Introduction
Hepatocellular carcinoma (HCC) is the fifth most
prevalent malignant tumor in men worldwide and the second most
frequent cause of cancer-related mortality (1). Although surgical resection and liver
transplantation are the main modalities of curative treatment for
HCC, most patients are at the late stages of the disease, when
curative treatment is not feasible and the outcome is likely to be
poor (2). Certain agents such as
sorafenib and cisplatin have been approved for chemotherapeutic
treatment of HCC, but their use is associated with limited
survival, due to the resistance of HCC cells to these agents and
their multiple toxic effects (3,4).
Therefore, it is important to identify new compounds with improved
effectiveness and lower toxicity, in order to provide a wide range
of clinical options for HCC patients.
Immunosuppression can be clearly detected in both
cancer patients and tumor-bearing animals (5). During hepatocyte carcinogenesis,
numerous immune tolerogenic factors induced by virus infection or
chronic inflammatory response may accumulate, which facilitates an
aggressive and effective counterattack against the anti-HCC immune
responses of the host (6). For
example, the frequencies of myeloid dendritic cells in HCC patients
are reduced compared with their normal counterparts. This event
impairs production of IL-12 and limits the allostimulatory
activities of dendritic cells (7).
Decreased numbers of circulating natural killer (NK) cells and
their cytotoxic activities against HCC cells have also been
described in HCC patients (8,9).
Notably, some agents targeting specific molecules have exhibited
off-target effects on immune cells, including T cells, NK cells,
monocytes and dendritic cells, in addition to their direct effects
on tumor cells. Previous studies have demonstrated that sorafenib
inhibits the function of NK cells, rendering the host more
susceptible to tumor growth and metastasis (10,11).
Overall, these studies suggest that stimulating anti-HCC immunity
may be a promising alternative strategy for the treatment of
HCC.
Kanglaite (KLT) is an oily substance extracted from
the plant species Coix lacryma-jobi (family, Poaceae). There
is some evidence that medical use of coix seed and its extracts is
beneficial in the treatment of cancer metastasis, hypertension,
arthritis, asthma and immunological disorders (12). KLT was clinically used in China and
significantly improved the lifespan and quality of life of patients
when combined with chemotherapy, radiotherapy or surgery (13). A number of studies showed that KLT
principally blocks the G2þM phase of the cell cycle, thereby
reducing the mitotic division rates and inhibiting the
proliferation of tumor cells, while it can also activate
pro-apoptotic factors, leading to apoptosis (12). A recent study in mice with Lewis
lung carcinoma further demonstrated that KLT exhibits antitumor
activity in vivo, via its immunomodulatory effects (14). The nuclear factor κB (NF-κB) is an
important regulator of transcription that controls the expression
of various genes involved in the immune system, cancer and
inflammatory functions (15,16).
KLT increases the interleukin (IL)-2 level in the supernatant of
isolated splenocytes, and decreases the NF-κB level in the nucleus
of lung tumor cells (14).
In this study, we firstly evaluated the effects of
KLT treatment on the immune response of HCC patients. In addition,
we used C57BL/6 mice transplanted with HepG2 cells to investigate
the effects of KLT treatment on HCC. Specifically, we assessed the
tumor growth inhibition rate, liver indexes, and the number of
immune system cells in tumor-bearing mice, and explored the
molecular mechanisms underlying the effects of KLT.
Materials and methods
Human blood samples
KLT was provided by Zhejiang Kanglaite
Pharmaceutical Co., Ltd. (Hangzhou, China). Human blood samples of
10 patients with HCC (aged between 35 and 67, six male and four
female) treated with KLT were obtained from The First Affiliated
Hospital of Nanjing Medical University. The study protocol was
approved by the Institutional Ethics Committee of The First
Affiliated Hospital of the Nanjing Medical University.
Flow cytometric analysis (FACS)
The percentages of NK cells, CD4+ and
CD8+ T cells were measured by flow cytometry (BD
FACSCalibur™ platform; BD Biosciences, Franklin Lakes, NJ, USA) as
previously described (17).
Briefly, purified CD4+ and CD8+ T cells were
fixed with cold 70% ethanol (stored at −20°C) for at least 2 h at
4°C. The cells were resuspended and washed twice with
phosphate-buffered saline (PBS) containing 20 mM EDTA.
Intercellular RNA was removed by incubating the samples with RNase
A (Sigma-Aldrich, St. Louis, MO, USA; 1 mg/ml) at 37°C for at least
1 h. Cells were then stained with propidium iodide (30 μg/ml) and
cell cycle distribution was analyzed by FACS. The percentages of
the cells were estimated relative to the percentage of the subG1
DNA content.
Enzyme-linked immunosorbent assay
(ELISA)
The production of the cytokines IL-2 and interferon
(IFN)-γ in the blood serum was measured by a standard sandwich
ELISA assay. The assay was performed according to the instructions
of the corresponding mouse IL-2 and IFN-γ kits, purchased from
R&D Systems, Inc. (Minneapolis, MN, USA). Absorbance of the
samples was measured on an ELISA microplate reader (Multiskan Ex;
Thermo Labsystems Oy, Helsinki, Finland).
Nude mouse xenograft assay
Male nude BALB/c mice (6 weeks-old) were obtained
from the Shanghai Experimental Animal Center (Chinese Academy of
Sciences, Shanghai, China), and housed under pathogen-free
conditions. All the animal-handling procedures were conducted in
accordance with experimental animal guidelines established by the
Experimental Animal Management Committee of Jiangsu Province. HepG2
cells were obtained from the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). HepG2 cells were cultured in
DMEM containing 10% fetal bovine serum, 100 units/ml penicillin,
100 μg/ml streptomycin and 5.5 mM D-glucose at 37°C with 5%
CO2. To induce tumor growth, 5×106 HepG2
cells (200 μl) were subcutaneously injected into the right flank of
the mice. When the tumor areas had reached ~30 mm2 (at 4
days after inoculation), the mice were randomly assigned to four
groups, with ten mice in each group. The positive control group
received 5 mg/kg cisplatin (Sigma-Aldrich). The vehicle control
group received 0.9% normal saline. The KLT groups received 6.25
(KLT low) and 12.5 (KLT high) mg/kg KLT by intraperitoneal
injection for 12 days. Tumor growth was assessed every 3 days by
measuring the tumor area, calculated as V = lw, where l is the
length and w is the width. Then, all mice were sacrificed, and the
tumors were immediately weighed.
Determination of blood indexes of liver
function
Blood samples were collected in heparinized tubes
from the mice prior to sacrifice, under diethyl ether anesthesia.
Serum was prepared by centrifugation of the blood samples at 4,000
× g for 15 min. Serum aspartate aminotransferase (AST) and alanine
aminotransferase (ALT) levels were determined using an automatic
biochemical analyzer (Sysmex Corp., Tokyo, Japan) according to the
manufacturer’s instructions.
Isolation of subsets of immune cells
Splenic cells were isolated from harvested mouse
spleen tissue samples in a sterile environment. CD4+ T,
CD8+ T and NK cells were purified from single-cell
suspensions of mouse splenocytes using a magnetic-activated cell
sorting (MACS) system, the rat anti-mouse CD4, CD8, CD49b, and the
goat anti-rat IgG microbeads, purchased from BD Biosciences.
Cytotoxic activity determination
The cytotoxic activity of mouse NK and
CD8+ T cells against HepG2 cells was measured based on
the activity of lactate dehydrogenase, which is released from
damaged cells. For this purpose, we used the CytoTox 96®
Non-Radioactive Cytotoxicity Assay kit (Promega BioSciences, inc.,
San Luis Obispo, CA, USA) according to the manufacturer’s
instructions.
Quantitative reverse transcription
(qRT)-PCR
Total RNA was isolated using the TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) following the manufacturer’s
instructions. Complementary (c)DNA was synthesized from 2 mg RNA
using the M-MLV reverse transcriptase (Promega, Madison, WI, USA)
and an oligo(dT) primer, following the manufacturer’s instructions.
The levels of the mRNA encoding B-cell lymphoma (Bcl)-2, Bcl-xL and
IL-2 were determined by qPCR on these cDNAs, using the TransStart
SYBR green qPCR kit (Beijing TransGen Biotech Co., Ltd., Beijing,
China) on a MyiQ thermocycler (Bio-Rad, Hercules, CA, USA). Each
PCR reaction mixture (20 ml) contained 10 ml of 2 SYBR-Green Master
mix and 7 ml of the forward and reverse primers (5 nM). The
reactions were incubated for 10 min at 95°C, followed by 45 cycles
of incubation at 95°C for 15 sec, 60°C for 15 sec and 72°C for 15
sec. The expression levels of the target genes were normalized to
that of the glyceraldehyde-3-phosphate dehydrogenase gene
(GAPDH). The primer pair sets used were the following
(5′↔3′): Bcl-2 forward, ATG CCT TTG TGG AAC TAT ATG GC, and
reverse, GGT ATG CAC CCA GAG TGA TGC; Bcl-xL forward, GCT GGG ACA
CTT TTG TGG AT, and reverse, CTA GGC CCA ACC CTG TGA TA; IL-2
forward, TGA GCA GGA TGG AGA ATT ACA GG, and reverse, ATG TGT TGT
CAG AGC CCT TTA G; GAPDH forward, GCA GTG GCA AAG TGG AGA TT, and
reverse, GGA GAC AAC CTG GTC CTC AG.
Chromatin immunoprecipitation (ChIP)
assay
ChIP assay was performed using polyclonal rabbit
anti-p65 antibody purchased from Santa Cruz Biotechnology Inc.,
(Santa Cruz, CA, USA). ChIP assay was performed using a ChIP assay
kit according to the manufacturer’s instructions (Millipore,
Darmstadt, Germany).
Statistical analysis
Continuous data are expressed as the mean ± standard
error. Comparisons were made using unpaired, two-tailed Student’s
t-test or one-way analysis of variance. All statistical analyses
were conducted using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Effects of KLT on host lymphocyte
subgroups in the blood and on serum cytokine profiles
Following treatment of HCC patients with KLT, we
examined the number of T and NK cells in the peripheral blood at
week 2, and months 1 and 2. As shown in Fig. 1A–C, KLT treatment increased the
number of NK cells (CD3+ and NK1.1+), as well
as that of T cells (CD4+ and CD8+) in a
time-dependent manner. The IFN-γ and IL-2 proteins function as
stimulators of immune responses. We evaluated the effect of KLT
treatment on the serum profile of these two cytokines. The
concentration of serum IFN-γ and IL-2 was also time-dependently
increased upon KLT treatment in HCC patients (Fig. 1D).
Effects of KLT on growth of the
inoculated tumor
To confirm the in vivo anti-HCC effects of
KLT, we tested tumor-suppressing activity of KLT at different
doses, using cisplatin, a conventional chemotherapeutic drug, as a
positive control. The results showed that transplantation of HepG2
cells significantly reduced the body weight of mice (Fig. 2A) while the area of tumors
gradually increased (Fig. 2B). The
area of tumors was significantly decreased in the KLT high group
(treated with 12.5 mg/kg KLT) compared with the group treated with
saline (Fig. 2B), without
significantly affecting the body weight (Fig. 2A). Treatment with both doses of KLT
also significantly reduced the tumor weight (P<0.01) compared
with saline treatment (Table I).
By contrast, although cisplatin administration was associated with
a higher tumor growth inhibitory rate compared with KLT, the
average tumor weight in the cisplatin group was significantly
decreased (P<0.05) compared with saline treatment.
 | Table IInhibitory effects of KLT on growth
of HCC. |
Table I
Inhibitory effects of KLT on growth
of HCC.
| Group | N | Dose (mg/kg) | Tumor weight
(g) | Tumor growth
inhibition rate % |
|---|
| Non-tumor | 10/10 | - | - | - |
| Saline | 10/10 | - | 1.71±0.37 | - |
| Cisplatin | 10/7 | 5 | 0.79±0.41b | 53.8 |
| KLT low | 10/10 | 6.25 | 1.19±0.50a | 30.4 |
| KLT high | 10/10 | 12.5 | 1.15±0.31a | 49.7 |
Effects of KLT on liver function
A vital aim of cancer chemotherapy is to repress the
growth of malignant cells without affecting their normal
counterparts. To evaluate the toxicological effects of KLT
injection on normal hepatocytes, we measured the serum levels of
hepatic function markers i.e., the activities of ALT and AST.
Cisplatin treatment significantly increased the levels of serum ALT
and AST (P<0.01) compared with the non-tumor group, but these
two indexes were not clearly affected by KLT injection (Table II).
 | Table IIEffects of KLT on serum indexes of
liver function. |
Table II
Effects of KLT on serum indexes of
liver function.
| Group | Dose (mg/kg) | ALT (U/l) | AST (U/l) |
|---|
| Non-tumor | - | 127.8±12.3 | 478.4±27.9 |
| Saline | - | 124.6±10.3 | 473.2±25.5 |
| Cisplatin | 5 | 312.4±23.4a | 796.4±23.1a |
| KLT low | 6.25 | 119.0±11.5 | 483.8±21.6 |
| KLT high | 12.5 | 115.7±13.7 | 468.5±26.7 |
Effects of KLT on lymphocyte homeostasis
and cytokine profiles in mice spleens
As shown in Fig.
3A, transplantation of HepG2 cells reduced the concentrations
of IFN-γ and IL-2 in the serum (P<0.01 and P<0.05, compared
with non-tumor mice), indicating that systemic immunosuppression
occurs upon transplantation. Following KLT injection, the serum
levels of IFN-γ and IL-2 in tumor-bearing mice were significantly
higher than those in the saline-treated tumor-bearing mice.
However, cisplatin treatment failed to reverse the reduced IFN-γ
and IL-2 levels in tumor-bearing mice (Fig. 3A). These results suggested that KLT
treatment has the potential to alleviate tumor
transplantation-induced cytokine dysregulation.
FACS assessment of the percentages of
CD4+ T, CD8+ T and NK cells in single-cell
suspensions of mice spleens
The results of FACS analysis showed that the
percentages of CD8+ T and NK cells remained almost
unchanged in all experimental groups (Fig. 3B). By contrast, as shown in
Fig. 3B, the percentage of
CD4+ T cells in the spleen was significantly reduced in
the tumor-bearing mice treated with saline as compared with their
non-tumor counterparts (P<0.01). This effect was reversed upon
KLT injection (P<0.01 and P<0.001 for the two KLT-treated
groups as compared with the saline group, respectively). Cisplatin
treatment did not reverse the tumor transplantation-induced
reduction in the CD4+ T cell percentage (Fig. 3B).
KLT enhances the cytotoxic activity of NK
and CD8+ T cells against HepG2 cells
CD4+ T cells can mediate antitumor
responses by stimulating the cytotoxic activity of NK and
CD8+ T cells. We therefore determined the effects of KLT
treatment on the cytotoxic activities of NK and CD8+ T
cells against HepG2 cells. As shown in Fig. 4, HepG2 tumor cell transplantation
markedly reduced the cytotoxic activities of NK and CD8+
T cells as compared with the non-tumor control group (P<0.01 and
P<0.05, respectively). Cisplatin treatment also significantly
(P<0.01) reduced the cytotoxic activities of NK cells and
CD8+ T cells (Fig. 4).
By contrast, KLT treatment increased the cytotoxic activities of
these cells (p<0.05 in the low-dose group and P<0.001 in the
high-dose group) compared with the saline-treated group.
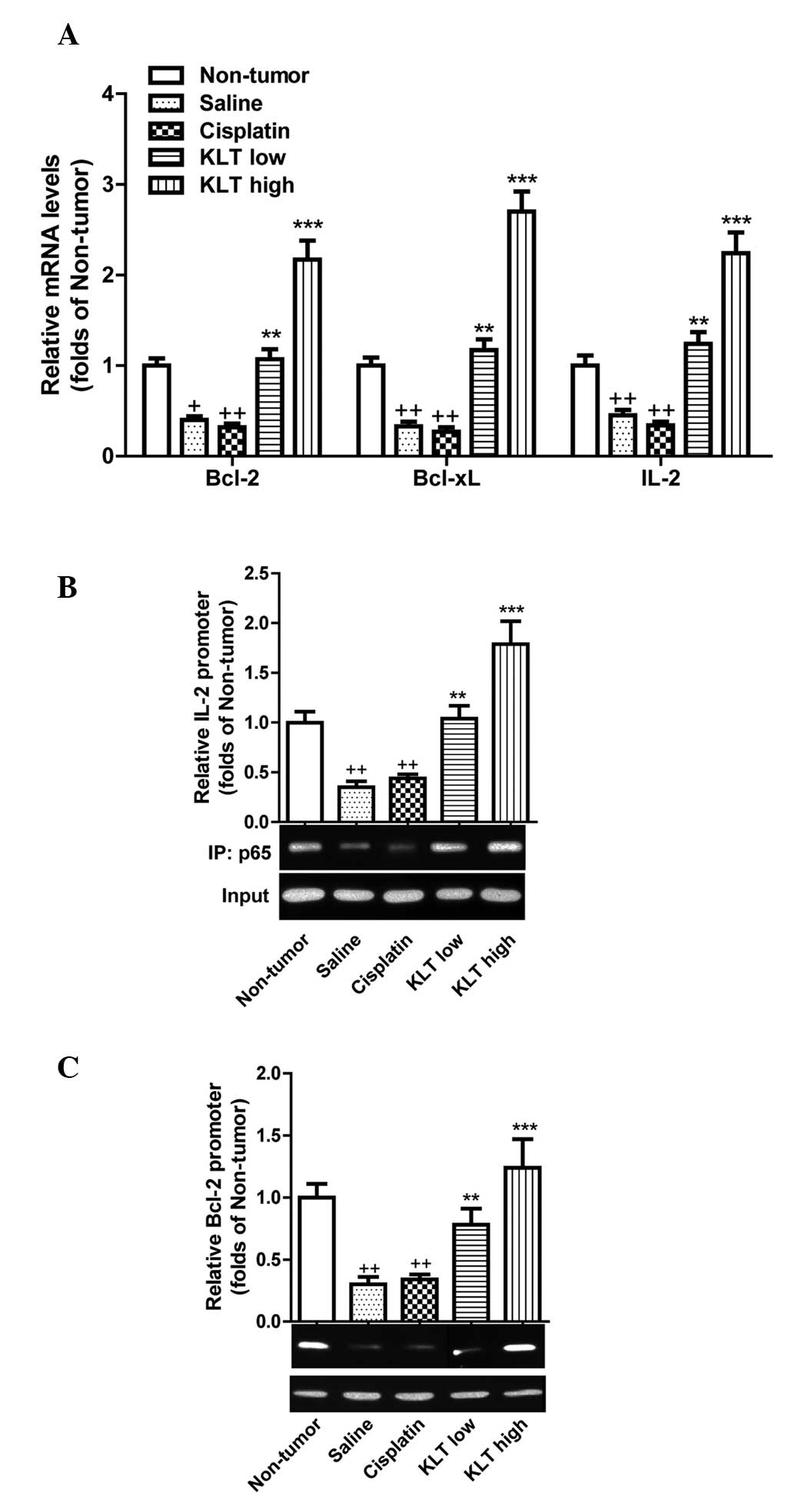
KLT activates NF-κB signaling in
CD4+ T cells
NF-κB is a pivotal transcription factor that both
promotes cell survival and stimulates immune response. Therefore,
we tested whether KLT activates NF-κB signaling in the
CD4+ T cells of tumor-bearing mice. We examined the
effects of KLT injection on the transcriptional level of a subset
of NF-κB-regulated genes, Bcl-2, Bcl-xL and
IL-2 In CD4+ T cells. HepG2 tumor cell
transplantation markedly reduced the transcription of these
NF-κB-responsive genes (P<0.05 for Bcl-2 and P<0.01
for Bcl-xL and IL-2 compared with the non-tumor
group). The levels of Bcl-2, Bcl-xL and IL-2
were significantly increased by KLT treatment (P<0.01 in the
low-dose group and P<0.001 in the high-dose group) compared with
saline treatment (Fig. 5A). By
contrast, cisplatin treatment did not reverse the reduction in
Bcl-2, Bcl-xL and IL-2 levels induced by the
tumor transplantation (Fig. 5A).
Additionally, the ChIP assay demonstrated that following ALT
treatment, more NF-κB p65 proteins associated with the promoter
regions of IL-2 and Bcl-2 encoding genes in CD4+ T cells
(Fig. 5B and C). Collectively,
these results suggested that KLT activates part of NF-κB signaling
in CD4+ T cells of the host.
Discussion
Herbal products are used in traditional medicine for
the prevention or treatment of cancer in various societies
worldwide. Since one of the most important adverse effects of
chemotherapeutic drugs is the reduced immunity of the patients, it
is highly desirable to treat patients with drugs that not only
exert antitumor effects, but also enhance their immunity (18,19).
A previous study demonstrated the antitumor activity and
immunomodulatory effects of KLT in vivo, using mice with
Lewis lung carcinoma (14). Our
study also indicated that KLT treatment may enhance the immune
system of patients with HCC. The present study also demonstrated
that KLT significantly inhibits tumor growth in mice transplanted
with HepG2 cells. KLT increased the percentage of CD4+ T
cells, the serum concentrations of IFN-γ and IL-2, and the
cytotoxic activity of NK and CD8+ T cells against HepG2
cells. Furthermore, KLT increased the expression of the
NF-κB-responsive genes Bcl-2, Bcl-xL and IL-2
in CD4+ T cells. Cisplatin did not enhance the immune
system (IFN-γ and IL-2 levels, spleen lymphocyte subgroup
percentages) and did not rescue the expression of Bcl-2,
Bcl-xL and IL-2 in the mouse model of HCC. Liver
function indexes, i.e., the activities of ALT and AST, were the
only features that were significantly improved by cisplatin in
contrast to KLT treatment. Overall, although KLT showed a reduced
antitumor activity (tumor growth inhibition rate, tumor weight)
compared with cisplatin, it had an apparent advantage in enhancing
the patients’ immunity in our experiments.
Natural regulatory T cells are present in high
frequencies in tumor-infiltrating lymphocytes and in draining lymph
nodes, and are thought to facilitate tumor development.
CD4+ T cells are a key subset of immune cells mediating
antitumor immune defense (20,21).
It has been demonstrated that cancer cells can induce
CD4+ T cell apoptosis, which contributes to immune
evasion during tumor progression (22,23).
Consistent with these reports, we found that following
transplantation of the hepatic HepG2 tumor cells, the percentage of
CD4+ T cells, but not CD8+ T or NK cells, was
decreased in the spleen. KLT administration alleviated the tumor
cell transplantation-induced reduction of CD4+ T cell
percentage, which suggests that KLT has the potential to ameliorate
tumor-induced immunodeficiency. T helper 1 (Th1) cells, a subtype
of CD4+ T cells, were shown to secrete the IFN-γ and
IL-2 cytokines (22), which
improve the cytotoxicity of CD8+ T and NK cells and
promote antitumor immune responses (24,25).
Similarly in this study, we showed that following transplantation
of HepG2 tumor cells, the serum levels of the Th1 cytokines IFN-γ
and IL-2 were decreased. The cytotoxic activities of
CD8+ T and NK cells against HepG2 cells were also
impaired. KLT treatment significantly increased the cytotoxic
activity of both NK cells and CD8+ T cells against HepG2
cells. These events rescue the systemic immunosuppression induced
by tumor transplantation and stimulate the host anticancer immune
response, which may explain the in vivo KLT anticancer
activity.
NF-κB is crucial for the regulation of numerous
biological processes, including cell apoptosis and immune response.
NF-κB signaling inhibits cell apoptosis by inducing the
transcription of various anti-apoptotic genes, such as Bcl-2
and Bcl-xL (26). The
expression of Bcl-2 was demonstrated to play a pivotal role in the
control of CD4+ T cell survival (27). Moreover, the induction, by NF-κB,
of the transcription of the IFN-γ and IL-2 genes,
which modulate the immune response, is well established (28). In line with the inhibitory effect
of NF-κB previously reported, we found that KLT increases the serum
levels of IFN-γ and IL-2. We also assessed the effects of KLT on
the transcription of the NF-κB-responsive genes Bcl-2,
Bcl-xL and IL-2 by qRT-PCR. Consistently, our ChIP
assay revealed that KLT administration increased the interaction
between the NF-κB p65 subunit and the promoter regions of Bcl-2 and
IL-2 encoding genes in CD4+ T cells. The results
demonstrated that KLT treatment upregulates these genes in
CD4+ T cells of tumor-bearing mice.
Notably, KLT treatment did not affect the percentage
of CD8+ T cells or the transcription of NF-κB-regulated
genes in these cells (data not shown). This observation suggests
that KLT selectively activates NF-κB signaling in CD4+ T
cells. The unique gene expression pattern observed in
CD4+ T cells may contribute to the cell-type specificity
of KLT, although the underlying molecular mechanisms deserve
further investigation. Collectively, our study provided a molecular
basis for the application of coix seed extract in HCC treatment. In
addition, our data support a future clinical use of KLT as an
adjuvant reagent that stimulates anticancer immune responses in
patients with HCC.
Acknowledgements
This study was supported by the research grant
P200901 from the Medical Science and Technology Development Fund of
the Health Bureau of Jiangsu Province.
References
|
1
|
Nordenstedt H, White DL and El-Serag HB:
The changing pattern of epidemiology in hepatocellular carcinoma.
Dig Liver Dis. 42(Suppl 3): S206–S214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clavien PA, Petrowsky H, DeOliveira ML and
Graf R: Strategies for safer liver surgery and partial liver
transplantation. N Engl J Med. 356:1545–1559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brunocilla PR, Brunello F, Carucci P, et
al: Sorafenib in hepatocellular carcinoma: prospective study on
adverse events, quality of life, and related feasibility under
daily conditions. Med Oncol. 30:3452013. View Article : Google Scholar
|
|
4
|
Pawlik TM, Reyes DK, Cosgrove D, Kamel IR,
Bhagat N and Geschwind JF: Phase II trial of sorafenib combined
with concurrent transarterial chemoembolization with drug-eluting
beads for hepatocellular carcinoma. J Clin Oncol. 29:3960–3967.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vasievich EA and Huang L: The suppressive
tumor microenvironment: a challenge in cancer immunotherapy. Mol
Pharm. 8:635–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pardee AD and Butterfield LH:
Immunotherapy of hepatocellular carcinoma: unique challenges and
clinical opportunities. Oncoimmunology. 1:48–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ormandy LA, Farber A, Cantz T, et al:
Direct ex vivo analysis of dendritic cells in patients with
hepatocellular carcinoma. World J Gastroenterol. 12:3275–3282.
2006.
|
|
8
|
Hoechst B, Voigtlaender T, Ormandy L, et
al: Myeloid derived suppressor cells inhibit natural killer cells
in patients with hepatocellular carcinoma via the NKp30 receptor.
Hepatology. 50:799–807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teitz-Tennenbaum S, Li Q, Okuyama R, et
al: Mechanisms involved in radiation enhancement of intratumoral
dendritic cell therapy. J Immunother. 31:345–358. 2008. View Article : Google Scholar
|
|
10
|
Krusch M, Salih J, Schlicke M, et al: The
kinase inhibitors sunitinib and sorafenib differentially affect NK
cell antitumor reactivity in vitro. J Immunol. 183:8286–8294. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang QB, Sun HC, Zhang KZ, et al:
Suppression of natural killer cells by sorafenib contributes to
prometastatic effects in hepatocellular carcinoma. PLoS One.
8:e559452013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu Y, Li CS and Dong Q: Chinese herb
related molecules of cancer-cell-apoptosis: a minireview of
progress between Kanglaite injection and related genes. J Exp Clin
Cancer Res. 27:312008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhan YP, Huang XE, Cao J, et al: Clinical
safety and efficacy of Kanglaite® (Coix Seed Oil) injection
combined with chemotherapy in treating patients with gastric
cancer. Asian Pac J Cancer Prev. 13:5319–5321. 2012.
|
|
14
|
Pan P, Wu Y, Guo ZY, Wang R, Wang YJ and
Yuan YF: Antitumor activity and immunomodulatory effects of the
intraperitoneal administration of Kanglaite in vivo in Lewis lung
carcinoma. J Ethnopharmacol. 143:680–685. 2012. View Article : Google Scholar
|
|
15
|
Wullaert A, Bonnet MC and Pasparakis M:
NF-κB in the regulation of epithelial homeostasis and inflammation.
Cell Res. 21:146–158. 2011.
|
|
16
|
Porta C, Larghi P, Rimoldi M, et al:
Cellular and molecular pathways linking inflammation and cancer.
Immunobiology. 214:761–777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Yu G, Wang D, Hu Y and Lei W:
ERK1/2 activation plays important roles in the opposite effects of
Trichostatin A in non-cancer and cancer cells. Toxicon. 57:932–937.
2011. View Article : Google Scholar
|
|
18
|
Lee S, Ra J, Song JY, et al: Extracts from
Citrus unshiu promote immune-mediated inhibition of tumor
growth in a murine renal cell carcinoma model. J Ethnopharmacol.
133:973–979. 2011.
|
|
19
|
Wang J, Tong X, Li P, Cao H and Su W:
Immuno-enhancement effects of shenqi fuzheng injection on
cyclophosphamide-induced immunosuppression in Balb/c mice. J
Ethnopharmacol. 139:788–795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang H, Hao S, Li F, Ye Z, Yang J and
Xiang J: CD4+ Th1 cells promote CD8+ Tc1 cell survival, memory
response, tumor localization and therapy by targeted delivery of
interleukin 2 via acquired pMHC I complexes. Immunology.
120:148–159. 2007. View Article : Google Scholar
|
|
21
|
Rakhra K, Bachireddy P, Zabuawala T, et
al: CD4+ T cells contribute to the remodeling of the
microenvironment required for sustained tumor regression upon
oncogene inactivation. Cancer Cell. 18:485–498. 2010.
|
|
22
|
Chaput N, Darrasse-Jeze G, Bergot AS, et
al: Regulatory T cells prevent CD8 T cell maturation by inhibiting
CD4 Th cells at tumor sites. J Immunol. 179:4969–4978. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Biswas K, Richmond A, Rayman P, et al: GM2
expression in renal cell carcinoma: potential role in tumor-induced
T-cell dysfunction. Cancer Res. 66:6816–6825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui G and Florholmen J: Polarization of
cytokine profile from Th1 into Th2 along colorectal
adenoma-carcinoma sequence: implications for the biotherapeutic
target? Inflamm Allergy Drug Targets. 7:94–97. 2008. View Article : Google Scholar
|
|
25
|
Grimm M, Gasser M, Bueter M, et al:
Evaluation of immunological escape mechanisms in a mouse model of
colorectal liver metastases. BMC Cancer. 10:822010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baldwin AS: Regulation of cell death and
autophagy by IKK and NF-κB: critical mechanisms in immune function
and cancer. Immunol Rev. 246:327–345. 2012.
|
|
27
|
Shi Y, Feng Y, Kang J, et al: Critical
regulation of CD4+ T cell survival and autoimmunity by
β-arrestin 1. Nat Immunol. 8:817–824. 2007.
|
|
28
|
Weil R and Israel A: T-cell-receptor- and
B-cell-receptor-mediated activation of NF-kappaB in lymphocytes.
Curr Opin Immunol. 16:374–381. 2004. View Article : Google Scholar : PubMed/NCBI
|