Introduction
Although intrinsic progenitor cell proliferation and
differentiation occur in response to cerebral ischemia in order to
provide functional benefits, this is not sufficient to prevent
irreversible brain damage. The transplantation of neural stem cells
(NSCs) into animal models of cerebral ischemia is able to produce
functional recovery; however, these cells are only available in the
fetal forebrain or the subventricular zone of the adult brain,
which raises ethical concerns and therefore, there is an inadequate
supply for therapeutic use (1).
Bone marrow stromal cells (BMSCs) have attracted interest as a
promising alternative for clinical application as they are easy to
isolate from bone marrow and are readily expanded in number in
vitro (2). They may also be
used for autotransplantation without ethical problems or inducing
any immune response.
A number of studies have shown that BMSC
transplantation is able to improve functional recovery following
cerebral ischemia (3–10). However, the mechanisms by which
BMSCs promote functional recovery remain to be elucidated. It has
been proposed that BMSCs secrete several trophic factors or
stimulate the host brain to express trophic factors (11–13).
Trophic factors that have been reported to be secreted by
mesenchymal stem cells include vascular endothelial growth factor
(VEGF), basic fibroblast growth factor (bFGF), insulin-like growth
factor 1 (IGF-1), neurotrophin-3 (NT-3), and brain-derived
neurotrophic factor (BDNF) (4).
VEGF promotes angiogenesis as well as neurogenesis (4). A stroke is able to induce
angiogenesis, which is associated with improved neurologic
recovery; however, under normal circumstances, angiogenesis induced
by stroke is insufficient for functional recovery. Since BMSCs
secrete VEGF, the secretion of this trophic factor by BMSCs or the
stimulation of host cells by BMSCs to secrete VEGF may have an
important role in the improvement of cerebral ischemia observed
following BMSC transplantation. The aim of the present study was to
investigate the changes that occur over time in a rat model of
middle cerebral artery occlusion (MCAO) when human BMSCs (hBMSCs)
are transplanted into the brain following reperfusion with a
particular focus on the expression of VEGF. hBMSCs were selected
for use in the present study as they are relevant to clinical
practice in humans and as transplantation of hBMSCs into rodents
has been demonstrated to be histocompatible (14,15).
Materials and methods
Experimental procedures were approved by the Animal
Experimentation Ethics Committee of the Medical College of Jilin
University (Changchun, China).
Culturing of hBMSCs
hBMSCs were isolated from healthy adult human ilium
bone marrow and incubated in low-glucose Dulbecco’s modified
Eagle’s medium (L-DMEM; Gibco, Life Technologies, Carlsbad, CA,
USA). Cells subcultured four times were characterized by flow
cytometry for CD34, CD45, CD29, and CD44 (R&D Systems, Inc.,
Minneapolis, MN, USA), and then co-cultured (4×105
cells/μl) with 5-bromo-2-deoxyuridine (BrdU; Sigma-Aldrich, St.
Louis, MO, USA) for 48 h prior to transplantation. The total number
of transplanted cells was 2×106 cells in 5 μl medium in
accordance with previous studies (14–17).
Middle cerebral artery occlusion rat
model
Male Wistar rats (Charles River Laboratories,
Beijing, China) weighing 250–280 g were kept at room temperature
(24°C) with a 12-h light-dark cycle and were given free access to
food and water. The MCAO procedure was a modification of the
methods described by Koizumi et al (18) and Longa et al (19). Briefly, under deep anesthesia
induced by 10% chloral hydrate (0.3 ml/100 g; Sigma-Aldrich), a
midline cervical incision was made and the left carotid bifurcation
was identified and exposed. A probe made of nylon thread with a
diameter of 0.225 mm was inserted into the ligated external carotid
artery and advanced into the internal carotid artery to a position
18±0.5 mm from the bifurcation. Following 90 min of ischemia, the
suture was slightly withdrawn for reperfusion for 1 h. During the
surgery, the rectal temperature was maintained between 37.5 and
38°C using a feedback heating pad through all the surgical and
postoperative procedures until the rats regained consciousness. The
sham rats were subjected to exposure of the internal carotid artery
(ICA) and external carotid artery, and the control rats were not
treated.
Transplantation
One hour following MCAO, the rats were anesthetized
by intraperitoneal injection of 10% chloral hydrate (0.3 ml/100 g)
and placed onto a stereotaxic frame. A suspension of
2×106 cells in 5 μl L-DMEM (Gibco, Life Technologies)
was stereotaxically injected into the ischemic boundary zone from 3
mm anterior to the bregma and 1 mm lateral to the midline.
Injections were given 4 mm below the cortical surface in each case.
Five groups of animals were prepared: i), Normal control group,
which did not receive any surgery (n=10); ii) sham-operated group,
in which a midline cervical incision was made and the right carotid
bifurcation was identified and exposed, but no probe was inserted
(n=10); iii) operated group, which received no transplantation
following ischemia/reperfusion (n=10); iv) DMEM-injected group,
which received only serum-free DMEM after ischemia/reperfusion
(n=10); v) hBMSC-transplantated group, which underwent
transplantation of BMSCs following ischemia/reperfusion (n=10).
Behavioral assessment
The severity of neurological damage was evaluated
using Chen’s Neurological Severity Score (NSS) system (Table I) (5). Neurologic function was graded on a
scale of 0–18 (0, normal score; 18, maximal deficit score). The NSS
is a composite of motoric, sensory, reflex, and balance
assessments. In the severity score of injury, one point was awarded
for the inability to perform a task or for the lack of an assessed
reflex; thus, the more severe the injury, the higher the score. The
recovery of neurologic function was observed and the scores of all
rats were recorded on days 1, 3, 7, and 28 following
transplantation.
 | Table INeurological Severity Score. |
Table I
Neurological Severity Score.
| Test | Points |
|---|
| Motor tests |
| Raising rat by the
tail | 3 |
| 1 Flexion of
forelimb | |
| 1 Flexion of
hindlimb | |
| 1 Head moved
>10° to vertical axis within 30 sec | |
| Placing rat on the
floor (normal=0; maximum=3) | 3 |
| 0 Normal walk | |
| 1 Inability to walk
straight | |
| 2 Circling towards
the paretic side | |
| 3 Falling down to
the paretic side | |
| Sensory tests | 2 |
| 1 Placing test
(visual and tactile test) | |
| 2 Proprioceptive
test (deep sensation, pushing the paw against the table edge to
stimulate limb muscles) | |
| Beam balance tests
(normal=0; maximum=6) | 6 |
| 0 Balances with
steady posture | |
| 1 Grasps side of
beam | |
| 2 Hugs the beam and
one limb falls down from the beam | |
| 3 Hugs the beam and
two limbs fall down from the beam, or spins on beam (>60
sec) | |
| 4 Attempts to
balance on the beam but falls off (>40 sec) | |
| 5 Attempts to
balance on the beam but falls off (>20 sec) | |
| 6 Falls off: No
attempt to balance or hang on to the beam (<20 sec) | |
| Reflexes absent and
abnormal movements | 4 |
| 1 Pinna reflex
(shakes head when touching the auditory meatus) | |
| 1 Corneal reflex
(blinks with eye when lightly touching the cornea with cotton) | |
| 1 Startle reflex
(motor response to a brief noise from snapping a clipboard
paper) | |
| 1 Seizures,
myoclonus, myodystony | |
| Maximum points | 18 |
Histological analysis
Rats from each group were sacrificed following each
behavioral assessment session to examine the existence and
distribution of human BMSCs and the expression of VEGF in the brain
tissue. The rats were sacrificed by administration of an overdose
of 10% chloral hydrate (Sigma-Aldrich) and then transcardially
perfused with 0.9% saline followed by 4% paraformaldehyde fixative
solution (Sigma-Aldrich). The brain was embedded in paraffin and
cut into coronal blocks of 5 μm using a brain slicer (Zivic Brain
Matrix; Zivic Instruments, Pittsburgh, PA, USA). For
immunofluorescence staining, sections were incubated with a mouse
monoclonal primary antibody to BrdU (1:100; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) or a rabbit polyclonal
primary antibody to VEGF (1:100; Santa Cruz Biotechnology, Inc.) at
4°C overnight. Following three washes with phosphate-buffered
saline (PBS), sections were incubated with an Alexa Fluor
488-labeled goat anti-mouse immunoglobuiln G (IgG) secondary
antibody (1:400; Zhongshan Belling Biotechnology, Guangdong, China)
or an Alexa Fluor 555-labeled goat anti-rabbit IgG secondary
antibody (1:400; Zhongshan Belling Biotechnology) for 1 h at room
temperature in the dark. Following washing with PBS, Hoechst 33342
(R&D Systems, Inc.) was used for nuclear staining and the
sections were mounted with glycerol and examined using fluorescence
microscopy. Five sequential slides from each block were examined
(magnification, ×200) and the number of VEGF-positive cells in each
slide was counted. The mean number of positive cells from the five
slides was recorded. This was considered to be representative of
the total number of VEGF-positive cells in the whole brain.
BrdU-positive cells were also observed using fluorescence
microscopy.
Western blot analysis
Rats from each group were sacrificed and perfused as
described above. Brains were dissected and frozen at −70°C. Western
blot analysis was performed using a primary polyclonal rabbit
anti-rat VEGF antibody (1:100; Santa Cruz Biotechnology) and a
secondary goat anti-rabbit IgG antibody (1:400 dilution; Zhongshan
Belling Biotechnology). The primary antibody used binds to the
amino acid splice variants 189, 165 and 121 of VEGF.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). One-way analysis of variance (ANOVA) with the Bonferroni
post-hoc test was performed to compare the difference between
groups at each time point. Statistical assessments were all
two-sided, and the statistical significance level was set as
P<0.05. Statistical analyses were performed using SPSS 15.0
statistics software (SPSS, Inc., Chicago, IL, USA).
Results
Culturing of hBMSCs
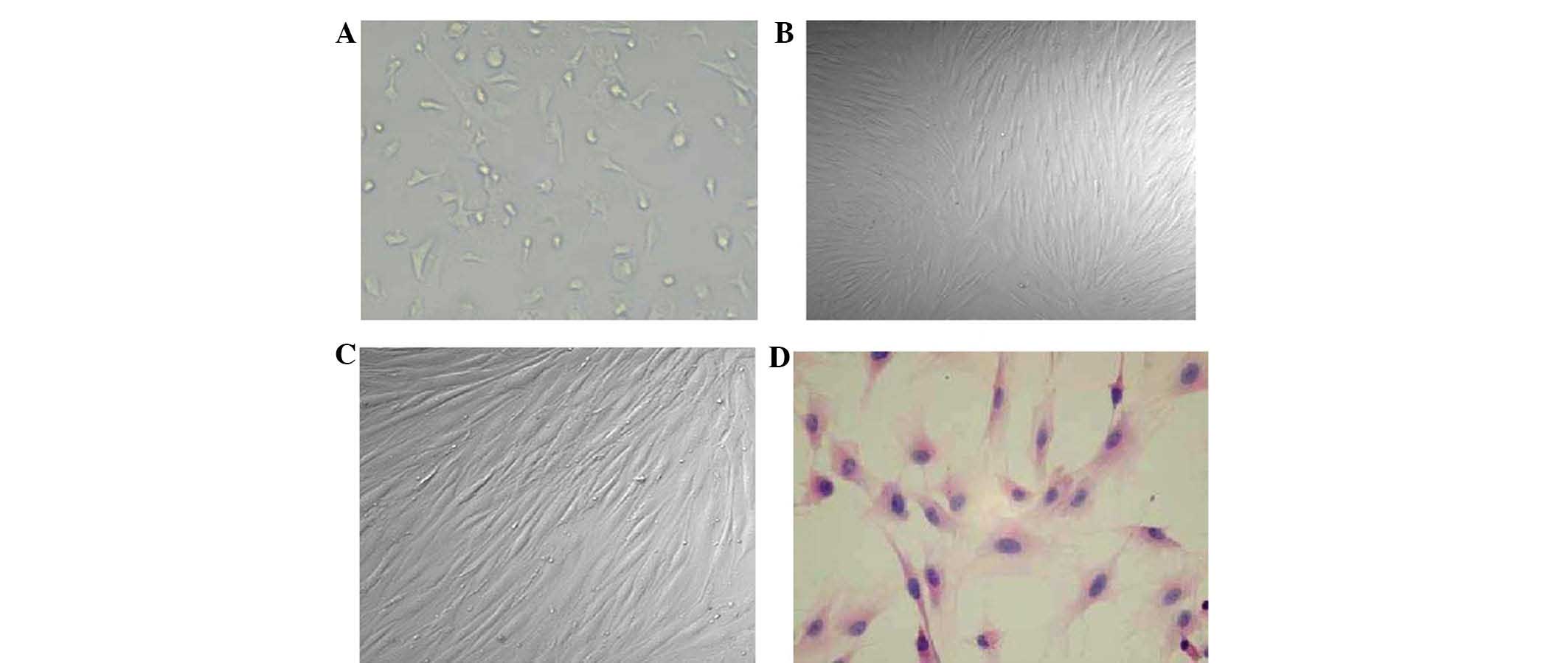
hBMSC cultures are shown in Fig. 1. The BMSC cell morphology at
initial plating, the third passage, and the sixth passage are shown
in Fig. 1A-C, respectively. A
representative hematoxylin and eosin (H&E)-stained hBMSC sample
is shown in Fig. 1D.
Histological study
The histology of the infarction area in the rats
from the three groups that underwent MCAO is shown in Fig. 2. The microscopy image shows that
neurons had large cytoblasts with clear nucleoli and abundant
cytoplasm. In the center of the ischemic region in MCAO rats, the
staining was light with loose tissue and mesenchymal edema
(Fig. 2A). The number of cells was
decreased. Cells were swollen and deformed with fractured
cytoblasts. Aberrations in the ischemic periphery were reduced, and
there were few infiltrating inflammatory and glial cells. Fig. 2A shows the center of the infarction
area in group C, which did not receive transplantation following
ischemia/reperfusion. The staining was lighter in the infarction
area compared with the normal brain with loose tissue and
mesenchymal edema (indicated by yellow arrow). The number of cells
was decreased. Cells were swollen (indicated by black arrow) and
deformed with cracked cytoblasts (indicated by green arrow).
Fig. 2B shows the adjacent normal
section of the corresponding tissue in group C.
Behavioral testing
The NSS for the five groups are shown in Fig. 3. The NSS in group A remained stable
from day one to day 28. In group B, the NSS decreased from day one
to day three and then remained stable from day seven to day 28. The
NSS in the three groups that underwent reperfusion decreased from
day one to day 28, among which group E showed the most significant
decrease.
The differences between the groups at each
time-point are summarized as follows: On day one, the NSS in the
groups with reperfusion (groups C-E) were significantly higher than
those in groups A and B, and similar results were observed on days
three, seven, and day 28. In addition, the NSS in group E was
significantly lower than that in group D on day three (8.75 versus
9.88), and the NSS in group E was significantly lower than that in
groups C and D on both days seven and 28.
VEGF
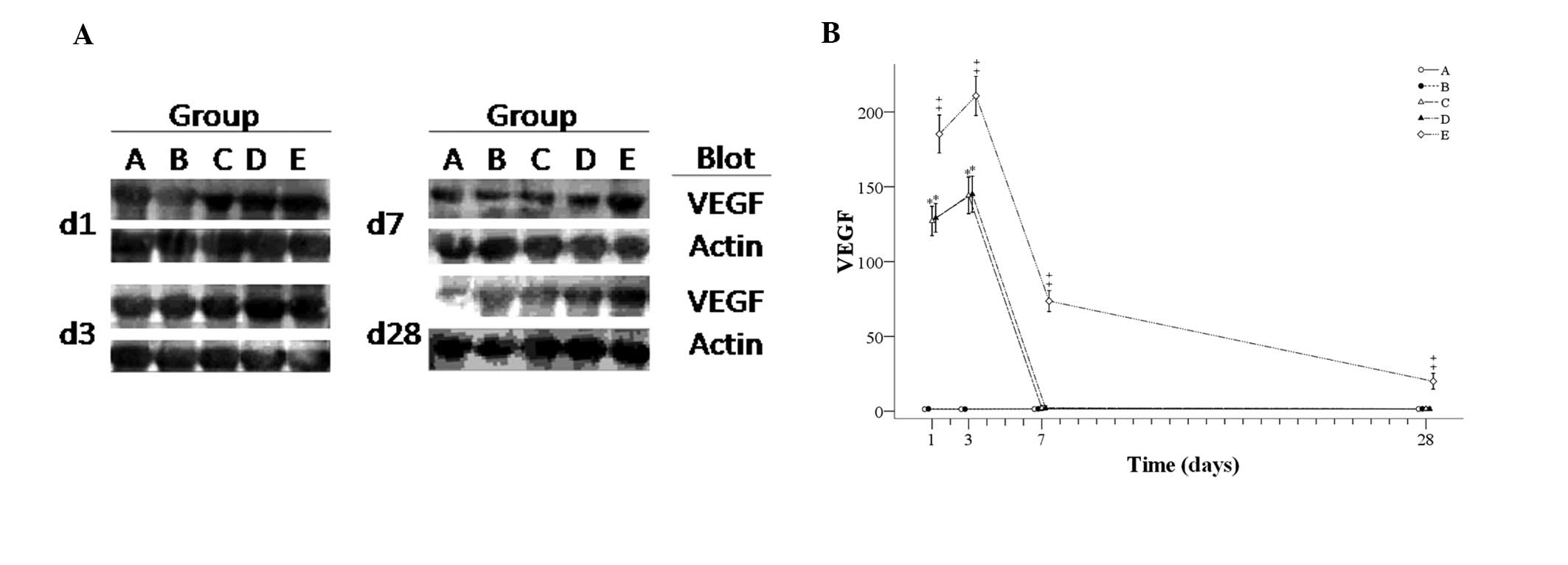
Fig. 4A shows
representative western blot images and Fig. 4B shows the number of VEGF-positive
cells for each group during the experiment. The number of
VEGF-positive cells in groups A and B remained stable from day one
to day 28. In the other three groups with reperfusion, similar
trends in the number of VEGF-positive cells were observed in groups
C and D, but the number of VEGF-positive cells in group E was
significantly higher than that in groups C and D from day one to
day 28. Amounts of VEGF-positive cells initially increased from day
one to day three, and steeply decreased to day seven, followed by
stabilization in groups C and D. However, in group E, the levels
increased from day one to day three and then continuously
decreased. The differences between the groups at each time-point
are summarized as follows: On days one and three, the number of
VEGF-positive cells in the groups with reperfusion (groups C-E) was
greater than that in groups A and B, and the number of
VEGF-positive cells in group E was significantly higher than that
in groups C and D. On days seven and 28, the number of
VEGF-positive cells in group E was significantly higher that in the
other four groups.
Immunostaining images of BrdU- and VEGF-positive
cells seven days following transplantation of BrdU-labeled hBMSCs
into the cortex of rat brains are shown in Fig. 5. The cell nuclei were stained blue,
whereas VEGF was stained red and was localized around the
karyotheca or in the cytoplasm. The hBMSCs were smaller than the
neurocytes, with elliptical or round nuclei, and they were stained
green due to BrdU labeling.
 | Figure 5Immunostaining images of BrdU- and
VEGF-positive cells seven days following transplantation of
BrdU-labeled hBMSCs into the cortex of rat brains (bar, 20 μm). The
cell nuclei are dark blue. VEGF is stained red and was expressed
around the karyotheca or in the cytoplasm. The hBMSCs (yellow
arrows in the non-merged image, white arrows in the merged image)
were smaller than the neurocytes, with elliptical or round nuclei
that were stained green due to BrdU labeling. Orange arrows point
to VEGF-positive cells, which tended to have dark blue-stained
nuclei, indicating a lack of BrdU staining and therefore, that
these were original host cells. VEGF, vascular endothelial growth
factor, hBMSCs, human bone marrow stromal cells. BrdU,
5-bromo-2-deoxyuridine. |
Discussion
In the present study, hBMSCs were injected into the
brain of a rat model of MCAO following reperfusion. A histological
study revealed that hBMSCs labeled with BrdU were predominantly
located at the boundary between intact tissue and the infarction
area. From day three the NSS of the rats that received BMSCs was
significantly lower than the score of rats that underwent MCAO but
did not receive BMSCs. From day one, the group that received BMSCs
had significantly higher expression levels of VEGF than the other
two groups that underwent MCAO. These results appear to support the
hypothesis that the secretion of VEGF by BMSCs or the stimulation
of host cells (rat cells) to secrete VEGF has an important role in
the recovery from cerebral ischemia.
The present study appears to confirm the findings of
previous studies in which BMSCs were transplanted into the rat
cerebral ischemia model. Wakabayashi et al (9) found that rats injected with a human
mesenchymal stem cell (MSC) line exerted a functional improvement
compared with the controls and VEGF expression in the host cells
was markedly increased, as well as epidermal growth factor (EGF)
and bFGF expression. The only neurotrophic factor expressed by the
MSCs appeared to be IGF-1. He et al (6) transplanted BMSCs and endothelial
progenitor cells (EPCs) and found that the combination produced
significantly greater functional improvement than BMSCs or EPCs
transplanted alone. Furthermore, the BMSC/EPC group showed a
significantly higher expression of VEGF, bFGF and BNDF. Bao et
al (4) transplanted hBMSCs
into rats with cerebral ischemia and found that following 14 days
the rats that received hBMSCs had increased levels of VEGF, BDNF
and NT-3. Wei et al (10)
used hypoxia preconditioned BMSCs for transplantation. Hypoxia
functionally improved BMSCs for transplantation, which may be due
to an enhanced expression of trophic factors.
Compared with other cell types, BMSCs are good
candidates for cell transplantation therapy. They are able to be
obtained easily from patients or a bone marrow bank, and are able
to be cultured and expanded in number with fewer ethical concerns
than fetal stem cells. Furthermore, autologous transplantation of
BMSCs or transplantation of BMSCs with the same human leukocyte
antigen (HLA) subtype from a healthy donor may minimize the risk of
rejection. For clinical application of cell transplantation therapy
for the injured brain, transvenous or intrathecal cell injection is
preferable as it is less invasive (20). However, the cell distribution into
the ischemic brain is uncertain. Therefore, stereotaxic injection
was used in the present study, and the results suggest that this
method was successful. With the advancement of imaging
technologies, the damaged tissue is able to be visualized easily.
The present study suggests that the direct injection of cells has
great potential in BMSC transplantation therapy for neurologic
disorders.
Therapeutic angiogenesis is considered to be crucial
for functional recovery following stroke. Angiogenesis requires a
stimulus such as ischemia, tumor and inflammation. VEGF is known to
be one of the most effective trophic factors that induces
angiogenesis following stroke, which contributes to the recovery of
the blood supply and functional recovery. In the present study, the
hBMSC-injected group showed significant improvement in the score of
the behavioral assessment tests compared with all the control
groups. Findings of previous studies have shown hBMSC
transplantation into the ischemic brain leads to improvement of the
behavioral outcome (12,13,19).
However, in these studies, the survival rate of BMSCs in the host
brain was >10%, and the proportion showing neuronal
differentiation was only 1–2%. The functional recovery in behavior
following MSC transplantation is thought to be mediated by
neurotrophic factors produced by BMSCs or by intrinsic parenchymal
cells stimulated by BMSCs (21–23).
The histological analysis in the present study revealed that the
BrdU-labeled hBMSCs survived and migrated to the nearby intact
tissue. The brains of BMSC-transplanted animals had a larger
proportion of VEGF-positive cells, which may have contributed to
the improvement in behavior. These results suggest that hBMSCs
cultured in vitro followed by transplantation are able to
survive and distribute widely following transplantation into the
brain via stereotaxic injection. This procedure is effective for
functional recovery from cerebral ischemia. Inducing the expression
of VEGF may have an important role in this process.
Another explanation for the improved recovery
following transplantation of hBMSCs is endogenous neurogenesis. Bao
et al (4) reported that
rats treated with hBMSCs showed enhanced endogenous cell
proliferation in the hippocampal subventricular and subgranular
zones, and an increased number of neuronal progenitor cells had
migrated to the ischemic boundary zone from the subventricular zone
and then differentiated into mature neurons with decreased levels
of apoptosis. Neurogenesis and angiogenesis induced by trophic
factors may have a role in the functional recovery following
treatment with hBMSCs.
One limitation of the present study was that the
sample size decreased at each assessed time-point. There were 10
animals in each group on day one, but only eight on day three, six
on day seven and four on day 28. Another limitation was that the
expression of only one trophic factor, VEGF, was assessed. Further
studies are required to examine other potentially important
underlying changes, including activation of VEGF signalling,
phosphorylation of the VEGF receptor, as well as any changes in
processes associated with angiogenesis and neurogenesis.
In conclusion, transplantation of hBMSCs into a rat
model of MCAO appeared to improve neurologic recovery and increase
the expression of VEGF in the ischemic region. VEGF may have an
important role in functional recovery by stimulating angiogenesis.
Transplantation of hBMSCs appears to be a promising stem cell
therapy for treatment of acute stroke. Future investigations should
focus on elucidating and confirming the mechanisms of the positive
effect of transplantation of hBMSCs on functional recovery
following cerebral ischemia.
References
|
1
|
Chen J, Li Y, Wang L, Lu M, Zhang X and
Chopp M: Therapeutic benefit of intracerebral transplantation of
bone marrow stromal cells after cerebral ischemia in rats. J Neurol
Sci. 189:49–57. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krebsbach PH, Kuznetsov SA, Bianco P and
Robey PG: Bone marrow stromal cells: characterization and clinical
application. Crit Rev Oral Biol Med. 10:165–181. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bang OY, Lee JS, Lee PH and Lee G:
Autologous mesenchymal stem cell transplantation in stroke
patients. Ann Neurol. 57:874–882. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bao X, Wei J, Feng M, et al:
Transplantation of human bone marrow-derived mesenchymal stem cells
promotes behavioral recovery and endogenous neurogenesis after
cerebral ischemia in rats. Brain Res. 1367:103–113. 2011.
View Article : Google Scholar
|
|
5
|
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M
and Chopp M: Therapeutic benefit of intravenous administration of
bone marrow stromal cells after cerebral ischemia in rats. Stroke.
32:1005–1011. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He XY, Chen ZZ, Cai YQ, et al: Expression
of cytokines in rat brain with focal cerebral ischemia after
grafting with bone marrow stromal cells and endothelial progenitor
cells. Cytotherapy. 13:46–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Chen J, Chen XG, et al: Human marrow
stromal cell therapy for stroke in rat: neurotrophins and
functional recovery. Neurology. 59:514–523. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Chopp M, Chen J, Wang L, Gautam SC,
Xu YX and Zhang Z: Intrastriatal transplantation of bone marrow
nonhematopoietic cells improves functional recovery after stroke in
adult mice. J Cereb Blood Flow Metab. 20:1311–1319. 2000.
View Article : Google Scholar
|
|
9
|
Wakabayashi K, Nagai A, Sheikh AM, Shiota
Y, Narantuya D, Watanabe T, Masuda J, Kobayashi S, Kim SU and
Yamaguchi S: Transplantation of human mesenchymal stem cells
promotes functional improvement and increased expression of
neurotrophic factors in a rat focal cerebral ischemia model. J
Neurosci Res. 88:1017–1025. 2010.
|
|
10
|
Wei L, Fraser JL, Lu ZY, Hu X and Yu SP:
Transplantation of hypoxia preconditioned bone marrow mesenchymal
stem cells enhances angiogenesis and neurogenesis after cerebral
ischemia in rats. Neurobiol Dis. 46:635–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen J, Zhang ZG, Li Y, Wang L, Xu YX,
Gautam SC, Lu M, Zhu Z and Chopp M: Intravenous administration of
human bone marrow stromal cells induces angiogenesis in the
ischemic boundary zone after stroke in rats. Circ Res. 92:692–699.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hess DC, Hill WD, Martin-Studdard A,
Carroll J, Brailer J and Carothers J: Bone marrow as a source of
endothelial cells and NeuN-expressing cells after stroke. Stroke.
33:1362–1368. 2002.PubMed/NCBI
|
|
13
|
Zhang ZG, Zhang L, Jiang Q and Chopp M:
Bone marrow-derived endothelial progenitor cells participate in
cerebral neovascularization after focal cerebral ischemia in the
adult mouse. Circ Res. 90:284–288. 2002. View Article : Google Scholar
|
|
14
|
Azizi SA, Stokes D, Augelli BJ, DiGirolamo
C and Prockop DJ: Engraftment and migration of human bone marrow
stromal cells implanted in the brains of albino rats - similarities
to astrocyte grafts. Proc Natl Acad Sci USA. 95:3908–3913. 1998.
View Article : Google Scholar
|
|
15
|
Zhao LR, Duan WM, Reyes M, Keene CD,
Verfaillie CM and Low WC: Human bone marrow stem cells exhibit
neural phenotypes and ameliorate neurological deficits after
grafting into the ischemic brain of rats. Exp Neurol. 174:11–20.
2002. View Article : Google Scholar
|
|
16
|
Gutiérrez-Fernández M, Rodríguez-Frutos B,
Ramos-Cejudo J, Teresa Vallejo-Cremades M, Fuentes B, Cerdán S and
Díez-Tejedor E: Effects of intravenous administration of allogenic
bone marrow- and adipose tissue-derived mesenchymal stem cells on
functional recovery and brain repair markers in experimental
ischemic stroke. Stem Cell Res Ther. 4:112013.(Epub ahead of
print).
|
|
17
|
Mitkari B, Kerkelä E, Nystedt J, Korhonen
M, Mikkonen V, Huhtala T and Jolkkonen J: Intra-arterial infusion
of human bone marrow-derived mesenchymal stem cells results in
transient localization in the brain after cerebral ischemia in
rats. Exp Neurol. 239:158–162. 2013. View Article : Google Scholar
|
|
18
|
Koizumi J, Yoshida Y, Nakazawa T and
Ooneda G: Experimental studies of ischemic brain edema, I: a new
experimental model of cerebral embolism in rats in which
recirculation can be introduced in the ischemic area. Jpn J Stroke.
8:1–8. 1986.
|
|
19
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dezawa M, Ishikawa H, Hoshino M, Itokazu Y
and Nabeshima Y: Potential of bone marrow stromal cells in
applications for neuro-degenerative, neuro-traumatic and muscle
degenerative diseases. Curr Neuropharmacol. 3:257–266. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Li Y, Wang L, Katakowski M, Zhang
L, Chen J, Xu Y, Gautam SC and Chopp M: Ischemic rat brain extracts
induce human marrow stromal cell growth factor production.
Neuropathology. 22:275–279. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin K, Minami M, Lan JQ, Mao XO, Batteur
S, Simon RP and Greenberg DA: Neurogenesis in dentate subgranular
zone and rostral subventricular zone after focal cerebral ischemia
in the rat. Proc Natl Acad Sci USA. 98:4710–4715. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kurozumi K, Nakamura K, Tamiya T, et al:
BDNF gene-modified mesenchymal stem cells promote functional
recovery and reduce infarct size in the rat middle cerebral artery
occlusion model. Mol Ther. 9:189–197. 2004. View Article : Google Scholar
|