Introduction
Osteoarthritis (OA) is a chronic cartilage and joint
disease among elderly individuals, primarily characterized by
articular cartilage breakdown, osteophyte formation, subchondral
sclerosis and synovium alterations (1). Cartilage degeneration, which impairs
function and causes pain and disability, is known to contribute to
major structural alterations in the joint. Inflammatory cytokines,
including interleukin-1 beta (IL-1β), cyclooxygenase-2 (COX-2),
prostaglandin E2 (PGE2) and nitric oxide (NO) as well as
catabolic factors, including matrix metalloproteinases (MMPs), a
disintegrin and metalloprotease with thrombospondin motifs (ADAMTS)
and cathepsins are major mediators of disturbed chondrocyte
function and cartilage degeneration (2). In addition, age, gender, injury and
obesity are considered to be major risk factors for the progression
of OA (3). Previous studies have
revealed that not only the knee and hip OA were closely associated
with obesity, but also the non-weight bearing joints, such as the
hand OA, were more frequent in obese individuals (4,5),
however, the reasons for this remain to be elucidated. Furthermore,
adipokines secreted by white adipose tissue are considered to be a
link between obesity and OA, particularly in non-weight bearing
joints (6). Accumulating evidence
suggested that adipokines, including leptin, adiponectin, resistin,
visfatin and apelin exert pro-inflammatory/anti-inflammatory and
catabolic/anabolic roles during the pathophysiology of OA (7).
Visceral adipose tissue-derived serine protease
inhibitor (vaspin) was identified as a novel adipocytokine, which
has been found to be expressed in the visceral adipose tissue of
Otsuka Long-Evans Tokushima Fatty rats at an age when obesity and
insulin resistance peaked (8).
Several human tissues, including adipose tissue (9,10),
skin (11), stomach (12), liver and pancreas (13) have been found to express vaspin.
Serum vaspin levels were paradoxically elevated in human subjects
with diabetes and obesity (14).
Notably, several studies demonstrated novel links between vaspin
and arthritis (15,16). Ozgen et al (15) found that serum vaspin levels were
higher in rheumatoid arthritis (RA) compared with healthy controls.
Furthermore, the serum vaspin level was increased following
glucocorticoid treatment but unaffected following adalimumab
treatment in RA patients (16). In
addition, another study (17)
identified increased levels of vaspin in the synovial fluid of
patients with RA compared with those with OA. These data suggest a
possible role of vaspin in the pathophysiology of arthritis.
Previously, Kamio et al (18) demonstrated that vaspin inhibited
receptor activator of nuclear factor-κB ligand (RANKL)-induced
osteoclastogenesis in RAW264.7 cells and bone marrow cells (BMCs).
Vaspin also inhibited the RANKL-induced upregulation of MMP-9 and
cathepsin K in RAW264.7 cells (18), which suggested that vaspin was
important in bone metabolism. However, to the best of our
knowledge, there are currently no studies investigating the effect
of vaspin on chondrocytes. The aim of the present study was to
detect the role of vaspin in chondrocytes. Initially, the effect of
vaspin on normal chondrocyte viability and gene expression of
MMP-2, MMP-9, ADAMTS-4, ADAMTS-5 and cathepsin was assessed, as
well as the effect of vaspin on the secretion of COX-2,
PGE2 and iNOS. Secondly, the effect of vaspin on
IL-1β-induced catabolic and inflammatory responses in chondrocytes
was evaluated. In addition, the present study investigated the
effect of vaspin on the IL-1β-induced nuclear factor-kappaB (NF-κB)
signaling pathway activation in chondrocytes.
Materials and methods
Isolation and culture of primary rat
articular chondrocytes
Rat articular chondrocytes for primary culture were
isolated from the knee joints of 4-week-old Sprague-Dawley rats
obtained from The Animal Center of Zhejiang University (Hangzhou,
China). The study was approved by the ethics committee of The
Second Hospital of Medical College, Zhejiang University (Hangzhou,
China). The harvested cartilage samples were cut into 1
mm3 cubes and digested for 0.5 h with 0.2% pronase
(Sigma, St. Louis, MO, USA), followed by digestion for 4 h with
0.1% collagenase (Sigma) at 37°C. The released chondrocytes were
cultured in 25 cm2 culture flasks in Dulbecco’s modified
Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/ml
penicillin and 100 μg/ml streptomycin (Invitrogen Life
Technologies, Carlsbad, CA, USA) in a 5% CO2 atmosphere
at 37°C. Chondrocytes were passaged at a ratio of 1:3 with 0.05%
trypsin (Life Technologies, Hangzhou, China) up to 80% confluence.
Cultured chondrocytes at passage 3 were used to avoid loss of
chondrocyte phenotype with successive passages.
Assessment of cell viability
Cytotoxicity of vaspin to chondrocytes was evaluated
using a Cell Counting kit-8 (CCK-8; Dojindo Laboratories, Kumamoto,
Japan) according to the manufacturer’s instructions. Chondrocytes
were cultured in 96-well plates at a density of 2×104
cells/cm3 and the cytotoxicity was assessed in the
presence of increasing concentrations of vaspin (0, 10, 50, 100,
250 and 500 ng/ml). Cell proliferation was examined 24 and 48 h
after the addition of vaspin. At the indicated time points, 100 μl
fresh medium and 10 μl CCK-8 solution were added to each well of
the plate, and the chondrocytes were incubated for 2 h. Absorbance
of 450 nm was measured using a microplate reader (Bio-Rad,
Hercules, CA, USA).
Quantitative polymerase chain reaction
(qPCR) analysis of MMP-2, MMP-9, ADAMTS-4, ADAMTS-5 and cathepsin
D
Rat chondrocytes were treated with various
concentrations of vaspin (0, 10, 50, 100, 250 and 500 ng/ml) for 24
h, while other chondrocytes were pretreated with various
concentrations of vaspin for 1 h prior to treatment with IL-1β (10
ng/ml) for 24 h. The gene expression was analyzed by qPCR. Total
RNA was isolated from monolayer chondrocytes using TRIzol reagent
(Invitrogen Life Technologies) according to the manufacturer’s
instructions, dissolved in diethylpyrocarbonate-treated water and
stored at -80°C prior to use. Total RNA (1 μg) was used for
synthesis of cDNA by reverse transcription, using the
PrimeScript-RT reagent kit (Takara Bio., Inc., Shiga, Japan), and
the reverse transcription reaction product was analyzed by qPCR
using the SYBR Premix Ex Taq (Takara Bio., Inc.), according to the
manufacturer’s instructions. The target gene primers used are shown
in Table I. A parallel
amplification of 18S (NR046237.1) was performed to normalize
expression data of the targeted gene transcript. Relative
expression level data were analyzed using the 2−ΔΔCt
method.
 | Table IPrimers of targeted genes. |
Table I
Primers of targeted genes.
| Targeted gene | Sequence
(5′-3′)a | Amplicon length
(bp) | Accession number |
|---|
| MMP-2 | F:
AGGATGGAGGCACGATTGG | | |
| R:
CTTGATGATGGGCGACGGT | 111 | NM031054 |
| MMP-9 | F:
ACCCCATGTATCACTACCACGAG | | |
| R:
TCAGGTTTAGAGCCACGACCAT | 91 | NM031055 |
| ADAMTS-4 | F:
GCCAGCAACCGAGGTCCCATA | | |
| R:
CCACCAGTGTCTCCACGAATCTAC | 113 | XM001053685 |
| ADAMTS-5 | F:
GGGGTCAGTGTTCTCGCTCTTG | | |
| R:
GCCGTTAGGTGGGCAGGGTAT | 146 | AY382879 |
| Cathepsin D | F:
CAGGCADATCGTAAGTGGC | | |
| R:
GTCGTGGAAAGGACAGTTGG | 51 | NM134334 |
| 18S | F:
TTGACGGAAGGGCACCA | | |
| R:
CAGACAAATCGCTCCACCAA | 165 | NR046237.1 |
ELISA analysis of COX-2, PGE2
and iNOS in the culture medium
Rat chondrocytes were treated with various
concentrations of vaspin (0, 10, 50, 100, 250 and 500 ng/ml) for 24
h, while other chondrocytes were pretreated with various
concentrations of vaspin for 1 h prior to IL-1β treatment (10
ng/ml) for 24 h. The effect of IL-1β and/or vaspin on the levels of
COX-2, PGE2 and iNOS secreted by articular chondrocytes
in the culture medium was further detected by commercially
available ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA)
according to the manufacturer’s instructions. All assays were
performed in duplicate.
Western blot analysis of p-NF-κB and
IκB-α
Confluent articular chondrocytes were incubated in
serum-free medium for 24 h. The chondrocytes were pre-treated with
various doses of vaspin (0, 10, 50, 100, 250 and 500 ng/ml) for 1 h
prior to IL-1β (10 ng/ml) for 24 h. All chondrocytes were washed
twice with ice-cold phosphate-buffered saline, harvested using a
scraper and then the cytoplasmic proteins were isolated using an
extraction kit (Beyotime Institute of Biotechnology, Jiangsu,
China). Samples were subjected to sodium dodecyl sulfate
polyacrylamide gel electrophoresis, transferred onto nitrocellulose
filters and probed with the following primary antibodies: p-NF-κB
and IκB-α (rabbit monoclonal anti-mouse; Cell Signaling Technology,
Hangzhou, China) overnight at 4°C. The membranes were washed and
incubated for 1 h at room temperature with horseradish
peroxidase-linked secondary antibodies (goat monoclonal
anti-rabbit). Detection was performed with enhanced
chemiluminescence using a commercially available kit according to
the manufacturer’s instructions (Cell Signaling Technology) and the
density of each band was measured by densitometry using Quantity
One Software (Bio-Rad Laboratories Inc., Munich, Germany).
Statistical analysis
All experiments were performed three times using
independent samples. Data are expressed as the mean ± standard
deviation. Statistical analyses were performed using SPSS 19.0 for
Windows software (SPSS, Inc., Chicago, IL, USA). Statistical
significance was determined using Student’s t-test and one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of vaspin on chondrocyte
viability
In order to rule out direct cytotoxic effects, a
CCK-8 test was performed. As shown in Fig. 1, the results indicated that vaspin
at concentrations of 10, 50, 100, 250 and 500 ng/ml after 24 and 48
h of culture demonstrated no significant cytotoxic effect on
chondrocytes. However, no chondrocyte proliferation effects of
vaspin were identified at any of these concentrations.
Effects of vaspin on the production of
catabolic factors and inflammatory cytokines in rat
chondrocytes
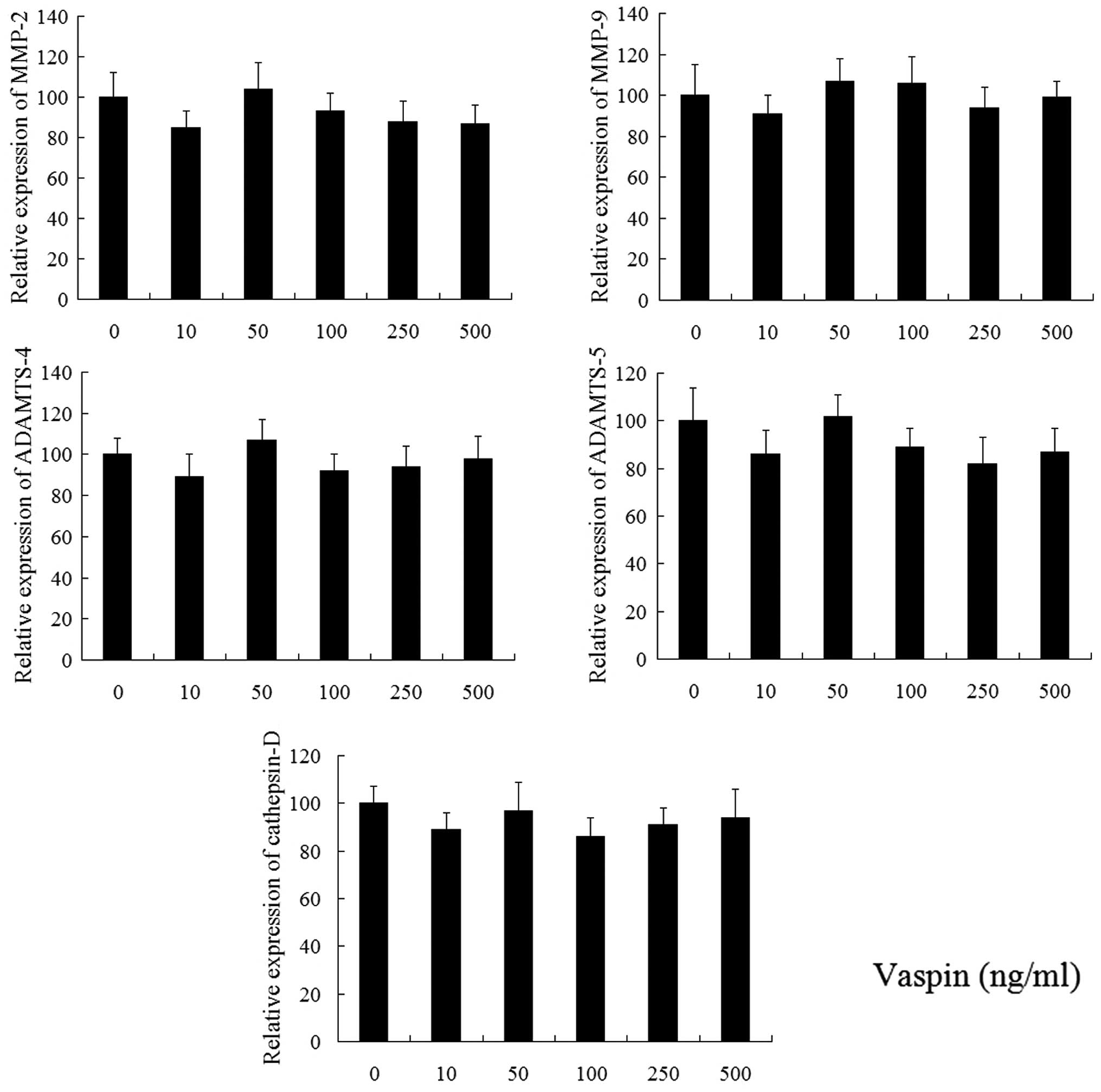
Following treatment with vaspin for 24 h, the
expression of MMP-2, MMP-9, ADAMTS-4, ADAMTS-5 and cathepsin D were
detected using qPCR. As shown in Fig.
2, vaspin (10, 50, 100, 250 and 500 ng/ml) alone did not affect
the gene expression of these catabolic factors. As noted in
Fig. 3, the secretion levels of
COX-2, PGE2 and iNOS in chondrocytes decreased following
treatment with vaspin, however, not significantly.
 | Figure 3Effects of vaspin on COX-2,
PGE2 and iNOS production. Chondrocytes were treated with
concentrations of vaspin (0, 10, 50, 100, 250 and 500 ng/ml) for 1
h prior to treatment with IL-1β (10 ng/ml) for 24 h. The results
are representative of three experiments. *P<0.05 and
**P<0.01, vaspin-treated chondrocytes compared with
chondrocytes stimulated with IL-1β alone. COX-2, cyclooxygenase-2;
PGE2 prostaglandin E2; iNOS, inducible nitrous oxide
synthase; vaspin, visceral adipose tissue-derived serine protease
inhibitor; IL-1β, interleukin-1β. |
Effects of vaspin on IL-1β-induced MMP-2,
MMP-9, ADAMTS-4, ADAMTS-5 and cathepsin D mRNA in rat
chondrocytes
Following stimulation with IL-1β (10 ng/ml; Fig. 4), rat chondrocytes demonstrated
marked upregulation of MMP-2, MMP-9, ADAMTS-4, ADAMTS-5 and
cathepsin D. Vaspin inhibited MMP-2, MMP-9, ADAMTS-5 and cathepsin
D but not ADAMTS-4 in low concentrations and failed to inhibit the
production of IL-1β-induced catabolic factors in high
concentrations.
 | Figure 4Effects of vaspin on IL-1β-induced
gene expression of MMP-2, MMP-9, ADAMTS-4, ADAMTS-5 and cathepsin D
in chondrocytes. Chondrocytes were pretreated with various
concentrations of vaspin (0, 10, 50, 100, 250 and 500 ng/ml) for 1
h prior to treatment with IL-1β (10 ng/ml) for 24 h. The results
are representative of three experiments. *P<0.05 and
**P<0.01, vaspin-treated chondrocytes compared with
chondrocytes stimulated with IL-1β alone. MMP, matrix
metalloproteinase; ADAMTS, a disintegrin and metalloproteinase with
thrombospondin motif; vaspin, visceral adipose tissue-derived
serine protease inhibitor. |
Effects of vaspin on IL-1β-induced COX-2,
PGE2 and iNOS production in rat chondrocytes
Following stimulation with IL-1β (10 ng/ml; Fig. 3), the production of COX-2,
PGE2 and iNOS from the culture medium of rat
chondrocytes significantly increased. Vaspin inhibited all these
inflammatory cytokines in a dose-dependent manner.
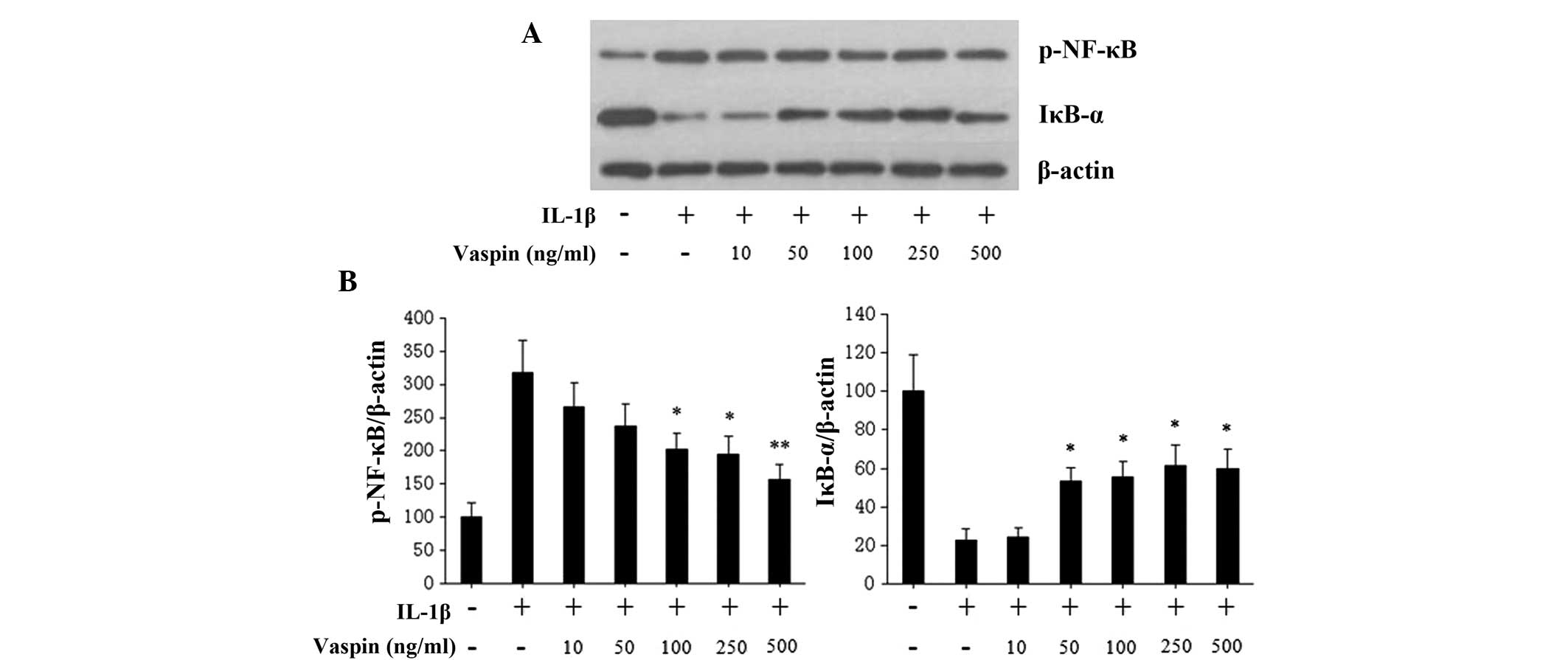
Effects of vaspin on the NF-κB pathway
induced by treatment with IL-1β in rat chondrocytes
In order to investigate the mechanisms of vaspin
mediated-inhibition of IL-1β-induced catabolic and inflammatory
responses, cultured rat chondrocytes were treated with either IL-1β
alone or with various concentrations of vaspin for 24 h. The
phosphorylation of NF-κB and IκB-α were evaluated by western
blotting. Vaspin pretreatment at different concentrations (10, 50,
100, 250 and 500 ng/ml), prior to IL-1β stimulation, inhibited
IL-1β-induced NF-κB phosphorylation in a dose-dependent manner
(Fig. 5). In addition, the
degradation of IκB-α induced by IL-1β was also inhibited by vaspin
(Fig. 5).
Discussion
Vaspin has been suggested to be a ‘good’ adipokine
similar to adiponectin (19).
Administration of vaspin to obese mice improved glucose tolerance
and elevated insulin sensitivity (20). A study by Kadoglou et al
demonstrated a protective effect of vaspin against atherosclerosis
and cardiovascular events (21).
In addition, several studies have demonstrated that vaspin may act
as an anti-apoptotic and anti-inflammatory cytokine in certain cell
types (22–24). Phalitakul et al (22) demonstrated that vaspin inhibited
methylglyoxal-induced endothelial cell apoptosis by preventing
caspase-3 activation via the inhibition of NADPH oxidase-derived
reactive oxygen species (ROS) generation. Another study by the same
group (23) revealed that vaspin
prevented tumor necrosis factor-α (TNF-α)-induced intracellular
adhesion molecule-1 via inhibiting ROS-dependent NF-κB and protein
kinase C-θ (PKCθ) activation in cultured rat vascular smooth muscle
cells (SMCs). This suggested that vaspin had an inhibitory effect
on the inflammatory state of SMCs. Furthermore, vaspin was able to
suppress the expression of pro-inflammatory cytokines, including
leptin, TNF-α and resistin in chronic hepatitis (24). However, another study by Fu et
al (25) failed to demonstrate
that vaspin inhibited TNF-α-induced inflammation in human umbilical
vein endothelial cells. These results strongly suggested that
vaspin may act as an anti-inflammatory adipokine in metabolic
diseases.
As a newly identified adipokine, the role of vaspin
on chondrocytes and on the pathophysiology of OA remains to be
elucidated. To the best of our knowledge, the present study is the
first to investigate the effect of vaspin on chondrocytes. Our
previous studies demonstrated that two important adipokines, leptin
and apelin, had a catabolic effect on the articular cartilage by
stimulating the expression of inflammatory and catabolic factors
(26,27). However, another adipokine
adiponectin was suggested to have a protective role in the
development of OA (28,29). The present study aimed to reveal
the precise role of this novel adipokine on chondrocytes.
The present study demonstrated that vaspin had no
cytotoxicity when the concentration reached 500 ng/ml. Following
treatment with different concentrations of vaspin, no significant
change was observed in the gene expression of catabolic factors
MMP-2, MMP-9, ADAMTS-4, ADAMTS-5 and cathepsin. The present data
indicated that vaspin alone in chondrocytes demonstrated neither
catabolic nor anti-catabolic effects. However, low concentrations
of vaspin significantly inhibited IL-1β-induced MMP-2, MMP-9,
ADAMTS-5 and cathepsin D but not ADAMTS-4 gene expression.
Increased expression of MMP-2 and MMP-9 in pathological chondral,
meniscal and synovial lesions of OA was considered to be important
in OA pathophysiology (30). These
two key MMPs, also termed gelatinase-A and gelatinase-B, could
degrade numerous substrates, including collagens type IV, V, VII,
X, cartilage-specific type XI, aggrecan core protein and
significant collagen type II (31,32).
In addition, another important aggrecanase ADAMTS-5 (aggrecanase-2)
was also suppressed markedly by vaspin in IL-1β-induced
chondrocytes. Notably, ADAMTS-4 and -5 were the most efficient
aggrecanases and the most likely candidates to have a role in OA
(33). In addition, low
concentrations of vaspin also inhibited IL-1β-induced production of
cathepsin D, which contribute to the proteolytic processing of the
core protein of aggrecan in the initial stages of OA (34). These results suggested that low
concentrations of vaspin have an anti-catabolic effect on
chondrocytes in the presence of the proinflammatory agent IL-1β, by
inhibiting MMPs, aggrecanases and cathespin production.
Following treatment with different concentrations of
vaspin, the inflammatory mediators COX-2, PGE2 and iNOS
secreted by chondrocytes decreased but not significantly, which
suggested that vaspin may have an anti-inflammatory effect on the
metabolism of chondrocytes. Furthermore, the production of COX-2,
PGE2 and iNOS induced by IL-1β was significantly
suppressed by vaspin in a dose-dependent manner in the present
study. COX-2 and iNOS are two important inflammatory factors
involved in the pathophysiology of OA, whose expression could be
induced by IL-1β (35). COX-2 and
iNOS led to the release of PGE2 and NO in chondrocytes,
which has been demonstrated to increase MMP production and be
implicated in joint pain in OA (36,37).
PGE2 has also been implicated in the inflammation and
cartilage degradation associated with OA (38,39).
Several studies have reported that vaspin exhibits
anti-inflammatory properties in a variety of cell types (23,24).
The present study demonstrated for the first time, to the best of
our knowledge, that vaspin prevented IL-1β-induced COX-2,
PGE2 and iNOS production in rat normal chondrocytes,
which suggested that vaspin has anti-inflammatory effects in the
presence of the pro-inflammatory agent IL-1β on chondrocytes.
The current study also investigated the molecular
mechanisms by which vaspin inhibited the inflammatory mediators in
response to IL-1β in chondrocytes. The results suggested that
vaspin suppressed IL-1β-induced phosphorylation of NF-κB and
inhibited IL-1β-induced IκB-α degradation in chondrocytes. NF-κB is
a crucial signaling molecule in regulating the expression of
inflammatory and catabolic factors in chondrocytes (40). The NF-κB family exists in
unstimulated cells bound to the IκB family protein, and the
NF-κB/IκB complex is not able translocate to the nucleus. IκB
degradation is tightly regulated by pro-inflammatory cytokines,
including IL-1β, which then phosphorylate NF-κB and transport it
from the cytoplasm to the nucleus, where it binds to the promoter
regions of target genes, including inflammatory mediators and
catabolic factors (41,42). In the current study, IL-1β induced
the phosphorylation of NF-κB in chondrocytes and this was inhibited
by vaspin. These results were partly supported by Phalitakul et
al (23), who revealed that
vaspin significantly inhibited TNF-α-induced phosphorylation of
NF-κB in SMCs. Additionally, Li et al also reported that
vaspin inhibited high glucose-induced SMC proliferation and
chemokinesis by preventing ROS activation and mitogen-activated
protein kinase (MAPK) phosphatidylinositol 3-kinase (PI3K/Akt) and
NF-κB signaling (43). The
previous studies together with the present study suggest that the
anti-catabolic and anti-inflammatory effects of vaspin may partly
be associated with the inhibition of NF-κB activation. However, the
exact mechanism and the effects of vaspin on other signaling
pathways, including PKCθ, MAPK and PI3K/Akt remain to be
elucidated. Further studies are required to elucidate the precise
mechanism underlying the effect of vaspin on the chondrocyte
inflammatory process.
In conclusion, the present study demonstrated that
low concentrations of vaspin inhibited the IL-1β-induced expression
of catabolic factors, including MMP-2, MMP-9, ADAMTS-5 and
cathepsin D. In addition, the present study revealed that the
production of inflammatory mediators COX-2, PGE2 and
iNOS induced by IL-1β could be suppressed by vaspin in a
dose-dependent manner in chondrocytes suggesting that vaspin has
anti-catabolic and anti-inflammatory effects and that it may also
be a potential protective cytokine during the development of OA.
Furthermore, this role may be in part due to the inhibition of
IL-1β-induced activation of the NF-κB signaling pathway.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81201429).
References
|
1
|
Krasnokutsky S, Samuels J and Abramson SB:
Osteoarthritis in 2007. Bull NYU Hosp Jt Dis. 65:222–228. 2007.
|
|
2
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 71:33–42.
2011. View Article : Google Scholar
|
|
3
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yusuf E: Metabolic factors in
osteoarthritis: obese people do not walk on their hands. Arthritis
Res Ther. 14:1232012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cicuttini FM, Baker JR and Spector TD: The
association of obesity with osteoarthritis of the hand and knee in
women: a twin study. J Rheumatol. 23:1221–1226. 1996.PubMed/NCBI
|
|
6
|
Yusuf E: Metabolic factors in
osteoarthritis: obese people do not walk on their hands. Arthritis
Res Ther. 14:1232012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bay-Jensen AC, Slagboom E, Chen-An P, et
al: Role of hormones in cartilage and joint metabolism:
understanding an unhealthy metabolic phenotype in osteoarthritis.
Menopause. 20:578–586. 2013.PubMed/NCBI
|
|
8
|
Hida K, Wada J, Eguchi J, et al: Visceral
adipose tissue-derived serine protease inhibitor: a unique
insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci
USA. 102:10610–10615. 2005. View Article : Google Scholar
|
|
9
|
Fain JN, Buehrer B, Bahouth SW, Tichansky
DS and Madan AK: Comparison of messenger RNA distribution for 60
proteins in fat cells vs the nonfat cells of human omental adipose
tissue. Metabolism. 57:1005–1015. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klöting N, Berndt J, Kralisch S, et al:
Vaspin gene expression in human adipose tissue: association with
obesity and type 2 diabetes. Biochem Biophys Res Commun.
339:430–436. 2006.
|
|
11
|
Meyer-Hoffert U: Reddish, scaly, and
itchy: how proteases and their inhibitors contribute to
inflammatory skin diseases. Arch Immunol Ther Exp (Warsz).
57:345–354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klöting N, Kovacs P, Kern M, et al:
Central vaspin administration acutely reduces food intake and has
sustained blood glucose-lowering effects. Diabetologia.
54:1819–1823. 2011.
|
|
13
|
Körner A, Neef M, Friebe D, et al: Vaspin
is related to gender, puberty and deteriorating insulin sensitivity
in children. Int J Obes (Lond). 35:578–586. 2011.PubMed/NCBI
|
|
14
|
Youn BS, Klöting N, Kratzsch J, et al:
Serum vaspin concentrations in human obesity and type 2 diabetes.
Diabetes. 57:372–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ozgen M, Koca SS, Dagli N, Balin M,
Ustundag B and Isik A: Serum adiponectin and vaspin levels in
rheumatoid arthritis. Arch Med Res. 41:457–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klaasen R, Herenius MM, Wijbrandts CA, et
al: Treatment-specific changes in circulating adipocytokines: a
comparison between tumour necrosis factor blockade and
glucocorticoid treatment for rheumatoid arthritis. Ann Rheum Dis.
71:1510–1516. 2012. View Article : Google Scholar
|
|
17
|
Senolt L, Polanská M, Filková M, et al:
Vaspin and omentin: new adipokines differentially regulated at the
site of inflammation in rheumatoid arthritis. Ann Rheum Dis.
69:1410–1411. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamio N, Kawato T, Tanabe N, et al: Vaspin
attenuates RANKL-induced osteoclast formation in RAW264.7 cells.
Connect Tissue Res. 54:147–152. 2013. View Article : Google Scholar
|
|
19
|
Choi SH, Hong ES and Lim S: Clinical
implications of adipocytokines and newly emerging metabolic factors
with relation to insulin resistance and cardiovascular health.
Front Endocrinol (Lausanne). 4:972013.PubMed/NCBI
|
|
20
|
Wada J: Vaspin: a novel serpin with
insulin-sensitizing effects. Expert Opin Investig Drugs.
17:327–333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kadoglou NP, Gkontopoulos A, Kapelouzou A,
et al: Serum levels of vaspin and visfatin in patients with
coronary artery disease-Kozani study. Clin Chim Acta. 412:48–52.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Phalitakul S, Okada M, Hara Y and Yamawaki
H: Vaspin prevents methylglyoxal-induced apoptosis in human
vascular endothelial cells by inhibiting reactive oxygen species
generation. Acta Physiol (Oxf). 209:212–219. 2013.
|
|
23
|
Phalitakul S, Okada M, Hara Y and Yamawaki
H: Vaspin prevents TNF-α-induced intracellular adhesion molecule-1
via inhibiting reactive oxygen species-dependent NF-κB and PKCθ
activation in cultured rat vascular smooth muscle cells. Pharmacol
Res. 64:493–500. 2011.PubMed/NCBI
|
|
24
|
Kukla M, Mazur W, Bułdak RJ and
Zwirska-Korczala K: Potential role of leptin, adiponectin and three
novel adipokines - visfatin, chemerin and vaspin - in chronic
hepatitis. Mol Med. 17:1397–1410. 2011. View Article : Google Scholar
|
|
25
|
Fu BD, Yamawaki H, Okada M and Hara Y:
Vaspin can not inhibit TNF-α-induced inflammation of human
umbilical vein endothelial cells. J Vet Med Sci. 71:1201–1207.
2009.
|
|
26
|
Bao JP, Chen WP, Feng J, Hu PF, Shi ZL and
Wu LD: Leptin plays a catabolic role on articular cartilage. Mol
Biol Rep. 37:3265–3272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu PF, Chen WP, Tang JL, Bao JP and Wu LD:
Apelin plays a catabolic role on articular cartilage: in vivo and
in vitro studies. Int J Mol Med. 26:357–363. 2010.PubMed/NCBI
|
|
28
|
Uchida K, Urabe K, Naruse K, Ogawa Z,
Mabuchi K and Itoman M: Hyperlipidemia and hyperinsulinemia in the
spontaneous osteoarthritis mouse model, STR/Ort. Exp Anim.
58:181–187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen TH, Chen L, Hsieh MS, Chang CP, Chou
DT and Tsai SH: Evidence for a protective role for adiponectin in
osteoarthritis. Biochim Biophys Acta. 1762:711–718. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsieh YS, Yang SF, Chu SC, et al:
Expression changes of gelatinases in human osteoarthritic knees and
arthroscopic debridement. Arthroscopy. 20:482–488. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Birkedal-Hansen H, Moore WG, Bodden MK, et
al: Matrix metalloproteinases: a review. Crit Rev Oral Biol Med.
4:197–250. 1993.PubMed/NCBI
|
|
32
|
Kozaci LD, Buttle DJ and Hollander AP:
Degradation of type II collagen, but not proteoglycan, correlates
with matrix metalloproteinase activity in cartilage explant
cultures. Arthritis Rheum. 40:164–174. 1997. View Article : Google Scholar
|
|
33
|
Fushimi K, Troeberg L, Nakamura H, Lim NH
and Nagase H: Functional differences of the catalytic and
non-catalytic domains in human ADAMTS-4 and ADAMTS-5 in
aggrecanolytic activity. J Biol Chem. 283:6706–6716. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Handley CJ, Mok MT, Ilic MZ, Adcocks C,
Buttle DJ and Robinson HC: Cathepsin D cleaves aggrecan at unique
sites within the interglobular domain and chondroitin sulfate
attachment regions that are also cleaved when cartilage is
maintained at acid pH. Matrix Biol. 20:543–553. 2001. View Article : Google Scholar
|
|
35
|
Chabane N, Zayed N, Afif H, et al: Histone
deacetylase inhibitors suppress interleukin-1β-induced nitric oxide
and prostaglandin E2 production in human chondrocytes.
Osteoarthritis Cartilage. 16:1267–1274. 2008.
|
|
36
|
Salvemini D, Misko TP, Masferrer JL,
Seibert K, Currie MG and Needleman P: Nitric oxide activates
cyclooxygenase enzymes. Proc Natl Acad Sci USA. 90:7240–7244. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sasaki K, Hattori T, Fujisawa T, Takahashi
K, Inoue H and Takigawa M: Nitric xide mediates
interleukin-1-induced gene expression of matrix metalloproteinases
and basic fibroblast growth factor in cultured rabbit articular
chondrocytes. J Biochem. 123:431–439. 1998. View Article : Google Scholar
|
|
38
|
Amin AR, Attur M, Patel RN, et al:
Superinduction of cyclooxygenase-2 activity in human
osteoarthritis-affected cartilage. Influence of nitric oxide. J
Clin Invest. 99:1231–1237. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang P, Zhu F and Konstantopoulos K:
Prostaglandin E2 induces interleukin-6 expression in human
chondrocytes via cAMP/protein kinase A- and phosphatidylinositol
3-kinase-dependent NF-κB activation. Am J Physiol Cell Physio.
298:C1445–C1456. 2010.PubMed/NCBI
|
|
40
|
Vincenti MP and Brinckerhoff CE:
Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in
arthritis: integration of complex signaling pathways for the
recruitment of gene-specific transcription factors. Arthritis Res.
4:157–164. 2002. View
Article : Google Scholar
|
|
41
|
Tian B and Brasier AR: Identification of a
nuclear factor κB-dependent gene network. Recent Prog Horm Res.
58:95–130. 2003.
|
|
42
|
Hayden MS and Ghosh S: Signaling to NF-κB.
Genes Dev. 18:2195–2224. 2004.
|
|
43
|
Li H, Peng W, Zhuang J, et al: Vaspin
attenuates high glucose-induced vascular smooth muscle cells
proliferation and chemokinesis by inhibiting the MAPK, PI3K/Akt,
and NF-κB signaling pathways. Atherosclerosis. 228:61–68.
2013.PubMed/NCBI
|