoxia
exposure downregulated runt-related transcription factor 2 (Runx2)
and bone morphogenetic protein 2 expression. This resulted in the
inhibition of key steps in the differentiation of pluripotent
mesenchymal precursors and committed osteoblasts into the
osteogenic lineage. Through microarray profiling, the
downregulation of Runx2 was indicated to be a potential mechanism
underlying the anoxia-induced inhibition of differentiation
(9). Similar results were reported
by Holzwarth et al (16),
who showed that human MSCs (hMSCs) exhibited a reduced rate of
proliferation under reduced oxygen tension compared with that under
21% oxygen; the cells instead accumulated in the G1
phase. Adipogenic and osteogenic differentiation were also shown to
be severely impaired, although the increase in oxygen levels from 1
to 3% restored the capacity of the cells for osteogenic
differentiation (16). Potier
et al (17) indicated that
temporary exposure (48 h) of hMSCs to hypoxia led to the limited
stimulation of angiogenic factor secretion but to the persistent
(up to 14 days) downregulation of several osteoblastic markers.
This suggested that the exposure of MSCs in vivo to hypoxic
conditions following transplantation could affect their osteogenic
potential. By contrast, Grayson et al (18) demonstrated that pretreatment with
hypoxia could enhance the proliferation and tissue formation of
hMSCs. A 30-fold increase in hMSC expansion was obtained over six
weeks without loss of multi-lineage differentiation capabilities
when hMSCs were maintained in an atmosphere of 2% hypoxia (18). It is considered that the effect of
hypoxia on MSCs depends upon the severity of hypoxia, the origin of
the cells and the method of osteogenic induction (6,19).
However, the effect of the duration of hypoxia on the osteogenic
potential of MSCs is currently unknown.
In the present study, the effects of continuous
hypoxia (1% oxygen concentration) on the osteogenic potential of
MSCs in long-term culture were investigated. Four osteogenic
biomarkers, Runx2, osteopontin (OPN), osteocalcin (OCN) and
alkaline phosphatase (ALP), were detected at mRNA and protein
levels during the course of differentiation.
Materials and methods
MSC isolation and culture
The study protocol was approved by the Ethics
Committee of the Shanghai Sixth People’s Hospital (Shanghai,
China). Rat mesenchymal stromal cells (rMSCs) were isolated from
femoral marrow specimens and maintained according to local
regulations. Bone marrow samples were obtained from three rats and
numbered accordingly. rMSCs were harvested by gently flushing bone
marrow from the femora with α Minimum Essential Medium (Hyclone,
Logan, UT, USA) containing 10% fetal bovine serum (FBS; Sigma, St.
Louis, MO, USA) and 1% antibiotic solution. When rMSCs reached
60–70% confluence, they were detached and cryopreserved at passage
1 (P1) (90% FBS and 10% dimethylsulfoxide). For each
experiment, a new batch of rMSCs was thawed and cultured to
P3 under normoxia, for further use.
Multipotent differentiation assay
The potential of rMSCs for multipotent
differentiation into osteogenic and adipogenic lineages was
assessed. For the evaluation of induced osteogenesis, rMSCs were
seeded at a density of 5×104 cells/well and cultured in
osteogenic differentiation medium containing dexamethasone,
ascorbate and β-glycerophosphate (Sigma) for two weeks. Alizarin
Red staining was performed to assess cellular mineralization in the
differentiation medium. This was performed by fixing the cells with
100% ethanol for 15 min and then staining with 0.2% Alizarin Red S
solution, with a pH of 6.36–6.4, at room temperature for 1 h.
Adipogenic differentiation was performed according to conventional
methodology, using culture medium containing 1 μM dexamethasone and
5 μg/ml insulin. The cultured cells were stained with Oil Red
(Sigma) to detect fat droplets.
Conditions of hypoxia and osteogenic
induction
Methodology for the generation of hypoxic conditions
and osteogenic induction was based on that outlined in our previous
study (20). Normoxic (21%
O2) and hypoxic (1% O2) conditions were used
continuously for culturing the cells in a humidified atmosphere.
The proliferation and differentiation conditions of the cells were
equal for both levels of O2. When the rMSCs reached
P3 under normoxia, the cells were then either further
cultured under normoxia or were transferred to a hypoxia incubator
(Forma™ Series II 3110 Water Jacketed CO2 Incubator;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and maintained in
1% O2. Osteogenic differentiation was induced in 50%
confluent rMSC cultures by switching to osteogenic induction
medium. Control cells were cultured in parallel under hypoxia and
normoxia without osteogenic induction medium. After 3, 7, 14 and 21
days of culture, cells were harvested and cryopreserved for
subsequent analysis.
Quantitative polymerase chain reaction
(qPCR)
The expression level of the mRNA transcripts was
assessed by two-step qPCR. In brief, total RNA was prepared with
TRIzol® Plus (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions. A TURBO
DNA-free™ kit (Applied Biosystems, Foster City, CA, USA) was
employed for the removal of possible contaminated genomic DNA and
the subsequent removal of DNase itself from the total RNA
preparation. cDNA was then synthesized from total RNA using oligo
(dT) and ReverTra Ace® reverse transcriptase (Toyobo,
Osaka, Japan). qPCR was performed and analyzed by kinetic qPCR
using the ABI PRISM 7900 system (Applied Biosystems) with SYBR
Green Realtime PCR Master Mix Plus (Toyobo) for relative
quantification of the indicated genes. The transcript of β-actin
was used as an endogenous control for normalization.
The primers used for Runx2, OPN, OCN and ALP are
listed in Table I. All the PCR
primers were designed to span introns to discriminate the cDNA from
genomic amplicons, according to the manufacturer’s instructions.
The amplification efficacy of each PCR reaction was assessed
initially with a serial dilution of control samples, which fell
into the range of 95–105%. The comparative cycle threshold method
was utilized to assess the levels of each mRNA transcript relative
to the level of β-actin mRNA transcript in the same sample.
 | Table IPrimers used for the quantitative
polymerase chain reaction. |
Table I
Primers used for the quantitative
polymerase chain reaction.
| Gene | Orientation | Sequence
(5′-3′) |
|---|
| β-actin | Forward |
TTCTTTGCAGCTCCTTCGTTGCCG |
| Reverse |
TGGATGGCTACGTACATGGCTGGG |
| ALP | Forward |
TATGTCTGGAACCGCACTGAAC |
| Reverse |
CACTAGCAAGAAGAAGCCTTTGG |
| OCN | Forward |
GCCCTGACTGCATTCTGCCTCT |
| Reverse |
TCACCACCTTACTGCCCTCCTG |
| OPN | Forward |
CCAAGCGTGGAAACACACAGCC |
| Reverse |
GGCTTTGGAACTCGCCTGACTG |
| Runx2 | Forward |
ATCCAGCCACCTTCACTTACACC |
| Reverse |
GGGACCATTGGGAACTGATAGG |
Western blotting
Nuclear proteins were isolated from the rMSCs,
following cellular centrifugation at 4°C, using a nuclear protein
extraction kit (Biovision, Mountain View, CA, USA) according to the
manufacturer’s instructions. The protein contents of the cell
lysates were determined using a micro bicinchoninic acid assay kit
(Pierce; Thermo Fisher Scientific, Inc., Rockford, IL, USA).
Protein from the cell lysates was mixed with 4× loading buffer
containing 10 mM dithiothreitol and boiled for 10 min, prior to
electrophoresis by 10% SDS-PAGE. Following transfer onto
polyvinylidene fluoride membranes and blocking, the membranes were
incubated overnight at 4°C with monoclonal antibodies for
hypoxia-inducible factor 1α (HIF-1α; 1:3,000; Abcam, Cambridge, MA,
USA), Runx2 (1:1,000; Abcam), OPN (1:2,000; R&D Systems,
Minneapolis, MN, USA), OCN (1:3,000; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) or ALP (1:1,000; Santa Cruz
Biotechnology, Inc.). Following several washes in Tris-buffered
saline with Tween®20 (TBST), the membranes were
subsequently incubated for 1 h with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G antibody
(Sigma) diluted 1:3,000 in blocking buffer. The membranes were then
washed three times with TBST, and signals were detected by enhanced
chemiluminescence reagents (Thermo Fisher Scientific, Inc.) and
exposure to X-ray film. The density of the bands was quantified
using Quantity One software (Bio-Rad, Hercules, CA, USA). The
expression of HIF-1α was normalized to β-actin expression in the
same lane. Data from each experiment are presented as the fold
increase relative to the control group.
Alizarin Red staining
Alizarin Red staining was performed to assess the
mineralization of the cultured cells in the differentiation media
at the different stages. The methodology utilized was in accordance
with that described previously in the text.
Statistical analysis
All numerical data are expressed as the mean ±
standard error. A Student’s t-test was employed to compare
differences between groups with SPSS 17.0 software (SPSS, Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significantly difference. All experiments were performed in
triplicate.
Results
Morphology and surface antigen expression
of rMSCs
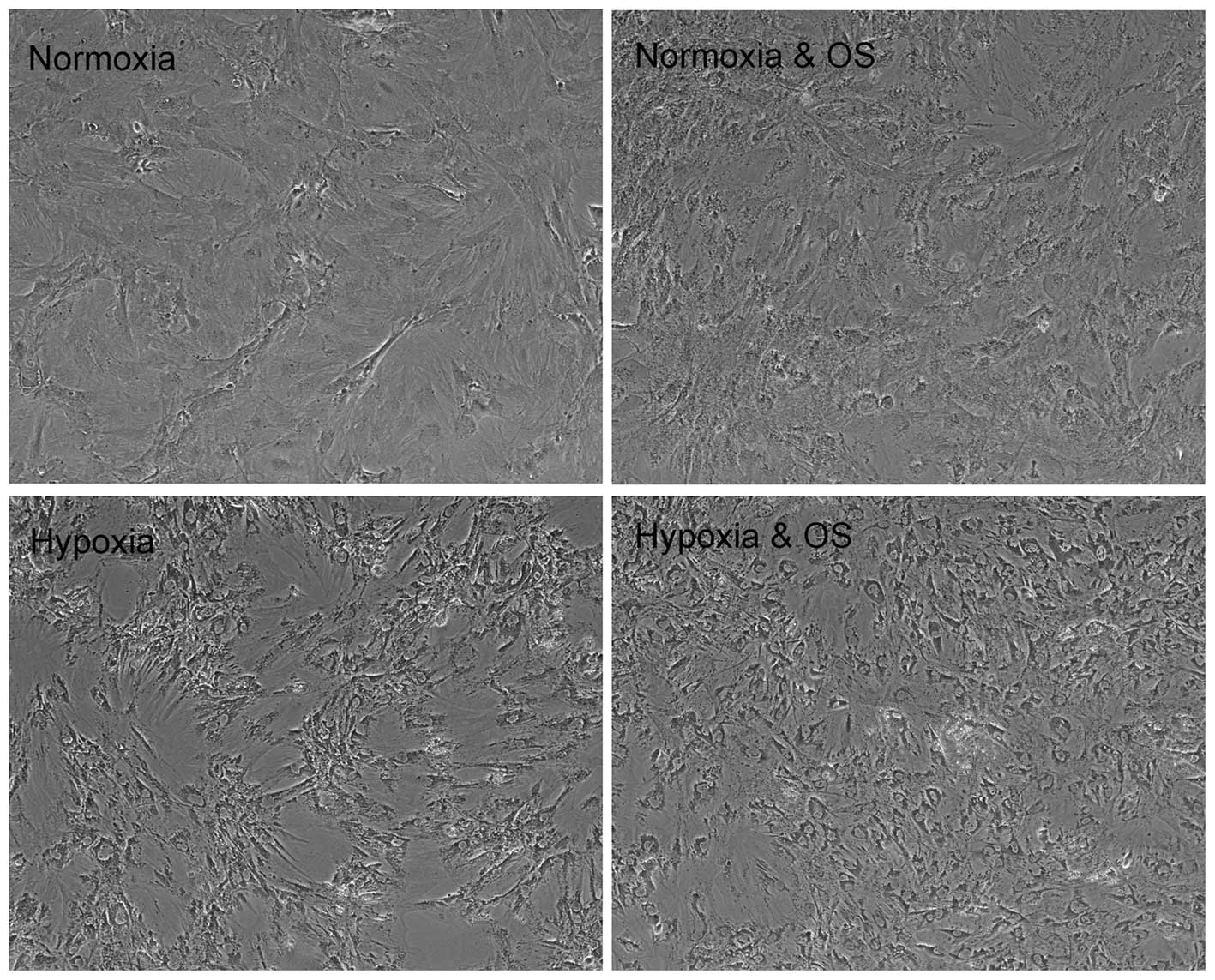
Cell morphology under 1% oxygen or atmospheric
conditions was observed by light microscopy, with photomicrographs
taken at 3, 7, 14 and 21 days. This showed that rMSCs cultured in
21% O2 without osteogenic induction were morphologically
indistinguishable up to passage P3. However, in
comparison with normoxic P1–P3 cells, rMSCs
under hypoxic conditions lost their spindle shape when treated with
osteogenic induction medium (Fig.
1).
Potency of induced multi-lineage
differentiation
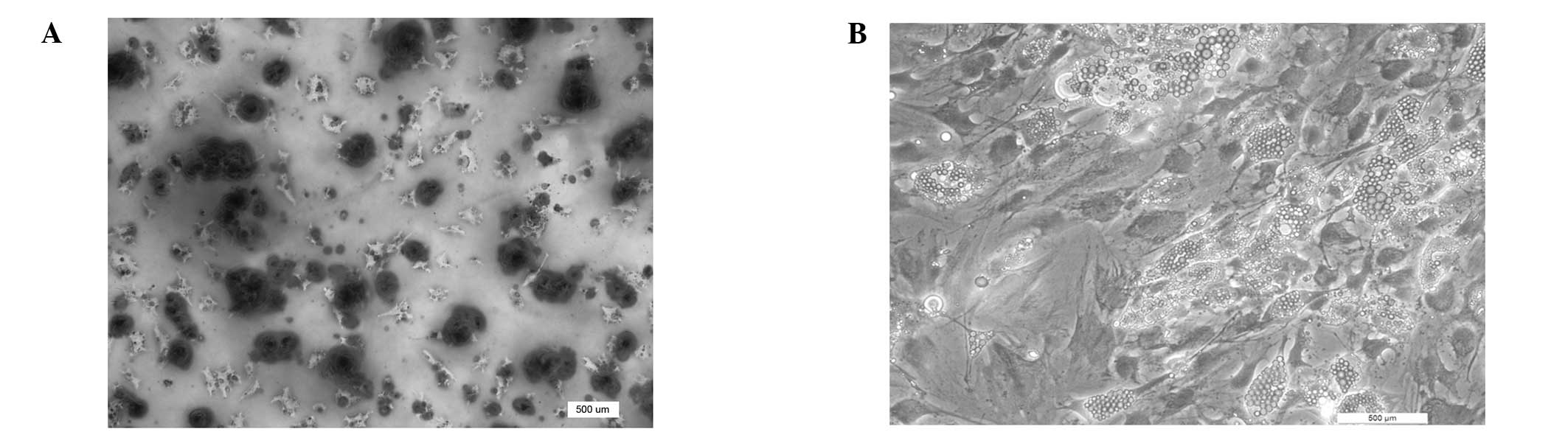
Alizarin Red S staining revealed that rMSCs cultured
in osteogenic medium for two weeks had significant calcium
deposition (Fig. 2A). In the case
of adipogenic differentiation, the accumulation of multiple
droplets, identified by Oil Red staining, occurred after three
weeks of culture (Fig. 2B). The
data indicated that the collected rMSCs had acquired the potency of
induced multi-lineage differentiation.
qPCR analysis of osteogenesis-related
biomarkers
A high Runx2 mRNA expression level was maintained in
normoxia-treated rMSCs, irrespective of whether they were cultured
in osteogenic induction medium. However, Runx2 mRNA expression was
low under hypoxic conditions. As an early biomarker of
osteogenesis, the suppression of Runx2 indicated that the
osteogenic capacity of the rMSCs was suppressed by hypoxia. The
expression pattern of OPN, OCN and ALP was similar to that of
Runx2, with significantly lower mRNA levels observed in
hypoxia-treated cells than in normoxia-treated cells (Fig. 3). Overall, early-, medium- and
mature-stage osteogenic biomarkers were all downregulated at the
mRNA level in a hypoxic environment.
 | Figure 3Quantitative polymerase chain reaction
of osteogenesis-related biomarkers. Runx2 expression remained
higher in normoxia-treated rat mesenchymal stromal cells,
irrespective of whether they were cultured in OS induction medium.
Runx2 expression remained at a low level under hypoxic conditions.
The pattern of expression of OPN, OCN and ALP was similar to that
of Runx2, with the levels of OPN, OCN and ALP all significantly
lower in hypoxia-treated cells. *P<0.05,
**P<0.01 and ***P<0.001 versus the
non-OS induction group; #P<0.05,
##P<0.01 and ###P<0.001 versus the
corresponding normoxia group. OS, osteogenic; Runx2, runt-related
transcription factor 2; OPN, osteopontin; OCN, osteocalcin; ALP,
alkaline phosphatase. |
Western blot analysis of
osteogenesis-related biomarkers
On the third day of culture, the expression of Runx2
was similar between cells cultured under normoxia and hypoxia,
irrespective of osteogenic induction; however, by days 7, 14 and
21, the protein expression of Runx2 was reduced in hypoxia-treated
cells. The protein expression levels of OCN and ALP peaked on day 7
in the hypoxia-treated cells. On day 14, the expression of ALP was
maintained at a similar level in the hypoxia-treated rMSCs, whereas
OCN expression was significantly downregulated in the hypoxia plus
OS induction group. OPN expression remained repressed in cells
cultured under hypoxia with osteogenic induction. On day 14, no
significant differences were observed in the expression levels of
OCN and ALP between the hypoxia- and normoxia-treated groups. under
the same conditions. The levels of OCN and ALP were higher in
hypoxia-treated cells than those in cells treated solely with
normoxia on day 21 (Fig. 4). It
was additionally observed that, in hypoxia-treated rMSCs, the
duration of Runx2 expression was relatively short and the
expression of OCN and ALP was advanced, indicating that hypoxia may
accelerate the osteogenic differentiation of rMSCs and osteocyte
maturation.
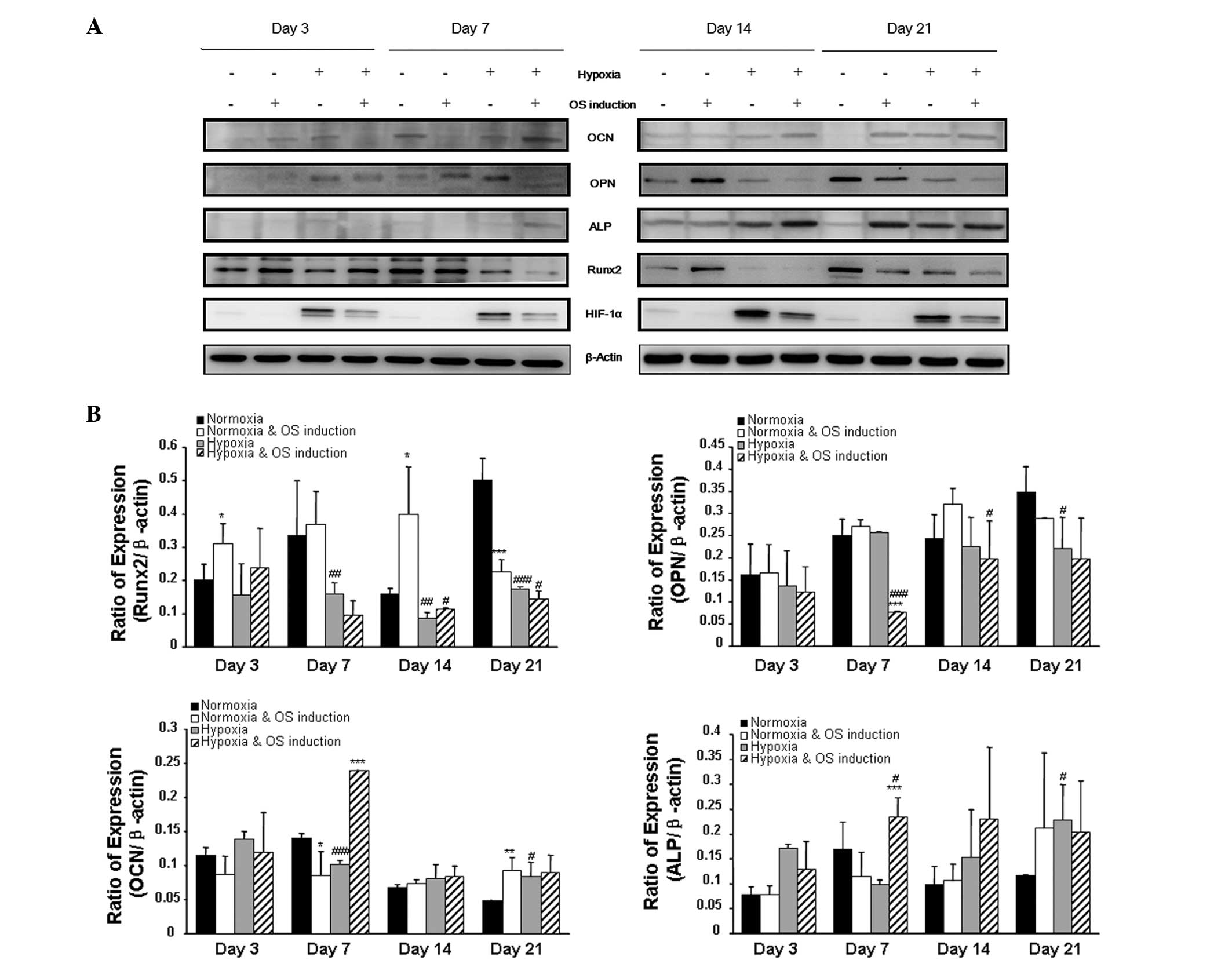 | Figure 4Western blot analysis of
osteogenesis-related biomarkers. (A) The pattern of expression of
osteogenic biomarkers at the protein level was shown on days 3, 7,
14 and 21, with β-actin expression set as the control. (B) The
protein bands were quantified using Quantity One image analysis
software. The expression of Runx2 was downregulated on days 7, 14
and 21 when cells were cultured under hypoxia. The expression of
OPN was similar on day 3 in all groups, and the level of OPN was
comparable when cells were exposed to OS induction under normoxia
or hypoxia. The levels of OCN were similar on days 3 and 14 in all
groups of cells; however, levels were higher in hypoxia-treated rat
mesenchymal stromal cells. In terms of ALP expression, the level of
ALP was higher on day 21 when the cells were treated by hypoxia.
*P<0.05, **P<0.01 and
***P<0.001 versus the non-OS induction group;
#P<0.05, ##P<0.01 and
###P<0.001 versus the corresponding normoxia group.
OS, osteogenic; Runx2, runt-related transcription factor 2; OPN,
osteopontin; OCN, osteocalcin; ALP, alkaline phosphatase. |
Alizarin Red staining
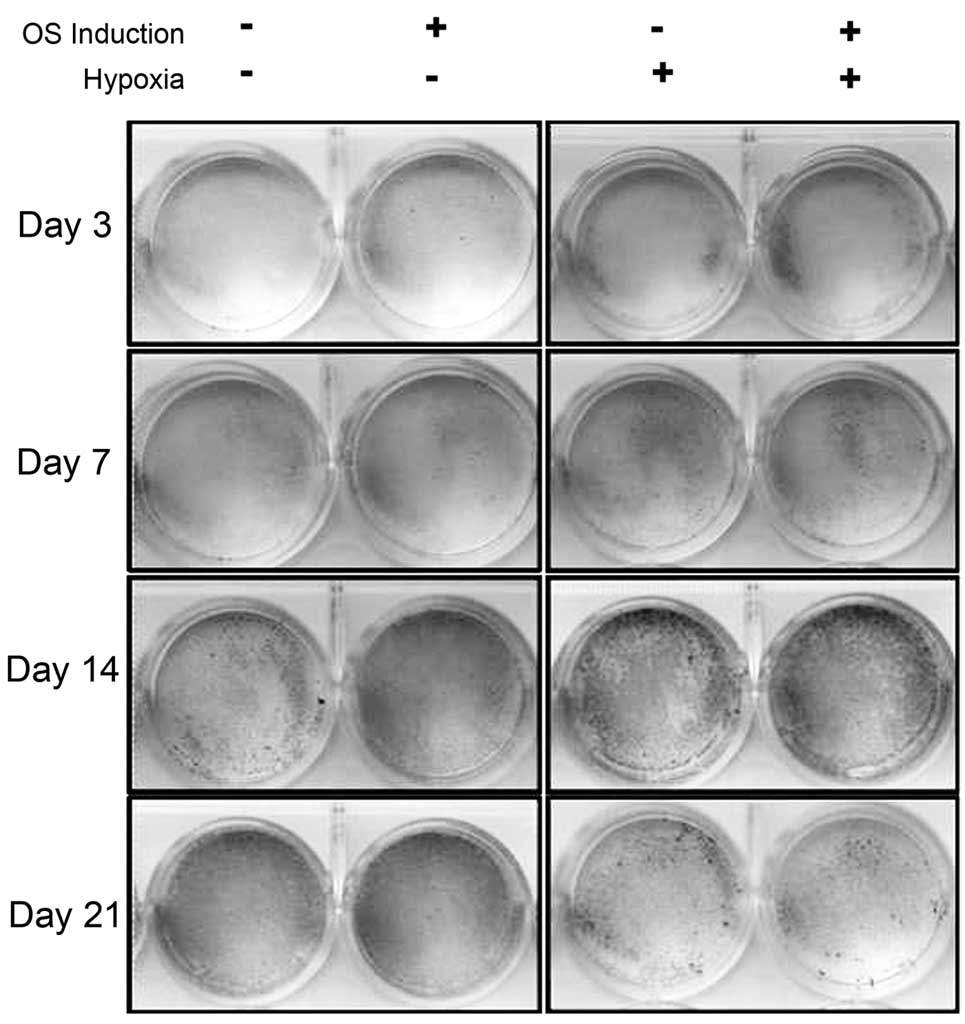
The results of the Alizarin Red staining directly
indicated that osteogenic ability was enhanced in hypoxia-treated
rMSCs as compared with controls on days 3, 7 and 14. Comparison
between the groups showed that osteogenesis was comparable between
the cells on the third day of culture in hypoxic conditions and
those on the seventh day of culture in normoxic conditions, and
similarly between the cells on the seventh and 14th days of hypoxia
and normoxia treatment, respectively, and those on the 14th and
21st days of hypoxia and normoxia treatment, respectively (Fig. 5). It was therefore speculated that
the osteogenic differentiation of rMSCs was accelerated following
culture in 1% oxygen. However, when the cells were maintained in
hypoxic culture until day 21, osteogenesis was impaired. It may
therefore be concluded that hypoxia can regulate the osteogenic
capacity of rMSCs in a time-dependent manner.
Discussion
MSCs are widely used as precursor cells for
regenerative medicine and in vitro tissue engineering.
Preliminary promising results have been obtained from the
application of cytotherapy with MSCs in the treatment of
osteonecrosis, cartilage defects and myocardial infarction
(21–23). Due to the hypoxic environment in
vivo (primary normoxia), it is speculated that cells under
normoxia in vitro may undergo considerable cellular stress
upon transplantation into in vivo conditions (24). Therefore, the study of the
characteristics of the survival, differentiation and apoptosis of
MSCs under hypoxia is highly significant.
It has previously been indicated that oxygen
concentration plays a key role in the determination of cell fate
and the maintenance of ‘stemness’ in adipose- and bone
marrow-derived MSCs (25).
However, whether hypoxia plays a positive or negative role in the
proliferation and differentiation of MSCs remains controversial. A
previous study by Grayson et al (18) found that hMSCs maintained under 2%
O2 for seven passages in vitro could result in a
30-fold higher level of hMSC expansion without the loss of
multi-lineage differentiation capacity (18). Furthermore, it has been
demonstrated that hypoxia can promote chondrogenesis of MSCs
(26) through the transcriptional
activity of SRY (sex determining region Y)-box 9 and HIF-1α
(27). In the context of tissue
engineering, when MSCs were seeded onto fibrin glue and cultured in
a 3% O2 atmosphere, Oct-4 was shown to be stably
expressed and an increase in directed differentiation was observed
(28). Conversely, a negative
effect of hypoxia on MSCs has also been noted (9). Physiological oxygen tension (1%
oxygen) during the in vitro culture of hMSCs slowed down
cell cycle progression and differentiation, which led to the
accumulation of MSCs in G1 phase (16). Similarly, the effect of hypoxia on
other precursors, including embryonic, neural and induced
pluripotent stem cells, is also the subject of great controversy
(29–31). It is currently believed that the
effect of hypoxia on MSCs is dependent on the severity and duration
of hypoxia, the cell origin and the method used for induction.
The effect of hypoxia on the differentiation of
rMSCs was evaluated in the present study by consecutive assessment
of the osteogenic biomarkers Runx2, OPN, OCN and ALP. The results
shown in Figs. 3 and 5 consistently demonstrate that hypoxia
regulates the osteogenesis of MSCs in a time-dependent manner. ALP
was analyzed as a biomarker of mature osteogenesis, and was
observed to peak in expression level faster when cells were
cultured under hypoxia. However, the data from mRNA expression
analysis differed from those from the analysis of individual
protein expression, which indicated that the post-translational
modification of osteogenic biomarkers was a critical step in the
differentiation of MSCs.
Alizarin Red staining indicated that 1% oxygen could
accelerate the osteogenic progress of rMSCs. However, culture in
continuous hypoxia resulted in reduced osteogenesis. The results
suggested that osteogenic induction had a protective function for
hypoxia-treated rMSCs, indicating that cells could be protected
from the effects of physiological hypoxia when transplanted into an
osteogenic environment in vivo. The effect of hypoxia on the
osteogenic differentiation of rMSCs may be in part dependent on the
duration of hypoxia. This study, to the best of our knowledge, is
the first to report of a time-dependent effect of hypoxia on MSCs,
although this effect has been preliminarily described in previous
studies (9,16–19,32).
The underlying molecular mechanisms of the
hypoxia-regulated differentiation of multipotent cells has
attracted the focus of a large number of studies, which have
revealed that HIF-1α plays an important role in the progress of
differentiation. HIFs regulate a number of stem cell effectors,
including Notch, Wnt and octamer-binding protein 4, that are
involved in the control of stem cell proliferation, differentiation
and pluripotency (6,7,33).
It has been shown that hypoxia-induced HIF-1α expression exerted a
protective effect on rMSCs, which may have contributed to the
accumulation of cells under hypoxia. Although previous findings
indicated that HIF had a limited role in enhancing MSC self-renewal
and growth factor secretion under hypoxia, the function of HIF
proved to be pivotal; HIF promoted self-renewal through enhancing
the preservation of early colony-forming progenitor cells and
maintaining the undifferentiated phenotype of MSCs (34).
At present, investigations in the field of stem cell
research are focusing on identifying therapeutic targets,
developing therapeutic tests, exploring cell differentiation and
the underlying physiological mechanisms, determining the optimal
culture conditions for pluripotent stem cells and ensuring efficacy
and safety (35). The aim of the
present study was to identify the optimal prerequisite for the
improved application of MSCs in in vitro engineering and
cytotherapy. Since hypoxia is key factor in the pathogenesis of
osteonecrosis, it is of great significance to study the effect of
hypoxia on the biological behavior of MSCs. As a result of these
findings and those from previous studies, traditional tissue
engineering under normoxia may undergo numerous changes in the near
future.
The limitations of the present study should also be
noted. Only a selection of representative osteogenic biomarkers was
investigated, and their intrinsic associations are not completely
understood. Furthermore, the transcriptional characteristics of
these biomarkers were not investigated; this is an aspect to be
investigated in future studies. The effects of hypoxia on the
behavior of MSCs, including the effects on migration, intercellular
communication and aging, also need to be taken into account
(36,37).
In conclusion, 1% oxygen was able to regulate the
osteogenic differentiation of rMSCs. Under the given conditions,
the osteogenesis of rMSCs was accelerated in the early period;
however, long-term hypoxia resulted in poor osteogenesis. The
effect of hypoxia on the osteogenic differentiation of rMSCs is
time-dependent. Therefore, when MSCs are used for tissue
engineering and cytotherapy, the duration of hypoxia pretreatment
should be controlled accurately to achieve improved efficiency and
the expected outcome.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of China (nos. 81272003 and
81301572).
References
|
1
|
Bianco P and Gehron Robey P: Marrow
stromal stem cells. J Clin Invest. 105:1663–1668. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parekkadan B and Milwid JM: Mesenchymal
stem cells as therapeutics. Annu Rev Biomed Eng. 12:87–117. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Philippe B, Luc S, Valérie PB, Jérôme R,
Alessandra BR and Louis C: Culture and use of mesenchymal stromal
cells in phase I and II clinical trials. Stem Cell Int.
503593:2010.
|
|
4
|
Meijer GJ, de Bruijn JD, Koole R and van
Blitterswijk CA: Cell-based bone tissue engineering. PLoS Med.
4:e92007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Griffin M, Iqbal SA and Bayat A: Exploring
the application of mesenchymal stem cells in bone repair and
regeneration. J Bone Joint Surg Br. 93:427–434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mohyeldin A, Garzón-Muvdi T and
Quiñones-Hinojosa A: Oxygen in stem cell biology: a critical
component of the stem cell niche. Cell Stem Cell. 7:150–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Araldi E and Schipani E: Hypoxia, HIFs and
bone development. Bone. 47:190–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdollahi H, Harris LJ, Zhang P, McIlhenny
S, Srinivas V, Tulenko T and DiMuzio PJ: The role of hypoxia in
stem cell differentiation and therapeutics. J Surg Res.
165:112–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salim A, Nacamuli RP, Morgan EF, et al:
Transient changes in oxygen tension inhibit osteogenic
differentiation and Runx2 expression in osteoblast. J Biol Chem.
279:40007–40016. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coyle CH, Izzo NJ and Chu CR: Sustained
hypoxia enhances chondrocyte matrix synthesis. J Orthop Res.
27:793–799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ivanovic Z: Hypoxia or in situ normoxia:
The stem cell paradigm. J Cell Phys. 219:271–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
El Tamer MK and Reis RL: Progenitor and
stem cells for bone and cartilage regeneration. J Tissue Eng Regen
Med. 3:327–337. 2009.PubMed/NCBI
|
|
13
|
Gao YS and Zhang CQ: Cytotherapy of
osteonecrosis of the femoral head: a mini review. Int Orthop.
34:779–782. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma T, Grayson WL, Fröhlich M and
Vunjak-Novakovic G: Hypoxia and stem cell-based engineering of
mesenchymal tissues. Biotechnol Prog. 25:32–42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mazumdar J, Dondeti V and Simon MC:
Hypoxia-inducible factors in stem cells and cancer. J Cell Mol Med.
13:4319–4328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holzwarth C, Vaegler M, Gieseke F, et al:
Low physiologic oxygen tensions reduce proliferation and
differentiation of human multipotent mesenchymal stromal cells. BMC
Cell Biol. 11:112010. View Article : Google Scholar
|
|
17
|
Potier E, Ferreira E, Andriamanalijaona R,
Pujol JP, Oudina K, Logeart-Avramoglou D and Petite H: Hypoxia
affects mesenchymal stromal cell osteogenic differentiation and
angiogenic factor expression. Bone. 40:1078–1087. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grayson WL, Zhao F, Bunnell B and Ma T:
Hypoxia enhances proliferation and tissue formation of human
mesenchymal stem cells. Biophys Biochem Res Commun. 358:948–953.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang S, Zhou Y, Seavey CN, Singh AK, Xu X,
Hunt T, Hoyt RF Jr and Horvath KA: Rapid and dynamic alterations of
gene expression profiles of adult porcine bone marrow-derived stem
cell in response to hypoxia. Stem Cell Res. 4:117–128. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao YS, Ding H, Xie XT and Zhang CQ:
Osteogenic induction protects rat bone morrow-derived mesenchymal
stem cells against hypoxia-induced apoptosis in vitro. J Surg Res.
184:873–879. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hauzeur JP and Gangji V: Phases 1–3
clinical trials using adult stem cells in osteonecrosis and
nonunion fractures. Stem Cells Int. 2010:4101702010.
|
|
22
|
Bianco P, Riminucci M, Gronthos S and
Robey PG: Bone marrow stromal stem cells: nature, biology, and
potential applications. Stem Cells. 19:180–192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khan M, Kwiatkowski P, Rivera BK and
Kuppusamy P: Oxygen and oxygenation in stem-cell therapy for
myocardial infarction. Life Sci. 87:269–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu W, Chen J, Cong X, Hu S and Chen X:
Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem
cells. Stem Cells. 24:416–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Basciano L, Nemos C, Foliguet B, de Isla
N, de Carvalho M, Tran N and Dalloul A: Long term culture of
mesenchymal stem cells in hypoxia promotes a genetic program
maintaining their undifferentiated and multipotent status. BMC Cell
Biol. 12:122011. View Article : Google Scholar
|
|
26
|
Müller J, Benz K, Ahlers M, Gaissmaier C
and Mollenhauer J: Hypoxic conditions during expansion culture
prime human mesenchymal stromal precursor cells for chondrogenic
differentiation in three-dimensional cultures. Cell Transplant.
20:1589–1602. 2011.
|
|
27
|
Robins JC, Akeno N, Mukherjee A, et al:
Hypoxia induces chondrocyte-specific gene expression in mesenchymal
cells in association with transcriptional activation of Sox9. Bone.
37:313–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baumgartner L, Arnhold S, Brixius K,
Addicks K and Bloch W: Human mesenchymal stem cells: Influence of
oxygen pressure on proliferation and chondrogenic differentiation
in fibrin glue in vitro. J Biomed Mater Res A. 93:930–940.
2010.PubMed/NCBI
|
|
29
|
Yoshida Y, Takahashi K, Okita K, Ichisaka
T and Yamanaka S: Hypoxia enhances the generation of induced
pluripotent stem cells. Cell Stem Cell. 5:237–241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Santilli G, Lamorte G, Carlessi L, Ferrari
D, Rota Nodari L, Binda E, Delia D, Vescovi AL and De Filippis L:
Mild hypoxia enhances proliferation and multipotency of human
neural stem cells. PLoS One. 5:e85752010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Prado-Lopez S, Conesa A, Armiñán A,
Martínez-Losa M, Escobedo-Lucea C, Gandia C, et al: Hypoxia
promotes efficient differentiation of human embryonic stem cells to
functional endothelium. Stem Cells. 28:407–418. 2010.PubMed/NCBI
|
|
32
|
Volkmer E, Kallukalam BC, Maertz J, Otto
S, Drosse I, Polzer H, et al: Hypoxic preconditioning of human
mesenchymal stem cells overcomes hypoxia-induced inhibition of
osteogenic differentiation. Tissue Eng Part A. 16:153–164. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goda N, Ryan HE, Khadivi B, McNulty W,
Rickert RC and Johnson RS: Hypoxia-inducible factor 1 alpha is
essential for cell cycle arrest during hypoxia. Mol Cell Biol.
23:359–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tamama K, Kawasaki H, Kerpedjieva SS, Guan
J, Ganju RK and Sen CK: Differential roles of hypoxia inducible
factor subunits in multipotential stromal cells under hypoxic
condition. J Cell Biochem. 112:804–817. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liras A: Future research and therapeutic
applications of human stem cells: general, regulatory, and
bioethical aspects. J Transl Med. 8:1312010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Raheja LF, Genetos DC, Wong A and
Yellowley CE: Hypoxic regulation of mesenchymal stem cell
migration: the role of RhoA and HIF-1α. Cell Biol Int. 35:981–989.
2011.PubMed/NCBI
|
|
37
|
Liu L and Rando TA: Manifestations and
mechanisms of stem cell aging. J Cell Biol. 193:257–266. 2011.
View Article : Google Scholar
|