Introduction
The gut endocrine cells constitute the largest
endocrine organ in the body and consist of at least 15 types of
endocrine cell (1–3). In addition to the endocrine mechanism
of action, the biologically active substances secreted by these
cells act locally on nearby structures through paracrine signaling
(4,5). The endocrine cells are scattered
among the epithelial cells that line the gut lumen. They have
specialized microvilli that project into the lumen and function as
sensors for the gut contents (mostly for nutrients), and they
respond to luminal stimuli by releasing their hormones into the
lamina propria (6–18).
The stomach comprises of four types of endocrine
cells, which produce serotonin, somatostatin, ghrelin and gastrin
(5). While serotonin and
somatostatin-secreting cells are present in the corpus and the
antrum of the stomach, ghrelin-secreting cells are localized to the
corpus and gastrin-secreting G cells are located in the antrum
(19).
Chromogranin A (CgA) is a common marker for gut
endocrine cells (20–22). In a previous study, the density of
CgA-secreting cells was observed to be abnormal in the stomach of
patients with irritable bowel syndrome (IBS) (23). Further studies have demonstrated
that the densities of all the gastric endocrine cells are affected
in patients with IBS (24).
Dietary management guidance given to patients with
IBS has been indicated to reduce symptoms and improve the quality
of life (25,26). The endocrine cells of the gut are
stimulated by the contents of the gut lumen, particularly nutrients
(27,28). Therefore, the present study was
undertaken to investigate whether the positive effects of dietary
management guidance observed in patients with IBS are associated
with changes in the densities of endocrine cells in the stomachs of
these patients.
Materials and methods
Patients and controls
Patients that were referred to our clinic at Stord
Helse-Fonna Hospital (Stord, Norway) and fulfilled the Rome-III
criteria (29) for IBS diagnosis
were considered for inclusion in the current study. Females and
males aged between 18 and 70 years were selected. Pregnant or
lactating females, and patients with organic gastrointestinal or
other systemic diseases, a history of drug abuse, or serious
psychiatric disturbances were excluded. Patients that had undergone
previous abdominal surgery, with the exception of appendectomy,
caesarean and hysterectomy, were also excluded.
The control group included healthy subjects that
underwent gastroscopy due to the following reasons:
Gastrointestinal bleeding, where the source of bleeding was
identified as hemorrhoids (n=3) or angiodysplasia (n=1); and health
concerns as a result of diagnosis of a family member with
gastrointestinal cancer (n=10). The control group consisted of nine
females and five males with a mean age of 54 years (range, 26–70
years).
The present study was performed in accordance with
the year 2000 edition of the Declaration of Helsinki and approved
by the Regional Committee for Medical Research Ethics of West
Norway. All patients submitted oral and written consent.
Study design
A total of 46 patients participated in the present
study, including 35 females and 11 males with a mean age of 35
years (range, 18–69 years). The patients were subjected to physical
examinations and blood tests in order to exclude inflammation,
infection or other organic diseases. Additionally, these patients
underwent colonoscopy with segmental biopsy samples to exclude
microscopic colitis. Each patient was scheduled for three sessions
of individual dietary management guidance with an experienced
nurse, lasting ~45 min each. These sessions were conducted with
intervals of at least 2 weeks. The patients underwent gastroscopy
prior to the first session and 3–9 months (median 4 months)
following the third session of dietary management guidance.
Individual dietary management
guidance
In the sessions, information was provided orally
using charts, in addition to written illustrated information. The
initial session included general information regarding IBS and the
importance of regular and healthy eating habits. The diets that
worsen IBS symptoms, such as insoluble dietary fibers and the
poorly absorbed highly fermentable oligosaccharides, disaccharides,
monosaccharaides and polyols (FODMAPs) were explained. The patients
were encouraged to consume dairy products daily and were informed
that milk and dairy products do not provoke IBS symptoms. The
patients were required to write a diary, in which they recorded
their daily food and drink intake, the frequency and degree of
abdominal pain, abdominal distension, stool frequency and
consistency, for 2 weeks. In addition the patients were asked to
test a protein-, fat- or carbohydrate-rich/poor diet. In the second
session, the information given in the first session was briefly
repeated. The nurse and the patient discussed the
symptom-triggering items based on the information noted in the
patient’s diary. The patients were then advised to alter the
proportions of protein, fat and carbohydrate, avoid items rich in
FODMAPs and insoluble fibers, and consume vegetables and fruits
containing less FODMAPs and insoluble fibers. During the last
session the patients informed the nurse of their experience of
dietary management. The nurse, along with the patient, designed a
suitable diet, which was strictly followed by the patient until the
end-point of the study.
Gastroscopy, tissue sampling,
histopathology and immunohistochemistry
Following an overnight fast, the patients and
control subjects underwent standard gastroscopy. Four biopsy
samples were taken from the corpus (major curvature) and another
four biopsy samples from the antrum of the stomach. Furthermore,
four biopsy samples were taken from the duodenum in order to
exclude celiac disease.
The biopsy samples were fixed in 4% buffered
paraformaldehyde overnight, embedded in paraffin wax, and were then
cut into 5-μm sections. Biopsy samples from the stomach and
duodenum underwent histopathological examinations. Biopsy samples
from the corpus and antrum were stained with hematoxylin and eosin
and immunostained with the avidin-biotin complex method using a
Vectastain ABC kit (Vector laboratories, Burlingame, CA, USA) and
the chromogen 3,3′-diaminobenzidine peroxidase substrate (DAB) kit
(Vector Laboratories) as described previously (30). Briefly, the sections were incubated
for 2 h at room temperature with a monoclonal mouse anti-N-terminal
of purified CgA primary antibody (code no. M869; Dako, Glostrup,
Denmark) diluted 1:1,000. Following incubation, the sections were
washed in phosphate-buffered saline (PBS; pH 7.4) and incubated for
30 min at room temperature with biotinylated swine anti-mouse IgG
diluted to 1:200 (Dako). The slides were washed with PBS, and
incubated for 30 min with avidin-biotin-peroxidase complex diluted
1:100, and then submerged in 3,3′-diaminobenzidine, followed by
counterstaining with hematoxylin.
Computerized image analysis
The density of chromogranin A in the corpus and
antrum of patients with IBS and controls was measured using Olympus
Cell^D software, Olympus, (Tokyo, Japan). The number of
chromogranin A positive cells and the area of the epithelial cells
were measured in 10 randomly selected fields, magnification, ×40.
At this magnification each field represents a tissue area of 0.14
mm2. The chromogranin A cell density was expressed as
the number of cells/mm2 of the epithelium. All
quantification was conducted by the same scientist (Dr Tarek
Mazzawi), who was blinded to the identity of the sections.
Statistical analysis
The paired t-test was used to compare the results of
patients prior to and following dietary guidance with results in
the control subjects. The data are presented as the mean ± standard
error. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients and controls
A total of 25 patients withdrew their consent at
various stages of the study. The majority of these were due to a
lack of motivation when their symptoms improved following dietary
guidance and/or unwillingness to undergo a second gastroscopy. Two
patients were excluded due to non-compliance. Five patients were
excluded as they were diagnosed with celiac disease (n=2), lupus
(n=1), became pregnant (n=1) or moved abroad (n=1) during the
course of the study. Thus, fourteen patients completed the study.
These patients consisted of nine females and five males with a mean
age of 34 years (range, 20–45 years). Biopsies from one female
patient were obtained only from the antrum.
Gastroscopy, histopathology and
immunohistochemistry
The macroscopic appearance of the esophagus, stomach
and duodenum was normal in patients and controls. Histopathological
examination displayed normal histology of the stomach and duodenum
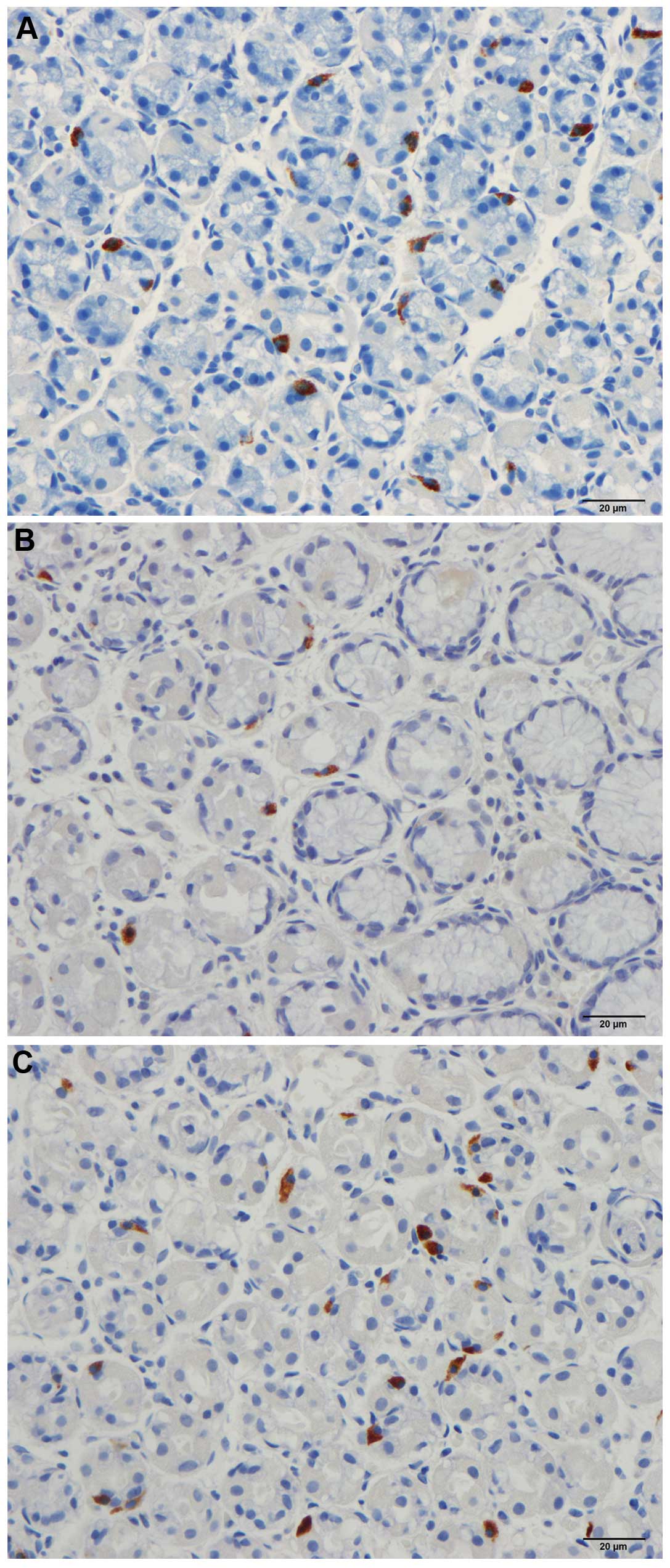
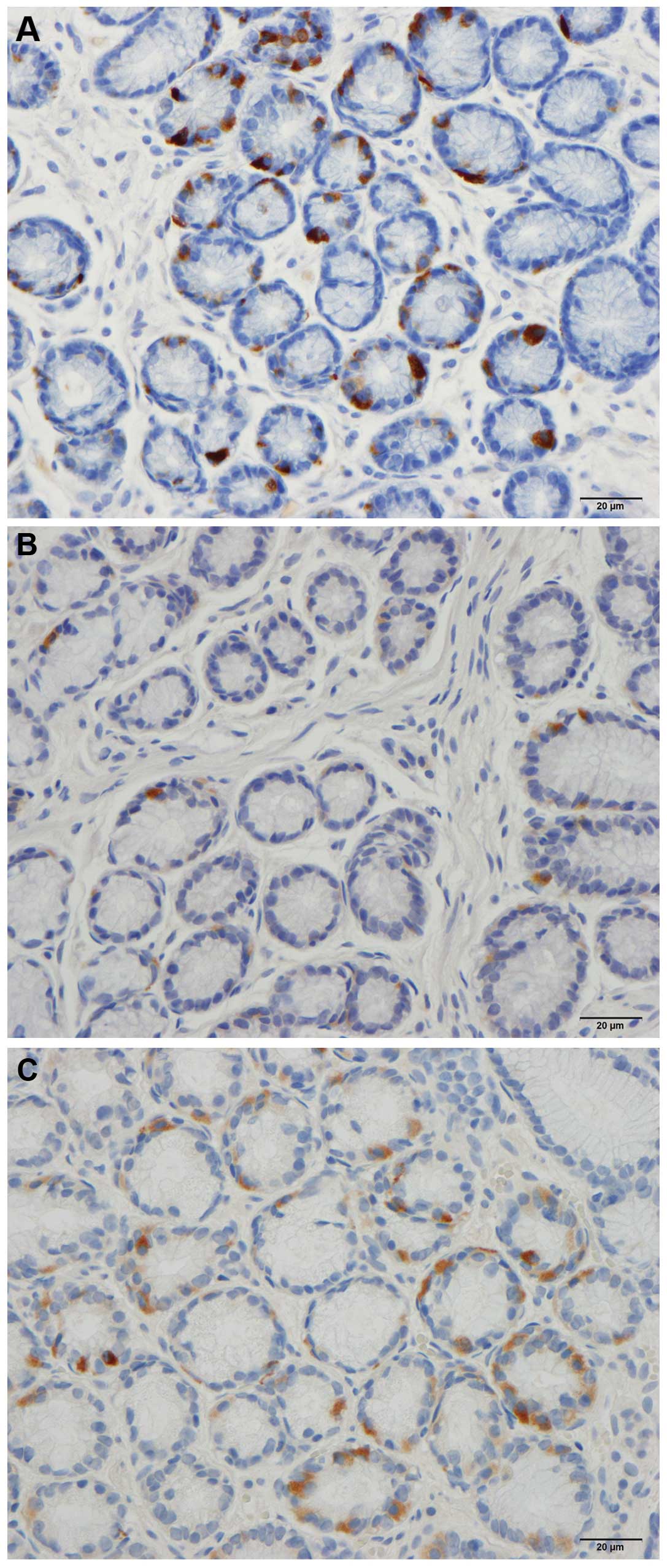
in patients and controls. CgA immunoreactive cells were identified
in the mucosa of the stomach in the two groups. These cells were
either basket- or flask-shaped, and a number of them had a long
basal cytoplasmic process.
Computerized image analyses
Corpus
The mean density of CgA-secreting cells in controls
was 147.9 cells/mm2 (95% CI: 113.8–182). The densities
of the CgA-secreting cells in patients with IBS prior to and
following dietary guidance were 62.6±9.3 and 102.3±14.3
cells/mm2, respectively (Figs. 1 and 2). The paired t-test indicated a
significant increase in the densities of CgA-secreting cells in IBS
patients following dietary guidance (P=0.0064).
Antrum
The mean density of the CgA-secreting cells in the
antrum was 87.7 cells/mm2 (95% CI: 43.8–131.7). The
densities of the CgA-secreting cells in patients with IBS prior to
and following dietary guidance was 28.5±6.5 and 46.5±11.1
cells/mm2, respectively (Figs. 3 and 4). No significant difference in the
densities of CgA-secreting cells was detected in IBS patients prior
to and following dietary guidance (P=0.2).
Discussion
Patients with IBS are recognized to have a high
dropout rate in clinical studies (26,31).
The design of the present study involved invasive gastroscopy
examinations, and adhering to a strict diet for a minimum of 3
months. This design may have contributed to the high rate of
dropout in addition to the non-compliance experienced in the
current study. Additionally, exclusions due to unexpected events
occurring during the study, such as pregnancy, moving abroad or
other diagnoses contributed to the low number of patients that
fulfilled the criteria required to complete the study. Regardless
of the small sample size, the present study indicated a clear
effect on gastric endocrine cell density following changes in
diet.
The density of CgA-secreting cells in the stomach
(in the corpus and antrum) was abnormal in IBS patients in the
present study prior to receiving dietary guidance, similar to
results reported in a previous study (23). Following dietary guidance and a
change of diet, the density of CgA-secreting cells significantly
increased in the corpus towards the values observed in healthy
controls. With regards to the antrum, these changes were also
observed but did not reach a significant level. This increase
demonstrates that changes in food intake via dietary guidance
alters the density of the total gastric endocrine cells towards a
normal level.
In a previous study on the same cohort of patients
investigated in the present study (25), a similar dietary program resulted
in a reduction of the symptoms and an improvement in the quality of
life of the patients. The observation in the current study, that
dietary changes can promote a density of endocrine cells that is
more similar to that of healthy patients, suggests that these
changes may be one of the causes for the amelioration of symptoms
and consequently the improvement in the quality of life in the
patients with IBS of the previous study. The findings of the
present study support the hypothesis that gut endocrine cells are
important in the pathogenesis of IBS (32,33).
Gut endocrine cells have microvilli extending to the
lumen that act as sensors for the contents of the gut and respond
to the luminal stimuli (32). It
has been reported that each intestinal crypt contains 4–6
pluripotent cells that differentiate through a series of cellular
precursors into all epithelial cell types, including the endocrine
cells (34–43). This differentiation is rapid and
takes 2–4 days (44,45).
In conclusion, the current study demonstrated that
the change in diet with the consequent change in the contents of
the gut lumen may be the cause of alterations to the
differentiation of endocrine cells observed in patients with IBS,
resulting in an increase in the density of endocrine cells.
Acknowledgements
The authors would like to thank Professor Hans Olav
Fadnes, Head of the Department of Medicine, Stord Helse-Fonna
Hospital, Norway, for his support in addition to reading and
commenting on the manuscript. The current study was supported by a
grant from Helse-Fonna.
References
|
1
|
Moran GW, Leslie FC, Levison SE,
Worthington J and McLaughlin JT: Enteroendocrine cells: neglected
players in gastrointestinal disorders? Therap Adv Gastroenterol.
1:51–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moran-Ramos S, Tovar AR and Torres N:
Diet: friend or foe of enteroendocrine cells - how it interacts
with enteroendocrine cells. Adv Nutr. 3:8–20. 2012. View Article : Google Scholar
|
|
3
|
Buffa R, Capella C, Fontana P, Usellini L
and Solcia E: Types of endocrine cells in the human colon and
rectum. Cell Tissue Res. 192:227–240. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Salhy M: Irritable bowel syndrome:
diagnosis and pathogenesis. World J Gastroenterol. 18:5151–5163.
2012.
|
|
5
|
El-Salhy M, Seim I, Chopin L, Gundersen D,
Hatlebakk JG and Hausken T: Irritable bowel syndrome: the role of
gut neuroendocrine peptides. Front Biosci (Elite Ed). 4:2783–2800.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sandstrom O and El-Salhy M: Ageing and
endocrine cells of human duodenum. Mech Ageing Dev. 108:39–48.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El-Salhy M: Ghrelin in gastrointestinal
diseases and disorders: a possible role in the pathophysiology and
clinical implications (review). Int J Mol Med. 24:727–732. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tolhurst G, Reimann F and Gribble FM:
Intestinal sensing of nutrients. Handb Exp Pharmacol. 309–335.
2012. View Article : Google Scholar
|
|
9
|
Lee J, Cummings BP, Martin E, et al:
Glucose sensing by gut endocrine cells and activation of the vagal
afferent pathway is impaired in a rodent model of type 2 diabetes
mellitus. Am J Physiol Regul Integr Comp Physiol. 302:R657–666.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parker HE, Reimann F and Gribble FM:
Molecular mechanisms underlying nutrient-stimulated incretin
secretion. Expert Rev Mol Med. 12:e12010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raybould HE: Nutrient sensing in the
gastrointestinal tract: possible role for nutrient transporters. J
Physiol Biochem. 64:349–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
San Gabriel A, Nakamura E, Uneyama H and
Torii K: Taste, visceral information and exocrine reflexes with
glutamate through umami receptors. J Med Invest. 56(Suppl):
209–217. 2009.PubMed/NCBI
|
|
13
|
Rudholm T, Wallin B, Theodorsson E,
Naslund E and Hellström PM: Release of regulatory gut peptides
somatostatin, neurotensin and vasoactive intestinal peptide by acid
and hyperosmolal solutions in the intestine in conscious rats.
Regul Pept. 152:8–12. 2009. View Article : Google Scholar
|
|
14
|
Sternini C, Anselmi L and Rozengurt E:
Enteroendocrine cells: a site of ‘taste’ in gastrointestinal
chemosensing. Curr Opin Endocrinol Diabetes Obes. 15:73–78.
2008.
|
|
15
|
Sternini C: Taste receptors in the
gastrointestinal tract. IV. Functional implications of bitter taste
receptors in gastrointestinal chemosensing. Am J Physiol
Gastrointest Liver Physiol. 292:G457–461. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buchan AM: Nutrient tasting and signaling
mechanisms in the gut III. Endocrine cell recognition of luminal
nutrients. Am J Physiol. 277:G1103–G1107. 1999.PubMed/NCBI
|
|
17
|
Montero-Hadjadje M, Elias S, Chevalier L,
et al: Chromogranin A promotes peptide hormone sorting to mobile
granules in constitutively and regulated secreting cells: role of
conserved N- and C-terminal peptides. J Biol Chem. 284:12420–12431.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shooshtarizadeh P, Zhang D, Chich JF, et
al: The antimicrobial peptides derived from
chromogranin/secretogranin family, new actors of innate immunity.
Regul Pept. 165:102–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Irritable bowel syndrome: diagnosis, pathogenesis and
treatment options. Nova Science Publishers Inc.; New York: 2012
|
|
20
|
Taupenot L, Harper KL and O’Connor DT: The
chromogranin-secretogranin family. N Engl J Med. 348:1134–1149.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wiedenmann B and Huttner WB: Synaptophysin
and chromogranins/secretogranins - widespread constituents of
distinct types of neuroendocrine vesicles and new tools in tumor
diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 58:95–121.
1989. View Article : Google Scholar
|
|
22
|
Deftos LJ: Chromogranin A: its role in
endocrine function and as an endocrine and neuroendocrine tumor
marker. Endocr Rev. 12:181–187. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El-Salhy M, Gilja OH and Hausken T:
Chromogranin A cells in the stomach of patients with sporadic
irritable bowel syndrome. Mol Med Rep. (In press).
|
|
24
|
El-Salhy M, Hatlebakk JG, Gundersen D and
Hausken T: Endocrine cells in the gastric oxyntic mucosa of
patients with irritable bowel syndrome. World J Gastroenterol.
6:176–185. 2014.
|
|
25
|
Mazzawi T, Hausken T, Gundersen D and
El-Salhy M: Effects of dietary guidance on the symptoms, quality of
life and habitual dietary intake of patients with irritable bowel
syndrome. Mol Med Rep. 8:845–852. 2013.PubMed/NCBI
|
|
26
|
Ostgaard H, Hausken T, Gundersen D and
El-Salhy M: Diet and effects of diet management on quality of life
and symptoms in patients with irritable bowel syndrome. Mol Med
Rep. 5:1382–1390. 2012.PubMed/NCBI
|
|
27
|
Spiller RC: Inflammation as a basis for
functional GI disorders. Best Pract Res Clin Gastroenterol.
18:641–661. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El-Salhy M, Gilja OH, Gundersen D,
Hatlebakk JG and Hausken T: Interaction between ingested nutrients
and gut endocrine cells in patients with irritable bowel syndrome.
(In press).
|
|
29
|
Spiller R, Aziz Q, Creed F, et al:
Guidelines on the irritable bowel syndrome: mechanisms and
practical management. Gut. 56:1770–1798. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
El-Salhy M, Mazzawi T, Gundersen D and
Hausken T: Chromogranin A cell density in the rectum of patients
with irritable bowel syndrome. Mol Med Rep. 6:1223–1225.
2012.PubMed/NCBI
|
|
31
|
Enck P, Klosterhalfen S and Kruis W:
Determination of placebo effect in irritable bowel syndrome. Dtsch
Med Wochenschr. 130:1934–1937. 2005.(In German).
|
|
32
|
El-Salhy M, Gundersen D, Gilja OH,
Hatlebakk JG and Hausken T: Is irritable bowel syndrome an organic
disorder? World J Gastroenterol. 20:384–400. 2014. View Article : Google Scholar
|
|
33
|
El-Salhy M, Hatlebakk JG, Gilja OH and
Hausken T: Irritable bowel syndrome: recent developments in
diagnosis, pathophysiology, and treatment. Expert Rev Gastroenterol
Hepatol. 8:435–443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barker N and Clevers H: Tracking down the
stem cells of the intestine: strategies to identify adult stem
cells. Gastroenterology. 133:1755–1760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barker N, van de Wetering M and Clevers H:
The intestinal stem cell. Genes Dev. 22:1856–1864. 2008. View Article : Google Scholar
|
|
36
|
Barker N, van Es JH, Kuipers J, et al:
Identification of stem cells in small intestine and colon by marker
gene Lgr5. Nature. 449:1003–1007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Korinek V, Barker N, Moerer P, et al:
Depletion of epithelial stem-cell compartments in the small
intestine of mice lacking Tcf-4. Nat Genet. 19:379–383. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng H and Leblond CP: Origin,
differentiation and renewal of the four main epithelial cell types
in the mouse small intestine. V. Unitarian Theory of the origin of
the four epithelial cell types. Am J Anat. 141:537–561. 1974.
View Article : Google Scholar
|
|
39
|
Fontaine J, Le Lièvre C and Le Douarin NM:
What is the developmental fate of the neural crest cells which
migrate into the pancreas in the avian embryo? Gen Comp Endocrinol.
33:394–404. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Le Douarin NM and Teillet MA: The
migration of neural crest cells to the wall of the digestive tract
in avian embryo. J Embryol Exp Morphol. 30:31–48. 1973.PubMed/NCBI
|
|
41
|
Rawdon BB and Andrew A: Origin and
differentiation of gut endocrine cells. Histol Histopathol.
8:567–580. 1993.PubMed/NCBI
|
|
42
|
Hoffman J, Kuhnert F, Davis CR and Kuo CJ:
Wnts as essential growth factors for the adult small intestine and
colon. Cell Cycle. 3:554–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
May CL and Kaestner KH: Gut endocrine cell
development. Mol Cell Endocrinol. 323:70–75. 2010. View Article : Google Scholar
|
|
44
|
Inokuchi H, Fujimoto S and Kawai K:
Cellular kinetics of gastrointestinal mucosa, with special
reference to gut endocrine cells. Arch Histol Jpn. 46:137–157.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Höcker M and Wiedenmann B: Molecular
mechanisms of enteroendocrine differentiation. Ann NY Acad Sci.
859:160–174. 1998.
|