Introduction
Lung cancer is the most common disease worldwide,
with high incidence and mortality (1–3).
Until 2008, an estimated 1.61 million new cases were reported,
representing 12.7% of all new cancer types (4–6). The
mortality rate (1.38 million cases) was estimated at 8.2% of the
total mortality due to cancer, which renders lung cancer the most
common type of cancer associated with mortality. Only in China,
lung cancer has been ranked the number one cause of death among
people with malignant tumors (7,8). The
registered mortality caused by lung cancer has increased by 464.84%
in the past 3 decades (9). It has
been reported that imbalance between cell proliferation and
apoptosis plays a vital role in the development of lung cancer,
along with mutations in tumor suppressor genes and oncogenes and
inactivation of multiple genes (10–13).
The programmed cell death 5 (Pdcd5) gene,
formerly designated as TF-1 cell apoptosis-related gene 19
(TFAR19), is involved in cell death and is upregulated
during apoptosis (14). The gene
was first cloned by the Peking University Center for Human Disease
Genomics in 1999. It is expressed in more than 50 tissues in adult
humans, and is highly expressed in tissues such as heart, kidney,
adrenal gland, testis and placenta (15). The Pdcd5 protein translocates
rapidly from the cytoplasm to the nucleus and plays an important
role in the inhibition of the proteasome-dependent degradation of
lysine acetyltransferase 5, which is involved in transcription,
DNA-damage response and cell-cycle control. Disorders in the
expression of PDCD5 have been associated with tumorigenesis
(16,17). Reduced Pdcd5 expression has been
reported in several types of tumor and has been associated with the
progression and prognosis of cancer. The protein showed potent
antitumor activity via the interaction with the histone
acetyltransferase Tip60 and the promotion of DNA damage-induced
apoptosis (16,18). However, the expression status and
clinical significance of Pdcd5 in lung cancer, and whether Pdcd5
can efficiently inhibit the progression of lung carcinomas, have
not yet been studied.
In the present study, we compared the expression
level of Pdcd5 in lung carcinoma and healthy lung tissues by
immunohistochemistry and western blotting. We further explored
whether the antitumor activity of Pdcd5 is regulated by the
mitochondria-related apoptotic pathway. This study provides new
perspectives for the early diagnosis, treatment and prognosis of
lung cancer.
Materials and methods
Cell cultures and transfection
A549 cells were cultured in Dulbecco’s modified
Eagle’s medium supplemented with 10% fetal bovine serum, 2 mM
L-glutamine, 100 U/ml penicillin, and 100 mM streptomycin, in an
atmosphere containing 5% CO2. The PCI-neo-Pdcd5 plasmid
was kindly provided by Dr Zhigang Liu (General Hospital of Jinan
Military Command, Jinan, Shandong, China) and was transfected into
the cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA),
according to the manufacturer’s protocol. Pdcd5 short hairpin RNA
(shRNA) lentiviral particles were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Immunohistochemical analysis
Immunohistochemical staining was performed as
described earlier (19,20). Briefly, 30 highly differentiated
lung adenocarcinoma tissues and 20 healthy lung tissues adajacent
to these were fixed in 4% paraformaldehyde for 24 h. The specimens
were obtained from the Department of Thoracic Surgery, General
Hospital of Jinan Military Command. The subjects and their families
provided written informed consent prior to the study. Our study was
performed in compliance with the Declaration of Helsinki and we
obtained approval for the study from the Ethics Committee of The
General Hospital of Jinan Military Command. The tissues were cut
from paraffin blocks in 2–5 μm thick sections using a microtome
(Microm HM 310, Microm International GmbH, Walldorf, Germany), and
were mounted on SuperFrost Plus slides (Carl Roth GmbH, Karlsruhe,
Germany). The primary rabbit anti-human polyclonal antibody
targeting Pdcd5 (1:100 dilution; Proteintech, Chicago, IL, USA) was
incubated overnight in a moist chamber at room temperature. The
secondary antibody, goat anti-rabbit, biotinylated anti-IgG (Vector
Laboratories Inc., Burlingame, CA, USA) was used at a 1:500
dilution. Paraffin-embeeded stained sections were observed under a
light microscope (NAZAR AM5, Germany).
MTT assay
The MTT assay was performed as previously described
(21–23). Briefly, A549 cells were placed into
48-well plates. Following cell adherence, the cells were
transfected with the PCI-neo-Pdcd5 plasmid or Pdcd5 shRNA
lentiviral particles for 24, 48 and 72 h. The proliferation of A549
cells was determined by measuring the optical density (OD) of the
samples at 570 nm.
Colony formation assay
For the colony formation assay, cells were seeded in
6-well plates (2×103 cells/well) and transfected with
PCI-neo-Pdcd5 or Pdcd5 shRNA for 24 h. The medium was changed every
two days, and the cells were cultured for ten days after
transfection of Pdcd5. Surviving colonies (≥50 cells/colony) were
fixed with methanol, stained with 1.25% crystal violet and counted
under the light microscope ??h after transfection and for a total
of ?? h.
Detection of apoptosis by
fluorescence-activated cell sorting (FACS)
A549 cells were trypsinized, washed three times with
cold phosphate-buffered saline, and resuspended in 200 μl binding
buffer. Fluorescein isothiocyanate (FITC)-conjugated Annexin V
(Biosea Biotechnology Co., Ltd., Beijing, China) was added
according to the manufacturer’s protocol, to a final concentration
of 0.5 μg/ml. Next, 1 μl of 100 μg/ml propidium iodide working
solution was added for incubation. Then, cells were incubated for
20 min at room temperature in the dark, and 400 μl of binding
buffer (5X Annexin binding buffer; 50 mM HEPES, 700 mM NaCl, 12.5
mM CaCl2, pH 7.4; Life Technologies, MA, USA) was added.
The samples were immediately analyzed on a FACSCalibur flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Western blotting
Protein samples were prepared and separated by
polyacrylamide gel electrophoresis as previously described
(24–26). We used primary antibodies targeting
Pdcd5, caspase-3 and -9, Bcl2-associated X protein (Bax), B-cell
lymphoma 2 (Bcl2), and β-actin (used as the loading control) at
dilutions 1:3,000, 1:5,000, 1:5,000, 1:3,000, 1:5,000 and
1:10,0000, respectively. As a secondary antibody, we used the
horseradish peroxidase-conjugated goat anti-mouse anti-IgG. All
antibodies were purchased from Santa Cruz Biothechnology, Inc.
In vivo tumor xenograft study
BalB/c mice were purchased from the Experimental
Animal Center of Shandong Medical University (Jinan, Shandong,
China) and kept in a pathogen-free environment with a 12-h
light/dark cycle. All experiments were conducted in conformation to
the Guidelines of the Animal Care and Use Committee of the General
Hospital of Jinan Military Command. A549 cells (5×105)
were subcutaneously injected into the back of the mice. The mice
were randomly divided into three groups (n>5): control (injected
with untransfected A549 cells), Pdcd5 shRNA (injected with A549
cells transfected with the Pdcd5 shRNA) and PCI-neo-Pdcd5 (injected
with A549 cells transfected with the PCI-neo-Pdcd5). The survival
of mice was recorded daily and the survival rate was determined as
100 × (number of survivors/total number of mice).
Statistical analysis
All the experiments were performed and repeated at
least three times. The data were analyzed by the SPSS statistical
package 11.5 (IBM, Armonk, NY, USA). The data were expressed as the
mean ± standard error of the mean. P<0.01 and P<0.05 denote
significantly statistical differences.
Results
Immunohistochemical detection of
Pdcd5
Formalin-fixed, paraffin-embedded specimens from 30
highly differentiated lung carcinoma and 20 healthy tissues were
analyzed by immunohistochemistry in order to detect the protein
expression of Pdcd5. As shown in Fig.
1, positive staining for Pdcd5 in healthy tissues was mainly
observed in the cytoplasm, uniformly distributed, and in some
cases, in the nucleus. By contrast, decreased immunoreactivity for
Pdcd5 was observed in lung carcinoma tissues.
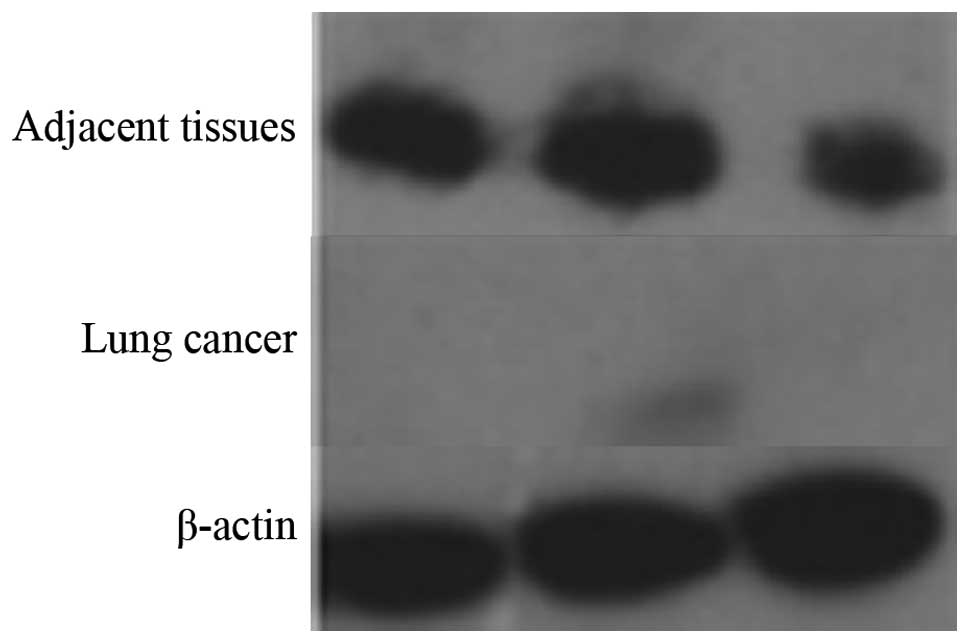
Western blot analysis of Pdcd5
expression
Next, we compared the expression level of Pdcd5
between lung carcinoma and healthy tissues by western blotting. As
shown in Fig. 2, the results of
three independent experiments showed that Pdcd5 expression is
markedly decreased in lung cancer tissues compared to healthy
ones.
The efficiency of overexpression and
interference of Pdcd5
We next used the lung adenocarcinoma cell line A549
as a cell model to study the effects of Pdcd5 gene
overexpression and silencing at the protein level by western
blotting. Pdcd5 overexpression was achieved by transfecting
A549 cells with the PCI-neo-Pdcd5 plasmid, and gene silencing by
using a Pdcd5-specific shRNA. As shown in Fig. 3, the protein expression of Pdcd5
was markedly reduced in cells transfected with the shRNA, while a
slight increase in the Pdcd5 level was observed in cells
transfected with the PCI-neo-Pdcd5 plasmid.
Overexpression of Pdcd5 inhibits
proliferation in the lung cancer cell line A549
The effects of Pdcd5 on cell viability and
proliferation of lung cancer cells were assessed by the MTT and
colony formation assays, respectively. As shown in Fig. 4, when Pdcd5 was overexpressed, a
significant and time-dependent increase in A549 cell death was
observed compared to untransfected cells (P<0.01). In addition,
the number of colonies was significantly decreased in
PCI-neo-Pdcd5-transfected cells in the colony formation assay.
Taken together, these results indicate that overexpression of Pdcd5
significantly inhibits A549 cell proliferation and that Pdcd5 may
act as a potential tumor suppressor.
Overexpression of Pdcd5 induces apoptosis
of the lung cancer cell line A549
In order to examine whether the inhibition of
proliferation in A549 cells overexpressing Pdcd5 is related to cell
apoptosis, FACS analysis was performed. A549 cells transfected with
PCI-neo-Pdcd5 or Pdcd5 shRNA were subjected to dual labeling with
Annexin V-FITC and propidium iodide (PI). As shown in Fig. 4C, the apoptotic rate was
significantly higher in the PCI-neo-Pdcd5 group (25.8%) compared
with the control (3.6%) (P<0.01).
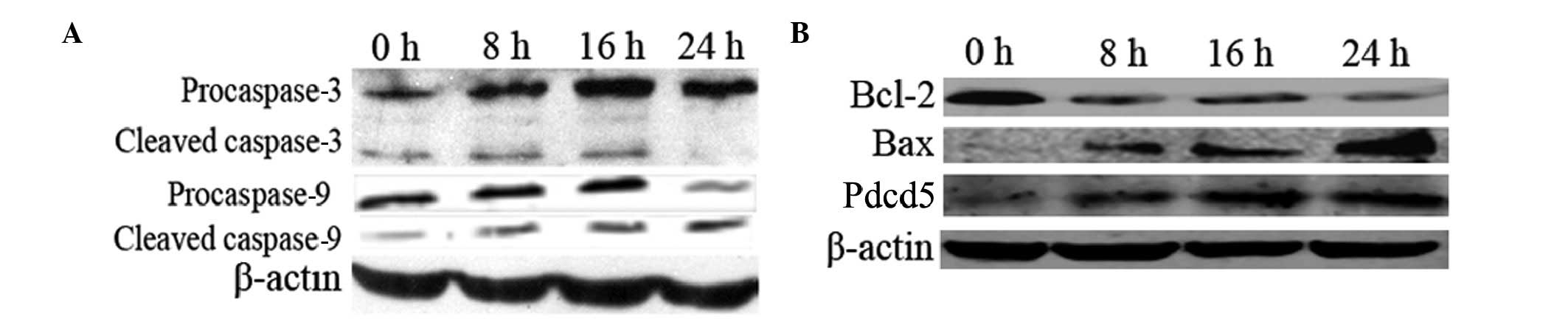
Activated caspase-3 and-9, increased Bax
and decreased Bcl-2 levels in Pdcd5-overexpressing cells
To further explore the mechanism by which expression
of Pdcd5 induces apoptosis, the levels of caspase-3, caspase-9 and
Bcl-2 family proteins were examined by western blot analysis. As
shown in Fig. 5A, both
procaspase-3 and -9 were cleaved into their characteristic active
forms, the relative level of which showed a time-dependent
increase, suggesting that the intrinsic mitochondrial apoptotic
pathway was activated. Moreover, the level of the Bcl-2 protein was
decreased and that of Bax was increased along with the increase in
the Pdcd5 level in PCI-neo-Pdcd5-transfected cells (Fig. 5B).
Overexpression of Pdcd5 exhibits
antitumor activity in a xenograft nude mouse model
In order to examine the ability of Pdcd5 to induce
apoptosis in vivo, a nude mice xenograft model was
established, and survival of mice injected with different types of
A549 cells was calculated. As shown in Fig. 6, stable expression of Pdcd5
significantly (P<0.01) increased the survival rate of mice
compared to Pdcd5 silencing or normal expression (control
group).
Discussion
Lung cancer is one of the most common malignant
tumor types in China. The incidence and mortality rates are rising
every year. Imbalance between cell proliferation and apoptosis
plays a vital role in the development of lung cancer, which
prompted us to focus on the Pdcd5 protein. Pdcd5 is an
apoptosis-regulated programmed cell death protein, first cloned in
1999 by Liu et al (14).
The gene is widely expressed in various tissues, except for the
hematopoietic system, and locates on chromosome 19q12-q1311
(27). The protein is composed of
125 amino acids, including 6 exons and 5 introns. Different
expression levels of Pdc5 have been reported in various diseases,
with reduced expression in leukemia (28), gastrointestinal stromal tumors
(29), astrocytic gliomas
(30) and prostate cancer
(31). In the present study, the
immunohistochemical analysis clearly showed positive staining of
Pdcd5 in healthy lung tissues, mostly in the cell cytoplasm, and
reduced staining in lung carcinoma tissues. This result was
consistent with western blot analysis.
The PCI-neo-Pdcd5 plasmid was transfected into the
human lung cancer cell line A549 to induce overexpression of Pdcd5.
Consequently, apoptosis was induced in cancer cells, as detected by
the MTT assay and flow cytometry analysis. It has been reported
that Pdcd5 enhances cisplatin-induced apoptosis in chondrosarcomas
(32,33), which is consistent with findings
from the present study. During the progression of apoptosis, the
expression level of anti-apoptotic and proapoptotic proteins is
tightly regulated. Here, the levels of Bcl-2 family proteins were
detected by western blot analysis. The expression of Bax was
increased and that of Bcl-2 was decreased after 24 h of
transfection with the PCI-neo-Pdcd5 plasmid. The ratio of Bax/Bcl-2
was thus increased, and apoptosis is expected to be promoted in
such conditions. In addition, the caspase-3 and -9 were activated
in A549 cells overexpressing Pdcd5, suggesting that Pdcd5
expression may activate the mitochondria-related apoptotic
pathway.
In summary, our study analyzed the expression and
clinical significance of Pdcd5 in lung cancer, but also provided
evidence for the mechanism of PDCD5-induced cell apoptosis, showing
that the mitochondria-related apoptotic signaling pathway may play
an important role in the process. However, the exact molecular
events of DCD5-induced cell apoptosis need to be explored in future
studies. The present study indicated that Pdcd5 may be a useful
target for the therapy of lung cancer.
References
|
1
|
Marshall HM, Bowman RV, Yang IA, Fong KM
and Berg CD: Screening for lung cancer with low-dose computed
tomography: a review of current status. J Thorac Dis. 5:S524–S539.
2013.PubMed/NCBI
|
|
2
|
Lee PN and Forey BA: Indirectly estimated
absolute lung cancer mortality rates by smoking status and
histological type based on a systematic review. BMC Cancer.
13:1892013. View Article : Google Scholar
|
|
3
|
Amorin Kajatt E: Lung cancer: a review of
current knowledge, diagnostic methods and therapeutic perspectives.
Rev Peru Med Exp Salud Publica. 30:85–92. 2013.(In Spanish).
|
|
4
|
Centers for Disease Control and Prevention
(CDC). State-specific trends in lung cancer incidence and smoking -
United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 60:1243–1247.
2011.PubMed/NCBI
|
|
5
|
Jankovic M, Samarzija M, Jakopovic M,
Kulis T and Znaor A: Trends in lung cancer incidence and mortality
in Croatia, 1988–2008. Croat Med J. 53:93–99. 2012.PubMed/NCBI
|
|
6
|
Kern DG, Kern E, Crausman RS and Clapp RW:
A retrospective cohort study of lung cancer incidence in nylon
flock workers, 1998–2008. Int J Occup Environ Health. 17:345–351.
2011.PubMed/NCBI
|
|
7
|
Han R, Zheng R, Zhang S, Wu M and Chen W:
Trend analyses on the differences of lung cancer incidence between
gender, area and average age in China during 1989–2008. Zhongguo
Fei Ai Za Zhi. 16:445–451. 2013.(In Chinese).
|
|
8
|
Chang S, Dai M, Ren JS, Chen YH and Guo
LW: Estimates and prediction on incidence, mortality and prevalence
of lung cancer in China in 2008. Zhonghua Liu Xing Bing Xue Za Zhi.
33:391–394. 2012.(In Chinese).
|
|
9
|
She J, Yang P, Hong Q and Bai C: Lung
cancer in China: challenges and interventions. Chest.
143:1117–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huncharek M, Muscat J and Geschwind JF:
K-ras oncogene mutation as a prognostic marker in non-small cell
lung cancer: a combined analysis of 881 cases. Carcinogenesis.
20:1507–1510. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suda K, Tomizawa K, Osada H, et al:
Conversion from the ‘oncogene addiction’ to ‘drug addiction’ by
intensive inhibition of the EGFR and MET in lung cancer with
activating EGFR mutation. Lung Cancer. 76:292–299. 2012.
|
|
12
|
You L, He B, Xu Z, et al: Inhibition of
Wnt-2-mediated signaling induces programmed cell death in
non-small-cell lung cancer cells. Oncogene. 23:6170–6174. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu L, Hu J, Zhao Y, et al: PDCD5 interacts
with p53 and functions as a positive regulator in the p53 pathway.
Apoptosis. 17:1235–1245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Wang Y, Zhang Y, et al: TFAR19, a
novel apoptosis-related gene cloned from human leukemia cell line
TF-1, could enhance apoptosis of some tumor cells induced by growth
factor withdrawal. Biochem Biophys Res Commun. 254:203–210. 1999.
View Article : Google Scholar
|
|
15
|
Chen CH, Jiang Z, Yan JH, et al: The
involvement of programmed cell death 5 (PDCD5) in the regulation of
apoptosis in cerebral ischemia/reperfusion injury. CNS Neurosci
Ther. 19:566–576. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao F, Ding L, Zhao M, Qu Z, Huang S and
Zhang L: The clinical significance of reduced programmed cell death
5 expression in human gastrointestinal stromal tumors. Oncol Rep.
28:2195–2199. 2012.PubMed/NCBI
|
|
17
|
Zhuge C, Chang Y, Li Y, Chen Y and Lei J:
PDCD5-regulated cell fate decision after
ultraviolet-irradiation-induced DNA damage. Biophys J.
101:2582–2591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi L, Song Q, Zhang Y, et al: Potent
antitumor activities of recombinant human PDCD5 protein in
combination with chemotherapy drugs in K562 cells. Biochem Biophys
Res Commun. 396:224–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saleem M, Maddodi N, Abu Zaid M, et al:
Lupeol inhibits growth of highly aggressive human metastatic
melanoma cells in vitro and in vivo by inducing apoptosis. Clin
Cancer Res. 14:2119–2127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adhami VM, Siddiqui IA, Ahmad N, Gupta S
and Mukhtar H: Oral consumption of green tea polyphenols inhibits
insulin-like growth factor-I-induced signaling in an autochthonous
mouse model of prostate cancer. Cancer Res. 64:8715–8722. 2004.
View Article : Google Scholar
|
|
21
|
Ahmadian S, Barar J, Saei AA, Fakhree MA
and Omidi Y: Cellular toxicity of nanogenomedicine in MCF-7 cell
line: MTT assay. J Vis Exp. 26:e11912009. View Article : Google Scholar
|
|
22
|
Verma A, Prasad KN, Singh AK, Nyati KK,
Gupta RK and Paliwal VK: Evaluation of the MTT lymphocyte
proliferation assay for the diagnosis of neurocysticercosis. J
Microbiol Methods. 81:175–178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seidl K and Zinkernagel AS: The MTT assay
is a rapid and reliable quantitative method to assess
Staphylococcus aureus induced endothelial cell damage. J
Microbiol Methods. 92:307–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishitani H, Sugimoto N, Roukos V, et al:
Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1
for proteolysis. EMBO J. 25:1126–1136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng L, Xu Z, Zhou Y, Yang T, Liang ZQ and
Zhang M: Effect of rosiglitazone on cells cycle, apoptosis and
expression of Skp2 and p27Kip1 in hepatocellular carcinoma cell
line. Zhonghua Gan Zang Bing Za Zhi. 18:148–149. 2010.(In
Chinese).
|
|
26
|
Schulman BA, Carrano AC, Jeffrey PD, et
al: Insights into SCF ubiquitin ligases from the structure of the
Skp1-Skp2 complex. Nature. 408:381–386. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng A, Wang Y, Ma D, Zhou H and Lou S:
Characterization of programmed cell death 5 (PDCD5) gene in human
cartilage and its possible significance. Beijing Da Xue Xue Bao.
35:481–484. 2003.(In Chinese).
|
|
28
|
Ruan GR, Qin YZ, Chen SS, et al: Abnormal
expression of the programmed cell death 5 gene in acute and chronic
myeloid leukemia. Leuk Res. 30:1159–1165. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li H, Wang Q, Gao F, et al: Reduced
expression of PDCD5 is associated with high-grade astrocytic
gliomas. Oncol Rep. 20:573–579. 2008.PubMed/NCBI
|
|
30
|
Du YJ, Xiong L, Lou Y, Tan WL and Zheng
SB: Reduced expression of programmed cell death 5 protein in tissue
of human prostate cancer. Chin Med Sci J. 24:241–245. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao F, Ding L, Zhao M, Qu Z, Huang S and
Zhang L: The clinical significance of reduced programmed cell death
5 expression in human gastrointestinal stromal tumors. Oncol Rep.
28:2195–2199. 2012.PubMed/NCBI
|
|
32
|
Chen C, Zhou H, Xu L, et al: Recombinant
human PDCD5 sensitizes chondrosarcomas to cisplatin chemotherapy in
vitro and in vivo. Apoptosis. 15:805–813. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin A, Jiang Y, Zhang X, Zhao J and Luo H:
Transfection of PDCD5 sensitizes colorectal cancer cells to
cisplatin-induced apoptosis in vitro and in vivo. Eur J Pharmacol.
649:120–126. 2010. View Article : Google Scholar : PubMed/NCBI
|