Introduction
Liver regeneration is an adaptive defense mechanism
to protect against external injury. Regeneration occurs through a
highly orchestrated and complex hepatic hyperplasia process,
involving numerous growth factor signaling cascades, cytokines,
extracellular matrix remodeling and an interactive network balance
between stimulatory and inhibitory factors for hepatocyte
proliferation (1). These processes
are precisely controlled by transcription factors to promote and
terminate liver tissue regeneration through three phases: i)
Initiation, ii) proliferation and iii) termination (2). The activities of transcription
factors allow their target genes to be transcribed rapidly. The
transcriptional targets encode various proteins that are essential
to induce hepatocytes to leave the quiescent state (G0)
and enter the cell cycle (2).
Following acute liver injury or hepatectomy, the loss of hepatic
parenchyma induces a high expression of tumor necrosis factor α,
lymphotoxin, interleukin-1 (IL-1) and IL-6 (3–5).
These cytokines and growth factors activate transcription factors
such as nuclear factor κ-light-chain-enhancer of activated B cells
(NF-κB), signal transducer and activator of transcription-3
(STAT3), activator protein 1 (AP-1) and CCAAT/enhancer-binding
protein β (C/EBPβ) in the remaining liver cells to initiate
proliferation and DNA synthesis. This process can be dynamically
adjusted through the increase of proliferative inhibitory factors
to maintain liver size during tissue restoration (6,7).
IL-6 is a critical initiation factor for liver
regeneration. During the initiation phase of liver regeneration,
IL-6 is secreted by activated Kupffer cells, enabling hepatocytes
to undergo mitosis by directly regulating proliferation-related
gene expression, such as hepatocyte growth factor expression, and
protecting hepatocytes from apoptosis (8). This process is primarily mediated by
the mitotic transcription factor STAT3, through the canonical
pathway, to regulate the liver regeneration process (8). Numerous binding sites for other
transcription factors are usually found adjacent to the STAT3
binding site and these factors cooperate to regulate gene
expression. It has been shown that STAT3 functions together with
C/EBPβ, AP-1 and NF-κB to regulate the expression of a large
network of liver regeneration of genes (9,10).
The C/EBP family of transcription factors is
characterized by a basic region/leucine zipper domain, which is
involved in the regulation of liver-specific gene expression, with
an important role in liver development and liver cell function
(11,12). C/EBPβ is an important member of the
C/EBP family as it has been shown that C/EBPβ deficiency can result
in liver regeneration failure, indicating an essential role for
C/EBPβ during regeneration (8,13).
C/EBPβ (also known as NF-IL-6) is a downstream target of IL-6
(14,15) and is activated through the
Ras/mitogen-activated protein kinase pathway to induce gene
expression during the acute phase of liver regeneration (16). A previous study also found that
C/EBPβ promoter sequences contain an acute phase response element,
and DNA binding experiments have demonstrated that STAT3 binds to
this element to activate transcription of C/EBPβ (17).
Although less comprehensively studied, pathways
leading to the inhibition of liver regeneration are considered to
be equally important as those initiating the process. Transmembrane
and ubiquitin-like domain containing 1 (Tmub1) is a candidate
molecule for involvement in the termination of liver regeneration;
Tmub1 was first identified by Della Fazia et al (18), who showed that its expression was
markedly elevated during liver regeneration. Additionally,
overexpression of Tmub1 has been shown to play a role in the
inhibition of cell proliferation (18). Our previous study found that
short-hairpin RNA knockdown of Tmub1 expression increased liver
cell proliferation, indicating that Tmub1 may play a negative role
in liver regeneration (19).
However, a regulatory function for Tmub1 expression is not clear.
The previous results demonstrated that IL-6 could enhance the
expression of Tmub1 in rat liver cells (19). C/EBPβ expression was significantly
increased 2 h after partial hepatectomy (20), whilst the peak of Tmub1 expression
appeared later, 48 h after surgery (18). This indicates that C/EBPβ may
participate in the regulation of Tmub1 gene expression. Through
bioinformatics analysis in the present study, it was found that the
Tmub1 promoter sequence contained C/EBPβ and STAT3 binding sites.
The aims of the present study were three-fold: i) To characterize
the association between C/EBPβ and Tmub1 gene expression; ii) to
determine whether C/EBPβ could bind the Tmub1 promoter by chromatin
immunoprecipitation (ChIP) assay; and iii) to analyze the effects
of C/EBPβ on Tmub1 promoter activity to predict the potential
binding sites of C/EBPβ in the Tmub1 gene.
Materials and methods
Cell culture
Normal BRL-3A rat hepatocytes (Cell Bank of Academia
Sinica, Shanghai, China) were cultured in high-glucose Dulbecco’s
Modified Eagle’s medium with 10% fetal bovine serum (Invitrogen
Life Technologies, Carlsbad, CA, USA) at 37°C with 5%
CO2.
Bioinformatics analysis of promoter
sequences of Tmub1 gene
According to available information on the rat Tmub1
gene (GenBank NC_005103.2; http://www.ncbi.nlm.nih.gov/genbank/), online software
(http://www-bimas.cit.nih.gov/molbio/proscan/; version
1.7) was used to predict the potential promoter regions of Tmub1,
using 53 as the cut-off value for prediction. Online software
(http://cbrc.jp/research/db/TFSEARCH.html) was used to
predict the potential transcription factor binding sites in the
upstream 5′ end sequence of the Tmub1 gene. The maximum score was
100.0 and the minimum score was 85.0.
Small interfering RNA (siRNA)
knockdown
Three targeted siRNAs and one scramble control siRNA
were synthesized by RiboBio Co. (Guangzhou, China) against rat
C/EBPβ. siRNA was transfected using Lipofectamine™ 2000 according
to the manufacturer’s instructions (Invitrogen Life Technologies).
Western blot analysis was performed to determine the optimal siRNA
sequence and concentration for the inhibition of C/EBPβ at 72 h
post-transfection.
Construction of the rat C/EBPβ
overexpression vector and cell transfection
mRNA was isolated from BRL-3A cells and reverse
transcribed into cDNA (Takara, Tokyo, Japan). The sense and
antisense primers for C/EBPβ amplification were as follows:
5′-ATATGGATCCATGCACCGCCTGCTGGCCT-3′ and
5′-ATAACTCGAGCTAGCAGTGACCCGCCGAGG-3′, respectively. The full-length
amplified C/EBPβ cDNA was inserted into a pCMVTag2B vector (Kang
Chen Bio-tech Inc., Shanghai, China) and the sequence was verified
by polymerase chain reaction (PCR) and sequencing. C/EBPβ
expression vectors were transfected using a Lipofectamine 2000 kit
(Invitrogen Life Technologies) and cells were harvested for testing
at 72 h post-transfection.
Quantitative PCR (qPCR) analysis
RNA was extracted from cells using
TRIzol® reagent (Takara) and used for reverse
transcription using a High Capacity RNA-to-cDNA kit (Takara)
according to the manufacturer’s instructions. PCR reactions were
prepared using the Brilliant SYBR Green qPCR Master Mix kit
(Takara) and amplified using a Bio-Rad CFX96 thermocycler (Bio-Rad,
Hercules, CA, USA). The sense and antisense primers were as
follows: Tmub1, 5′-GTAGGCGATGAGGTGACTGT-3′ and 5′-GCTGTGCTG
GTGTTGTGG-3′ (fragment length, 136 bp); C/EBPβ, 5′-CAC
CGGGTTTCGGGACTTG-3′ and 5′-CCCGCAGGA ACATCTTTAAGTG-3′ (fragment
length, 128 bp); β-actin internal reference,
5′-ACGTTGACATCCGTAAAGAC-3′ and 5′-GAAGGTGGACAGTGAGGC-3′ (fragment
length, 200 bp).
Western blot analysis
Cells were collected and washed with
phosphate-buffered saline, lysed in radioimmunoprecipitation assay
buffer on ice and disrupted by sonication. Protein samples were
separated by SDS-PAGE and transferred to a membrane. The membrane
was blocked with 5% non-fat dry milk buffer for 1 h and
immunoblotted with Tmub1 and anti-C/EBPβ primary and goat anti-rat
secondary antibodies (Santa Cruz Biotechnology Inc., Santa Cruz,
CA, USA). Protein bands were imaged by an Odyssey Two Infrared
Imaging system (LI-COR, Lincoln, NE, USA) and analyzed by Quantity
One software (Bio-Rad).
ChIP assay
A ChIP assay was performed using the EZ-ChIP kit
(Millipore, Billerica, MA, USA) according to the manufacturer’s
instructions. The following antibodies were used: Anti-RNA
polymerase as a positive control, normal rat immunoglobulin G as a
negative control (Santa Cruz Biotechnology Inc.) and anti-C/EBPβ
antibody in the experimental groups. Primer 5 software (Premier
Biosoft, Palo Alto, CA, USA) was used to design the following
primers for Tmub1 amplification: Forward primer,
5′-TCTGCAAGCATAAGGACCTC-3′ and reverse primer,
5′-ACTGAGCTAAATCCCCATCC-3′, (fragment length, 208 bp). PCR
reactions were carried out to validate whether C/EBPβ interacted
with the sequences within the promoter region of Tmub1.
Construction of Tmub1 reporter gene
vectors with varying promoter lengths
Genomic DNA from the BRL-3A cell line was used as a
template to amplify 12 fragments in the 5′ upstream sequence
(−2,007 to +50) of the Tmub1 gene. The forward primer is shown in
Table I and the reverse primer was
as follows: 5′-ATAACTCGAGACCCAGCTCCACCA GCTCCATT-3′. The promoter
fragments were subcloned into digested pGL3-Basic vectors (KangChen
Bio-tech Inc, Shanghai, China), and positive clones were identified
by PCR and sequencing.
 | Table IForward primer sequences for the
construction of Tmub1 reporter gene vectors. |
Table I
Forward primer sequences for the
construction of Tmub1 reporter gene vectors.
| Primer | Sequence (5′-3′) | Fragment length
(bp) |
|---|
| T1 |
ATAAACGCGTTACATATAGAAACCCCATCCTGAAA | 2058 |
| T2 |
ATAAACGCGTGCATATAAAACATATATATTTAA | 1784 |
| T3 |
TTAAACGCGTGCCCAGGGCAATATTTGAT | 1687 |
| T4 |
ATAAACGCGTGTAGAGTATTTTTAATTCAGACT | 1609 |
| T5 |
AAAAACGCGTCATGCAGATATAATATACAAACC | 1442 |
| T6 |
ATATACGCGTCAACCTTAGCAGAGGGAGCAG | 1317 |
| T7 |
ATAAACGCGTCTCTAGGTGGCCAAAGGTAGT | 926 |
| T8 |
ATATACGCGTCGTTTCTTCTTTCCAACTGA | 729 |
| T9 |
ATAAACGCGTCGAGGGCAATTCACGCCTCCT | 517 |
| T10 |
ATAAACGCGTAAGCTGGCGGCGGAAATAGAGT | 323 |
| T11 |
ATAAACGCGTCTACCGGTACCGGGAACATCT | 202 |
| T12 |
ATATACGCGTTCCCGGGCCGCGGCAGCA | 107 |
Detection of luciferase reporter gene
activity
BRL-3A cells were cultured in 48-well plates to
70–80% confluency prior to co-transfection with Tmub1
promoter-luciferase reporter vectors and C/EBPβ expression plasmid
or a negative control. Transfection was performed using
Lipofectamine 2000 according to the manufacturer’s instructions.
After 48 h in culture (depending on the experiment, 15 ng/ml IL-6
was added 12 h prior to the luciferase activity assay), cells were
harvested and assayed using a Dual Luciferase Assay kit (Promega
Corp., Madison, WI, USA) according to the manufacturer’s
instructions.
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis was performed with Sigma Plot 10.0
software (Systat Software Inc., San Jose, CA, USA). Differences
between individual groups were analyzed by least-significant
difference test. A value of P<0.05 was considered a
statistically significant difference.
Results
Bioinformatics analysis of the 5′
upstream sequence of Tmub1
Based on the information from GenBank, it was found
that the transcription start site of the rat Tmub1 gene was located
1,092 bp upstream of the translational initiation codon (ATG).
GC-rich regions were found at +50 bp upstream of the transcription
start point, which could be part of the first intron sequences and
binding sites for the promoter or enhancer of the Tmub1 gene.
Online software analysis revealed that the promoter binding sites
were more likely to be located within the −322 to −122 bp region
(score 69.64). TFSEARCH software analysis revealed that within the
−2,008 to +50 bp region, there were multiple potential binding
sites for transcription factors associated with liver regeneration
and IL-6 signaling pathways, including five C/EBP sites (5), three AP-1 sites (3), a STAT site (1) and two cAMP response element-binding
protein sites (2) (Table II).
 | Table IIDistribution of potential
transcription factor binding sites upstream of the Tmub1 gene. |
Table II
Distribution of potential
transcription factor binding sites upstream of the Tmub1 gene.
| Transcription
factor | Potential binding
sites (bp) |
|---|
| C/EBPβ | −1751 to −1738 |
| C/EBPβ | −1702 to −1689 |
| AP-1 | −1455 to −1445 |
| C/EBPβ | −1344 to −1331 |
| C/EBP | −794 to −781 |
| AP-1 | −727 to −717 |
| AP-1 | −664 to −654 |
| CREB | −411 to −404 |
| CREB | −240 to −137 |
| C/EBP | −240 to −228 |
| STAT | −145 to −137 |
IL-6 upregulation of Tmub1 expression may
correlate with C/EBPβ, STAT3 and AP-1 transcription factors
A series of Tmub1 luciferase reporter vectors, known
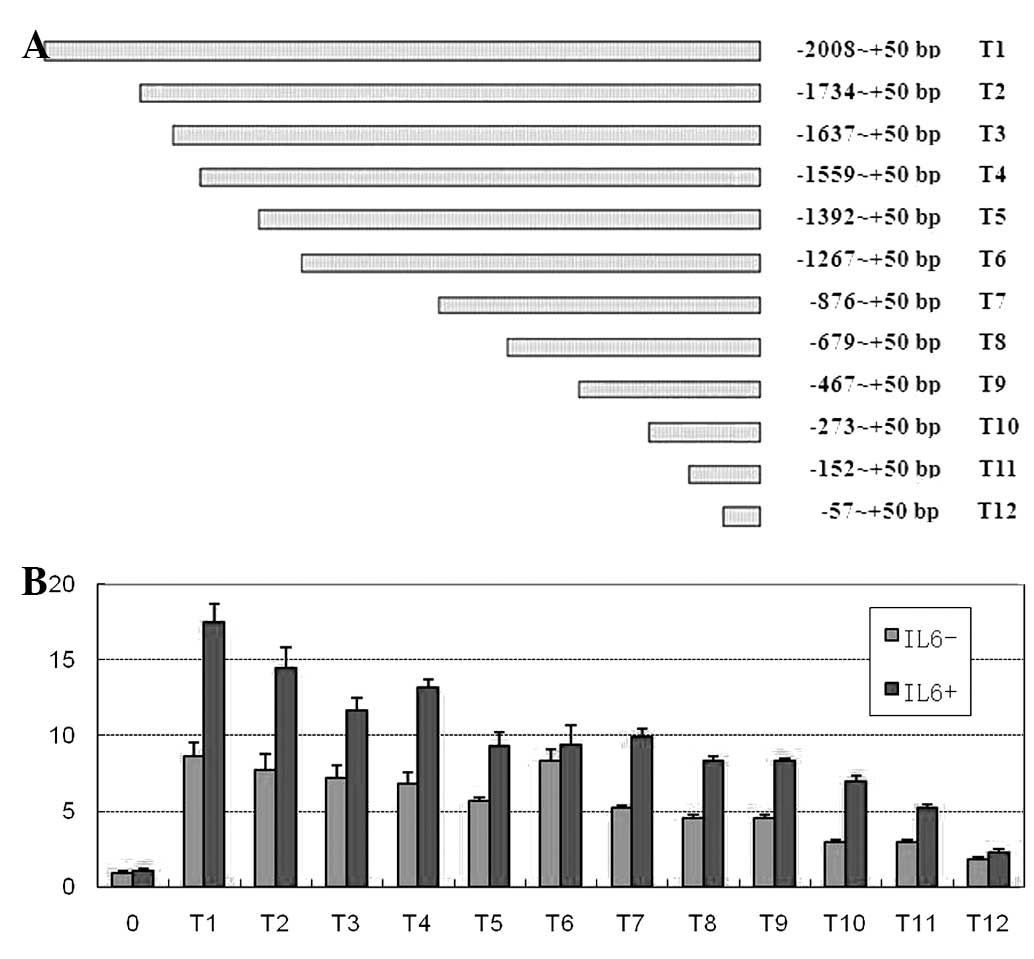
as T1–T12, were constructed (Fig.
1A) and transfected into BRL-3A cells to determine the Tmub1
promoter activity. In the absence of stimulating factors, all of
the sequence fragments showed significant transcriptional activity
(P<0.05). With the exception of the T4 and T6 promoter
fragments, which maintained promoter activity, the transcriptional
activities of the other promoter fragments were gradually decreased
by deleting the Tmub1 promoter region sequences. When the −1,392 to
−1,268 bp sequence was deleted, the transcriptional activity of the
T6 fragment was markedly elevated, indicating that there was a
potential repressive element of Tmub1 expression within the deleted
sequence (Fig. 1B). Following the
addition of IL-6, the luciferase reporter activity in most reporter
vectors was significantly increased (P<0.05), with the exception
of the T6 group. The luciferase activities among the groups were
also substantially variable (Fig.
1B). IL-6 could enhance transcriptional activity of the Tmub1
promoter, and deleting different sequences resulted in differential
effects on the IL-6-induced activities: Deletion of the −2,008 to
−1,735 (T2), −1,734 to 1,638 (T3), −1,559 to 1,393 (T5), −1,392 to
1,268 (T6), −273 to 153 (T11) and −152 to −58 bp (T12) sequences
had a more marked effect. These sequences contained potential
binding sites for C/EBPβ, STAT3 and AP-1. The majority of these
sites were C/EBPβ (or C/EBP) binding sites, which were located at
2,008 to −1,735, −1,734 to −1,638, −1,392 to −1,268 and −273 to
−153 bp.
C/EBPβ expression is positively
correlated with Tmub1 expression in rat liver cells
Upon siRNA knockdown of C/EBPβ expression, Tmub1
mRNA and protein levels significantly decreased in liver cells.
Conversely, upon overexpression of C/EBPβ, Tmub1 mRNA and protein
levels were increased (Fig. 2).
These results suggest that C/EBPβ levels are positively correlated
with Tmub1 expression and that C/EBPβ may be involved in the
regulation of Tmub1 expression during liver cell proliferation.
C/EBPβ can bind to 5′ upstream sequences
of the Tmub1 gene
ChIP assay was used to determine whether C/EBPβ
could bind to Tmub1 and directly regulate its expression in rat
liver cells. From bioinformatics analysis, a fragment sequence in
the Tmub1 promoter region was selected that contained one C/EBPβ
binding site (−1,344 to −1,331 bp) and this was used as a target
sequence in the experiment (Fig.
3). The PCR results demonstrated that the DNA sequence binding
to C/EBPβ contained the promoter sequence of the Tmub1 gene.
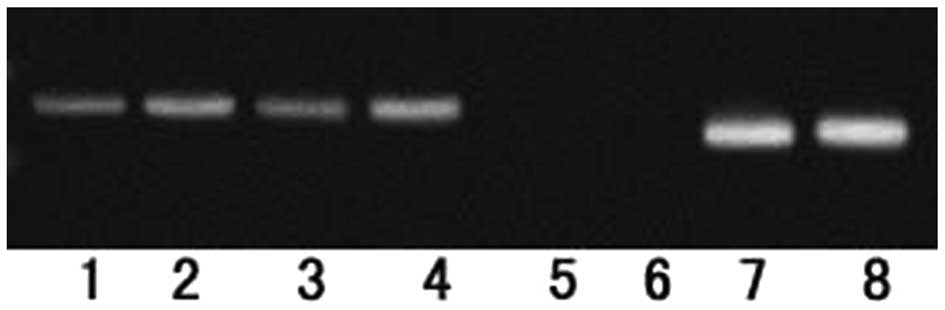 | Figure 3The Tmub1 gene promoter region
contains a C/EBPβ binding site. A chromatin immunoprecipitation
assay was performed using C/EBPβ-specific antibodies or control
immunoglobulin G in BRL-3A cells and the C/EBPβ-Tmub1 promoter
complex was amplified by polymerase chain reaction. Cells were
divided into eight groups: Lanes 1 and 2, experimental groups;
lanes 3 and 4, input controls; lanes 5 and 6, negative controls;
lanes 7 and 8, positive controls. The C/EBPβ overexpression vector
was transfected into the cells of the groups in lanes 2, 4, 6 and
8. A 208-base pair fragment was detected in the experimental (lanes
1 and 2), input (lanes 3 and 4) and positive control (lanes 7 and
8) groups, which was consistent with the predicted target fragment
in the promoter region of the Tmub1 gene. C/EBPβ,
CCAAT/enhancer-binding protein β; Tmub1, transmembrane and
ubiquitin-like domain containing 1. |
C/EBPβ can enhance the promoter activity
of Tmub1
To refine the potential binding sites of C/EBPβ in
Tmub1, luciferase reporter gene vectors were used to observe the
effects of C/EBPβ on the promoter activity of Tmub1 upon enhancing
C/EBPβ expression (Fig. 4). With
the exception of the T6 fragment, the transcriptional activities of
the other 11 constructs were significantly increased (P<0.05).
The most prominent effects on the transcriptional activity of the
promoter were obtained by deleting the −2,008 to −1,735 (T2), −273
to −153 (T11) and −152 to −58 bp (T12) sequences, indicating that
the binding sites of C/EBPβ may be located within these three
fragment sequences. Although the potential repressive element of
Tmub1 expression could lie within the −1,392 to −1,268 bp sequence,
the luciferase activity of the T5 reporter vector was also enhanced
following C/EBPβ overexpression, and the deletion of this fragment
sequence did not significantly alter luciferase activity. This
suggests that the C/EBPβ binding site may lie within this fragment
sequence and the transcriptional enhancement caused by C/EBPβ
interacting with the binding site can offset the inhibitory role of
the repressive element.
Discussion
Our previous study demonstrated that Tmub1 plays a
negative regulatory role in the hepatocyte proliferation process;
however, its expression can be upregulated by the positive
regulators of proliferation, such as IL-6 (19). There are little data regarding the
inhibitory factors affecting proliferation during hepatic
regeneration. Thus, clarifying the regulatory mechanism of Tmub1
expression is important in understanding the association between a
proliferative promoting factor and a proliferative inhibitory
factor in the hepatocyte regeneration process and the mechanism of
hepatocyte homeostasis.
The results of the present study indicated that IL-6
may regulate Tmub1 expression during liver cell proliferation. The
expression of IL-6 target genes ultimately depends on the
activities of its downstream transcription factors. In order to
explore the regulatory mechanism of Tmub1 expression, the
transcription factors involved in the regulation of Tmub1
expression by IL-6 were first identified. The effects of IL-6 on
the promoter activity of Tmub1 were assayed and it was identified
that, during the hepatic cell proliferation process, IL-6 may
enhance the expression of Tmub1 through the C/EBPβ, STAT3 and AP-1
transcription factors. These findings were consistent with previous
reports in the literature (9,10).
Among these transcription factors, STAT3 is a known characterized
downstream target of IL-6, and its mechanism has been studied
extensively (8). Notably, the
present study showed that Tmub1 contains multiple potential C/EBPβ
(or C/EBP) binding sites, and the deletion of these can
significantly influence the effect of IL-6 on enhancing the
transcription of Tmub1, thus indicating that IL-6 may regulate
Tmub1 expression through C/EBPβ.
C/EBPβ is an important transcription factor required
during liver regeneration and is a known downstream target of IL-6
(13,14). A search of the literature and our
own bioinformatics analysis have suggested that C/EBPβ is involved
in regulating the expression of Tmub1 (9,10,13,15,20),
and this is supported by the data presented in this study. It was
observed that inhibiting the expression of C/EBPβ by siRNA caused a
corresponding reduction in Tmub1 expression, whilst enhancing the
expression of C/EBPβ correlated with an increase in Tmub1
expression, indicating an involvement of C/EBPβ in the positive
regulation of Tmub1 gene expression. ChIP experiments confirmed
that C/EBPβ could bind with the promoter sequences of Tmub1 in
proliferating liver cells, indicating that C/EBPβ may directly
interact with Tmub1 to initiate or enhance Tmub1 expression.
Luciferase reporter experiments revealed that the fragment
sequences in the Tmub1 promoter region (e.g. −2,008 to −1,735,
−1,392 to −1,268, −273 to −153 and −152 to −58 bp) could
significantly influence the transcriptional effect mediated by
C/EBPβ, further suggesting that C/EBPβ binding sites may be present
in these sequences. All three regions contained predicted C/EBPβ
binding sites, with one binding site (−1,392 to −1,268 bp) fragment
confirmed by ChIP. The fragment sequence −152 to −58b bp had no
predicted C/EBPβ binding site, but contained a STAT3 binding site
that affected the transcriptional effect mediated by C/EBPβ. Taken
together, this indicated that C/EBPβ and STAT3 may collaboratively
regulate Tmub1 transcription.
This study has shown that Tmub1 expression is
regulated by IL-6, together with C/EBPβ. This demonstrates that
there is a close association between Tmub1, IL-6 and C/EBPβ, which
may play important roles in signal transduction during liver
regeneration. The activation of STAT3 through the Janus kinase
(JAK) pathway to induce downstream target gene expression is a
known regulatory mechanism of IL-6. C/EBPβ is also considered one
of the downstream targets of IL-6 and its expression can be
regulated by IL-6 during liver regeneration (21,22),
indicating that IL-6 can upregulate expression of C/EBPβ to enhance
the expression of Tmub1. The results of this study support these
described mechanisms reported in the literature. It is therefore
hypothesized that Tmub1 expression during the process of liver
regeneration may be regulated by the following mechanism:
IL-6-induced activation of STAT3 via the JAK pathway and
simultaneous activation of C/EBPβ to promote Tmub1 expression. It
is also possible that C/EBPβ and STAT3 transcription factors act
synergistically to enhance the expression of Tmub1.
In conclusion, C/EBPβ has been shown to be a key
transcription factor involved in the regulation of Tmub1
expression. During the process of liver regeneration, positive
regulators, such as IL-6 and C/EBPβ, can increase
proliferation-related gene expression and can, at the same time,
induce or enhance the expression of inhibitory factors such as
Tmub1 to limit excessive cell proliferation and maintain the
stability of liver regeneration.
Acknowledgements
This study was supported in part by a grant from the
Natural Science Foundation of China (no. 30972895) and from the
Natural Science Foundation of Chongqing (no. 2009BA5014). The
authors would like to thank Medjaden Bioscience Limited for
assisting in the preparation of this manuscript.
Abbreviations:
|
C/EBPβ
|
CCAAT/enhancer-binding protein β
|
|
Tmub1
|
transmembrane and ubiquitin-like
domain containing 1
|
|
STAT3
|
signal transducer and activator of
transcription-3
|
|
IL-6
|
interleukin-6
|
References
|
1
|
Duncan AW and Soto-Gutierrez A: Liver
repopulation and regeneration: new approaches to old questions.
Curr Opin Organ Transplant. 18:197–202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kurinna S and Barton MC: Cascades of
transcription regulation during liver regeneration. Int J Biochem
Cell Biol. 43:189–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michalopoulos GK and DeFrances MC: Liver
regeneration. Science. 276:60–66. 1997. View Article : Google Scholar
|
|
4
|
Stolz DB, Mars WM, Petersen BE, Kim TH and
Michalopoulos GK: Growth factor signal transduction immediately
after two-thirds partial hepatectomy in the rat. Cancer Res.
59:3954–3960. 1999.PubMed/NCBI
|
|
5
|
Fausto N: Liver regeneration. J Hepatol.
32:19–31. 2000. View Article : Google Scholar
|
|
6
|
Kountouras J, Boura P and Lygidakis NJ:
Liver regeneration after hepatectomy. Hepatogastroenterology.
48:556–562. 2001.
|
|
7
|
Pahlavan PS, Feldmann RE Jr, Zavos C and
Kountouras J: Prometheus’ challenge: molecular, cellular and
systemic aspects of liver regeneration. J Surg Res. 134:238–251.
2006.
|
|
8
|
Streetz KL, Luedde T, Manns MP and
Trautwein C: Interleukin 6 and liver regeneration. Gut. 47:309–312.
2000. View Article : Google Scholar
|
|
9
|
Schumann RR, Kirschning CJ, Unbehaun A,
Aberle HP, Knope HP, Lamping N, Ulevitch RJ and Herrmann F: The
lipopolysaccharide-binding protein is a secretory class 1
acute-phase protein whose gene is transcriptionally activated by
APRF/STAT/3 and other cytokine-inducible nuclear proteins. Mol Cell
Biol. 16:3490–3503. 1996.
|
|
10
|
Brown RT, Ades IZ and Nordan RP: An acute
phase response factor/NF-kappa B site downstream of the junB gene
that mediates responsiveness to interleukin-6 in a murine
plasmacytoma. J Biol Chem. 270:31129–31135. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Osada S, Yamamoto H, Nishihara T and
Imagawa M: DNA binding specificity of the CCAAT/enhancer-binding
protein transcription factor family. J Biol Chem. 271:3891–3896.
1996. View Article : Google Scholar
|
|
12
|
Takiguchi M: The C/EBP family of
transcription factors in the liver and other organs. Int J Exp
Pathol. 79:369–391. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Greenbaum LE, Li W, Cressman DE, Peng Y,
Ciliberto G, Poli V and Taub R: CCAAT enhancer-binding protein beta
is required for normal hepatocyte proliferation in mice after
partial hepatectomy. J Clin Invest. 102:996–1007. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akira S, Isshiki H, Sugita T, Tanabe O,
Kinoshita S, Nishio Y, Nakajima T, Hirano T and Kishimoto T: A
nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP
family. EMBO J. 9:1897–1906. 1990.PubMed/NCBI
|
|
15
|
Akira S and Kishimoto T: IL-6 and NF-IL6
in acute-phase response and viral infection. Immunol Rev.
127:25–50. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akira S: IL-6-regulated transcription
factors. Int J Biochem Cell Biol. 29:1401–1418. 1997. View Article : Google Scholar
|
|
17
|
Mayer C, Gruber HJ, Landl EM, Pailer S,
Scharnagl H, Truschnig-Wilders M and März W: Rosuvastatin reduces
interleukin-6-induced expression of C-reactive protein in human
hepatocytes in a STAT3- and C/EBP-dependent fashion. Int J Clin
Pharmacol Ther. 45:319–327. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Della Fazia MA, Castelli M, Bartoli D,
Pieroni S, Pettirossi V, Piobbico D, Viola-Magni M and Servillo G:
HOPS: a novel cAMP-dependent shuttling protein involved in protein
synthesis regulation. J Cell Sci. 118:3185–3194. 2005.PubMed/NCBI
|
|
19
|
Liu M, Liu H, Wang X, Chen P and Chen H:
IL-6-induction of hepatocyte proliferation through the
Tmub1-regulated gene pathway. Int J Mol Med. 29:1106–1112.
2012.PubMed/NCBI
|
|
20
|
Flodby P, Antonson P, Barlow C, Blanck A,
Porsch-Hällström I and Xanthopoulos KG: Differential patterns of
expression of three C/EBP isoforms, HNF-1, and HNF-4 after partial
hepatectomy in rats. Exp Cell Res. 208:248–256. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cressmann DE, Diamond RH and Taub R: Rapid
activation of the Stat3 transcription complex in liver
regeneration. Hepatology. 21:1443–1449. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niehof M, Streetz K, Rakemann T, Bischoff
SC, Manns MP, Horn F and Trautwein C: Interleukin-6-induced
tethering of STAT3 to the LAP/C/EBPbeta promoter suggests a new
mechanism of transcriptional regulation by STAT3. J Biol Chem.
276:9016–9127. 2001. View Article : Google Scholar
|