Introduction
Stem cell research is an emerging and actively
studied topic in life sciences. Despite the pluripotency of
embryonic stem cells (ESCs), their use is currently limited,
largely due to ethical issues. Since 2006, transformation of
fibroblasts into pluripotent cells has been successfully induced by
introduction of four genes: Oct4, Sox2, c-Myc
and Klf4. The resulting induced pluripotent stem (iPS)
cells, have a high similarity with ESCs in morphology,
proliferation, surface marker and gene expression profiles, and
differentiation potential (1,2).
Since then, a novel method to efficiently induce transformation of
skin cells into iPS cells was also identified, involving
introduction of the microRNA (mir) that is regulated by the the
Oct4 and Sox2 transcription factors, miR-302 (3–5).
Human umbilical cord mesenchymal stem cells
(hUCMSC), are a type of adult stem cells with multidirectional
differentiation potential that can differentiate into
cardiomyocytes (6), vascular
endothelial cells and osteoblasts (7), etc.; this type of mesenchymal stem
cells (MSCs) is widely used in research and clinical
applications.
The octamer transcription factor 4 (Oct4), also
known as Oct3/4 or Pou5fl (POU domain, class 5, transcription
factor 1) is a homeodomain transcription factor of the POU family,
and the most important transcriptional regulator of self-renewal
and differentiation in ESCs. Oct4 is considered a key factor of the
stem cellmultidirectional differentiation potential (8).
In this study, we infected hUCMSCs with a lentivirus
that carries the green fluorescent protein gene (GFP) to
assess its effects on Oct4 expression and explore the optimal
conditions for future transfections of hUCMSCs with the
GFP-conjugated exogenous mir-302.
Materials and methods
hUCMSC isolation and cultures
Five centimeters of the umbilical cord were
collected from full-term newborns delivered by caesarean section.
Informed consent of the mother, who faced no complications
throughout pregnancy was obtained. The umbilical cord was immersed
in a sterile culture flask containing Dulbecco’s modified Eagle’s
medium (DMEM)/F12 (Gibco-BRL, Grand Island, NY, USA) and was placed
on a clean bench; the arteries, veins and Wharton’s jelly were
removed from the umbilical cord, followed by washing with normal
saline to remove residual blood. Next, the umbilical cord was cut
into 1-mm3 pieces and seeded in a culture flask within a
small volume of DMEM/F12 medium containing 10% fetal bovine serum
(FBS). The flask was placed in a 37ºC incubator for 30 min; then,
part of the medium was added into wet tissue pieces, which were
incubated in a humidified 37ºC, 5% CO2 incubator. Half
of the culture medium was replaced three days later and cell growth
was observed daily under an inverted microscope.
Lentivirus packaging
A lentivirus packaging system, including
pLenO-DCE-Puro, pRSV-Rev, pMDlg-pRRE and pMD2.G was purchased from
(Invitrogen, Carlsbad, CA, USA). HEK-293T cells (Invitrogen) were
cultured until 60–70% confluence was reached, then washed with PBS
twice, isolated by treating with 0.25% trypsin containing 0.01%
EDTA (Sigma, St. Louis, USA) for 6–7 minutes. Cells were counted
with a hemocytometer and 2×106 were seeded into
10-cm2 petri dishes and incubated overnight in a 37ºC
incubator with 5% CO2. The medium was changed when the
cells had reached 60–70% confluence. The 10 μl plasmid (pRSV-Rev,
pMFlg-pRRE and pMD2.G; Invitrogen) and 3 μl calcium phosphate
(Sigma, St. Louis, MO, USA) mixture was transferred in medium
containing monolayer cells and was gently mixed; the medium was
changed after 6 h of culture. The supernatant containing the virus
was collected 48 h following the infection and centrifuged at 1,000
× g for 10 min at 4°C; the supernatant was passed through a 0.45-μm
filter. Following a new centrifugation at 6,000 × g for 2 h at 4°C,
the pellet containing the virus was dissolved in serum-free culture
medium, aliquoted and stored at −70°C prior to further use.
Lentivirus titer determination
One day before measurement, HEK-293T cells were
seeded into 96-well plates with 100 μl of culture medium in each
well. The lentivirus was serially diluted over the wells of the
plate; 8 μf/μl polybrene (Sigma) was simulteneously added to
increase the efficiency of infection. Cell growth was observed two
days later and the cells were collected for subsequent titer
determination.
Measurement of infection efficiency
hUCMSCs at passage 3 were isolated as described,
seeded into 24-well plates (5×104 cells/well) containing
DMEM/F12 medium supplemented with 10% FBS, and incubated in a 37°C
incubator with 5% CO2. Lentivirus [multiplicity of
infection (MOI)=0, 5, 10, 15, 20; triplicates for each MOI value]
was added to the wells when the cells had reached 70–80%
confluence. The medium was changed 24 h following the infection and
the fluorescence intensity was measured after 24, 48, 72 and 96 h.
Non-infected hUCMSCs were used as negative controls. The two cell
groups were respectively harvested by 0.25% trypsin (Hyclone,
Logan, UT, USA), digested for 2 min at room temperature and
resuspended in phosphate-buffered saline (PBS) after 96 h of
infection. The efficiency of infection was shown by the percentage
of cells containing GFP and was calculated by the following
formula: (Number of GFP positive cells/total number of cells) ×
100. The cell number was determined by a BD FACSCanto II cytometer
(BD Biosciences, San José, CA, USA).
MTT assay
hUCMSCs at passage 3 were isolated as described and
seeded into 96-well plates (2×103 cells/well). Five
wells of the non-infected and the lentivirus-infected group were
used for each assay, containing PBS and an equal volume of
lentivirus at a MOI of 20, respectively. At 24, 48, 72 and 96 h
after the infection, 20 μl of MTT (Beyotime Institute of
Biotechnology, Haimen, China) were added to each well. Following a
4-h incubation in a 37°C incubator with 5% CO2, the
culture medium was removed and 100 μl of dimethyl sulfoxide were
added to dissolve the formed crystals under low-speed vibration for
10 min. The optical density (OD) was measured at 490 nm with a
microplate reader 680 (Bio-Rad, Hercules, CA, USA).
Quantitative reverse
transcription-polymerase chain reaction (qRTPCR)
Total RNA was extracted from two- and eight-week
cultures of hUCMSCs with the TRIzol reagent (Invitrogen, New York,
NY, USA) following the manufacturer’s instructions. The RNA was
reverse transcribed into complementary DNA (cDNA) following
instructions of the Takara PrimeScript TM RT-RCR kit (DRR014A;
Takara Bio, Inc., Dalian, China). The forward and reverse primers
were: 5′-GTGAGAG GCAACCTGGAGAAT-3; 5′-TACAGAACCACACTCGGAC CAC-3′
for the gene Oct4 (Abcam, Cambridge, UK), and
5′-CTTTGGTATCGTGGAAGGACTC-3′; 5′-GTAGAGGCA GGGATGATGTTCT-3′ for the
glyceraldehyde 3-phosphate dehydrogenase gene (GAPDH),
respectively. The expected length of the amplified fragments was
118 and 132 bp, respectively. The qPCR reaction was performed with
the SYBR Premix Ex Taq™ II kit (Bio-Rad, Hercules, CA, USA) using
the following conditions: denaturation at 95°C for 30 sec, followed
by 40 cycles of denaturation at 95°C for 5 sec, annealing at 55°C
for 30 sec and elongation at 72°C for 30 sec. Analysis of the
amplification and melting curves was performed after the reaction.
The expression of Oct4 was calculated by the Δ (ΔCt) method
(9) and was expressed relative to
that of GAPDH.
Immunofluorescence
When cells in 24-well plates had reached 65–70%
confluence, the culture medium was discarded. The plates were
washed twice in PBS, fixation with 4% formaldehyde at room
temperature for 20 min, three washes in PBS for 5 min, treatment
with 0.2% Triton X-100 for 30 min, and three washes in PBS for 5
min. Next, blocking was performed in 1% bovine serum albumin for 1
h, and the blocking buffer was washed away prior to the incubation
with the 100X-diluted rabbit anti-human/mouse Oct4 antibody
(ab18976, Abcam) at 4°C in a humid box overnight; PBS instead of
the primary antibody was used as the blank control. The plates were
placed at 37°C for 30 min, followed by three PBS washes for 5 min,
incubation with the 50x-diluted tetramethylrhodamine
(TRITC)-labeled goat anti-rabbit secondary antibody IgG (Golden
Bridge Biotechnology Co., Ltd., Beijing, China) in the dark at 37°C
for 2 h, three PBS washes for 5 min, 4′,6-diamidino-2-phenylindole
(DAPI) nuclear staining for 1 min and a final PBS wash for 5 min.
Glass slides were mounted with glycerol and observed under a
fluorescence microscope (Olympus, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed with the SPSS
17.0 software (IBM, Armonk, NY, USA). Quantitative data were
presented as mean ± standard deviation (χ̄ ± SD), and comparisons
between groups was performed with t-tests. P<0.05 was considered
to indicate significant differences.
Results
hUCMSC growth and morphology
Half of the culture medium was replaced on the third
day of the culture, when most of the tissue pieces had attached to
the wells, and was again changed every two days afterwards. A few
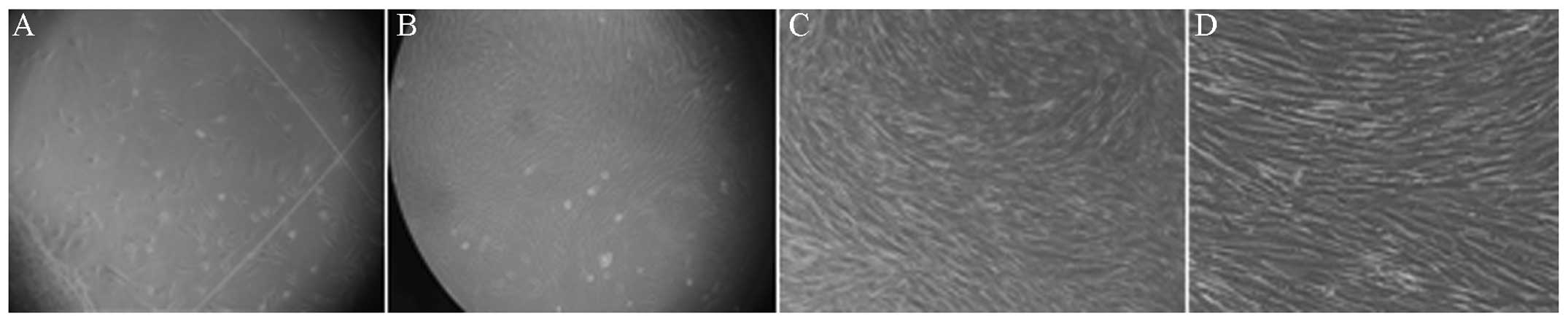
fusiform hUCMSCs dissociated from the tissue (Fig. 1A) after 10 days of culture, while a
high number of colonies was formed after 15 days, when hUCMSCs
displayed a typical fibroblast-like spindle shape (Fig. 1B). Cells were trypsinized and were
passaged after they had reached 80–90% confluence. Cell morphology
and properties, such as cell cycle phase (the majority were in
G0/G1 phases with only a small minority in the S phase),
self-renewal, pluripotency and differentiation did not
significantly change after passage 10 (Fig. 1C and D).
Analysis of hUCMSC surface markers
The analysis of hUCMSCs at the third passage by flow
cytometry showed that CD29, D90 and CD105 are expressed in these
cells (Fig. 2A–C and E), while the
cells are negative for CD34 and CD45 (Fig. 2F and G), which are the surface
markers of hematopoietic stem cells.
Lentivirus packaging and infection
efficiency
Bright green fluorescence was observed under the
fluorescence microscope 48 h following infection of the HEK-293T
cells (Fig. 3A and B), which
indicated successful packaging of the active lentivirus.
Hole-by-dilution was used to determine the virus titer and flow
cytometry was employed to detect the percentage of GFP-positive
cells. The titer of the GFP-carrying lentivirus was estimated at
2×108 TU/ml, which was considered suitable for infecting
hUCMSCs.
Following hUCMSC infection, all cells expressed the
GFP protein as shown by green fluorescence emitted at any MOI value
(except MOI = 0). The cell morphology observed under the
fluorescence microscope was similar to that observed under a light
microscope (Fig. 3C and D).
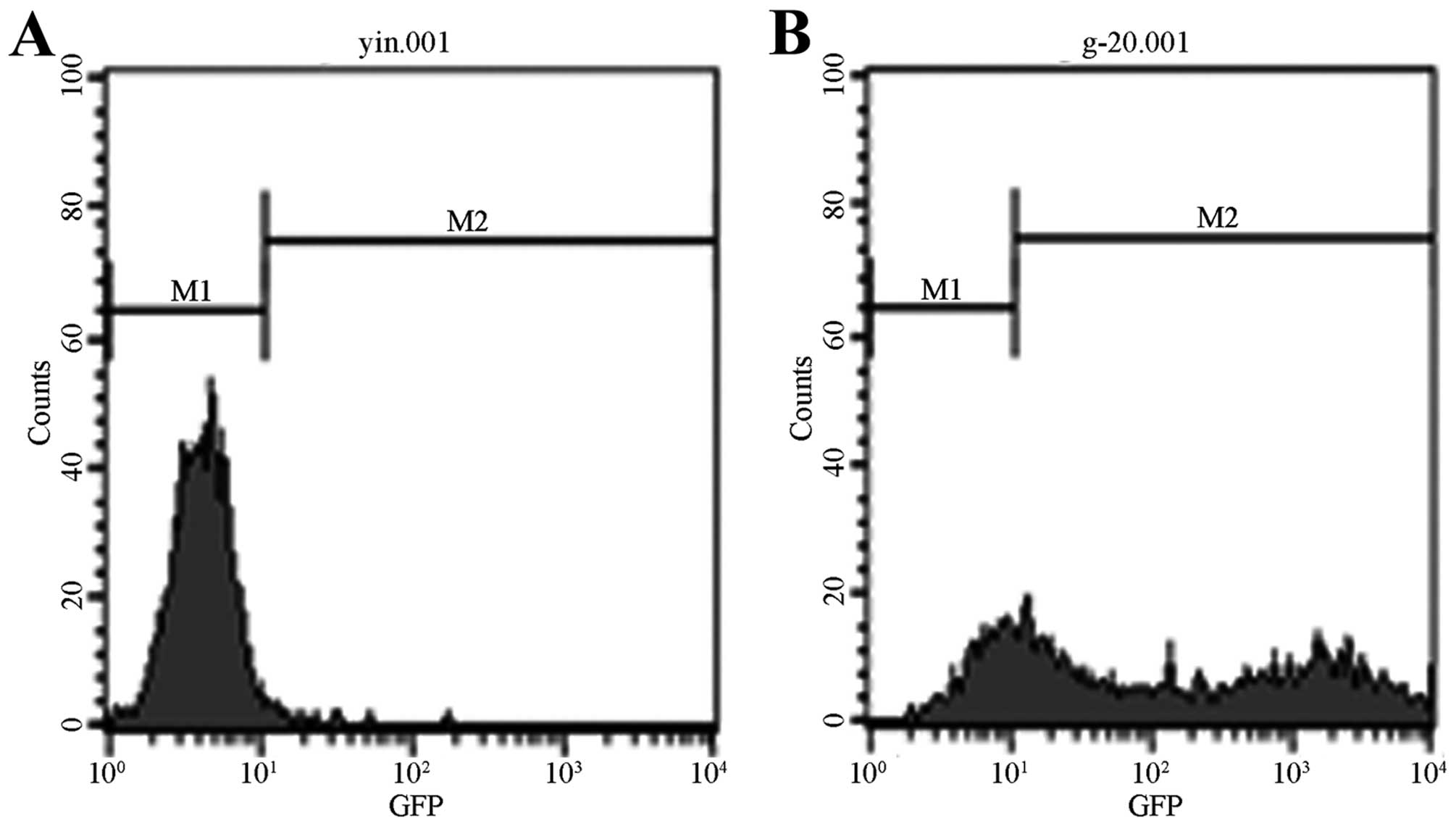
The fluorescence intensity was the strongest at 96 h
following the infection. With the increase of MOI and the
proliferation of cells, fluorescence became stronger, reaching its
highest level at MOI = 20. Flow cytometry analysis showed that the
infection efficiency is 75.85% at MOI = 20 and at 96 h following
the infection (Fig. 4).
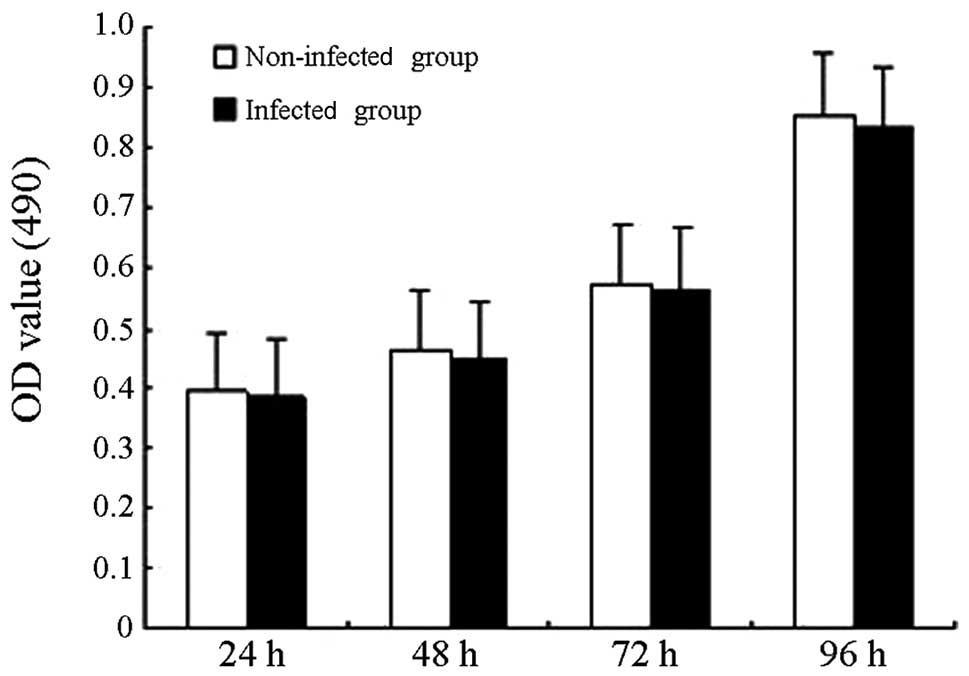
Cell proliferation
Cell proliferation was measured in the non-infected
and the lentivirus-infected group with the MTT assay (Fig. 5), and no statistically significant
difference was observed between the OD values of the two groups
(P>0.05), which suggested that the GFP gene carried by
the lentivirus does not markedly affect cell proliferation under
the tested conditions.
mRNA level of Oct4
The Oct4 expression level was defined as 1 in
the lentivirus-infected group of hUCMSCs after two weeks of
culture, and thus, the level of Oct4 was estimated at
0.9075±0.0124 after eight weeks of culture (Fig. 6). The relative expression of
Oct4 was not significantly different between cells cultured
for two and eight weeks (P>0.05).
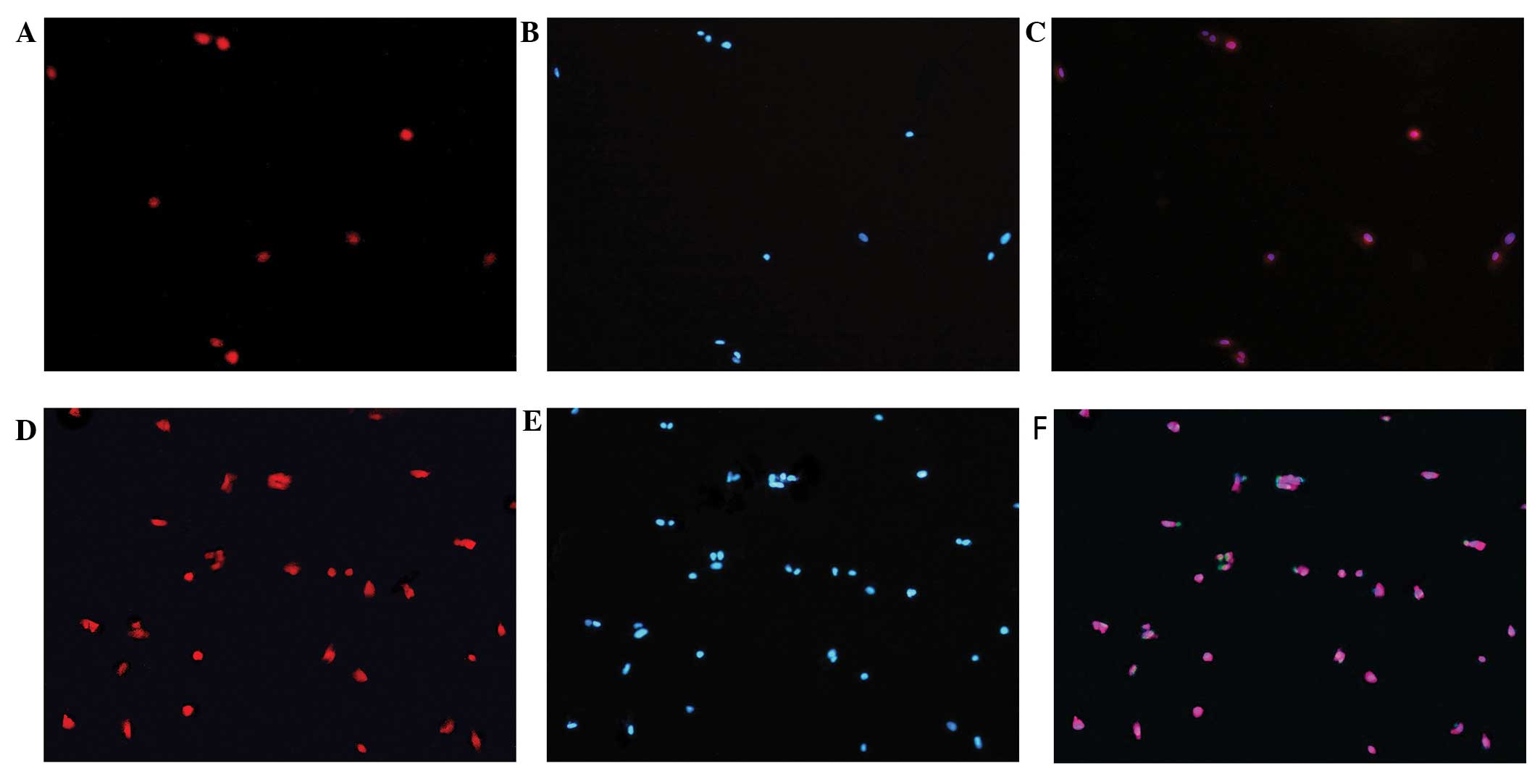
Oct4 protein expression
Immunofluorescence revealed that the Oct4 protein is
expressed in hUCMSCs of both the infected and the non-infected
group. There was no statistically significant difference in the
levels of Oct4 between the two groups. DAPI nuclear staining
experiments revealed that the Oct4 protein is mainly distributed in
the cell nuclei (Fig. 7).
Discussion
MSCs, an important member of the stem cell family,
are multipotent stem cells derived from the mesoderm at the early
stages of development, and show high self-renewal ability and
multidirectional differentiation potential. HUCMSCs, a type of
adult stem cells with multidirectional differentiation potential
(10), have several advantages
compared to other MSCs, including easy access and availability,
high proliferation ability, low immunogenicity, and no associated
ethical limitations. Moreover, hUCMSCs remain in their primitive
and undifferentiated state (11–14),
which renders introduction and expression of exogenous genes easy
during cell proliferation; this is one reason why hUCMSCs have
become the focus of numerous studies in recent years. In our
experiment, we cultured umbilical cord tissue sections in order to
isolate hUCMSCs.
Oct4 has been recognized as a specific marker of
stem cells and a key factor for cell totipotency, which is lost
during cell differentiation (15).
Oct4 is not only expressed in pluripotent embryonic cells, but also
in adult stem cells (16–19). Carlin et al (20) demonstrated that the embryonic stem
cell markers Oct4, Sox2 and Nanog are expressed in hUCMSCs. Can
et al (21) showed that the
Oct4 gene is expressed in hUCMSCs, indicating that hUCMSCs
possess stem cell properties.
Lentivirus is a non-oncogenic virus; in contrast to
other viral vectors, it can infect dividing and non-dividing cells,
especially cells that are difficult to transfect, such as primary
cells, stem cells and neurons, with an infection efficiency of
almost 100%. In addition, lentiviral vectors can effectively
integrate, and thus allow consistent expression of, exogenous genes
into the chromosome of host cells (22,23),
and have attracted increasing attention in the field of gene
transfer vectors (24). Miyoshi
et al (25) demonstrated
that GFP is a marker of infection efficiency. The lentiviral vector
used in our experiment bears the GFP-encoding gene, in order to
allow assessment of cell infection efficiency and optimization of
the infection conditions. Lentiviral vectors may integrate close to
promoters and insertion mutations, which may explain why infection
with the GFP-carrying lentivirus does not affect hUCMSC
proliferation. The low probability of lentivirus insertion near
promoters, minimizes the occurrence of insertion mutations, which
may partly explain the fact that proliferation of the infected
cells was not changed.
In our experiments, the GFP gene was
successfully introduced into hUCMSCs through a lentiviral vector,
which provides an ideal model for subsequent research on gene
infection. Green fluorescence was observed under a fluorescence
microscope and was the strongest at 96 h post-infection; the cell
morphology observed under the fluorescence microscope was similar
to that observed under a light microscope. Flow cytometry showed
that the infection efficiency was >75% at MOI = 20. The OD
values from the MTT assay did not show any significant difference
between the infected and the non-infected groups, which indicates
that infection with a GFP-carrying lentivirus has no effect on cell
proliferation. Oct4 expression was detected by qRT-PCR and
immunofluorescence. qRT-PCR revealed that the Oct4 mRNA
level is not significantly different between cells cultured for two
and eight weeks, which implies that infection of hUCMSCs with the
GFP-carrying lentivirus does not affect their pluripotency.
Immunofluorescence further showed that the Oct4 protein is
expressed in both infected and non-infected cells, with no apparent
difference between the two groups, and is mainly expressed in the
cell nuclei.
In conclusion, the GFP-carrying lentivirus can
effectively infect hUCMSCs and has no prominent effect on cell
pluripotency and proliferation. Our results lay a solid foundation
for future research using exogenous gene-carrying vectors.
References
|
1
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okita K, Ichisaka T and Yamanaka S:
Generation of germline-competent induced pluripotent stem cells.
Nature. 448:313–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin SL, Chang DC, Lin CH, Ying SY, Leu D
and Wu DT: Regulation of somatic cell reprogramming through
inducible mir-302 expression. Nucleic Acids Res. 39:1054–1065.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Card DA, Hebbar PB, Li L, et al:
Oct4/Sox2-regulated mir-302 targets cyclin D1 in human embryonic
stem cells. Mol Cell Biol. 28:6426–6438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zovoilis A, Pantazi A, Smoraq L, et al:
Embryonic stem cell-related miRNAs are involved in differentiation
of pluripotent cells originating from the germ line. Mol Hum
Reprod. 16:793–803. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmidt D, Mol A, Odermatt B, et al:
Engineering of biologically active living heart valve leaflets
using human umbilical cord-derived progenitor cells. Tissue Eng.
12:3223–3232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sarugaser R, Lickorish D, Baksh D,
Hosseini MM and Davies JE: Human umbilical cord perivascular
(HUCPV) cells: A source of mesenchymal progenitors. Stem Cells.
23:220–229. 2005.PubMed/NCBI
|
|
8
|
Boyer LA, Lee TI, Cole MF, et al: Core
transcriptional regulatory circuitry in human embryonic stem cells.
Cell. 122:947–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
10
|
Wang H, Yang Y, Ho G, et al: Programming
of human umbilical cord mesenchymal stem cells in vitro to
promote pancreatic gene expression. Mol Med Rep. 8:769–774.
2013.PubMed/NCBI
|
|
11
|
Phermthai T, Odglun Y, Julavijitphong S,
et al: A novel method to derive amniotic fluid stem cells for
therapeutic purposes. BMC Cell Biol. 11:792010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Troyer DL and Weiss ML: Wharton’s
jelly-derived cells are a primitive stromal cell population.
StemCells. 26:591–599. 2008.
|
|
13
|
Kim J, Lee Y, Kim H, et al: Human amniotic
fluid-derived stem cells have characteristics of multipotent stem
cells. Cell Prolif. 40:75–90. 2007.PubMed/NCBI
|
|
14
|
Schneider RK, Püllen A, Kramann R, et al:
Long-term survival and characterisation of human umbilical
cord-derived mesenchymal stem cells on dermal equivalents.
Differentiation. 79:182–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Atlasi Y, Mowla SJ, Ziaee SA and Bahrami
AR: OCT-4, an embryonic stem cell marker, is highly expressed in
bladder cancer. Int J Cancer. 120:1598–1602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hochedlinger K, Yamada Y, Beard C and
Jaenisch R: Ectopic expression of Oct-4 blocks progenitor-cell
differentiation and causes dysplasia in epithelial tissues. Cell.
121:465–477. 2005. View Article : Google Scholar
|
|
17
|
Mueller T, Luetzkendorf J, Nerger K,
Schmoll HJ and Mueller LP: Analysis of OCT4 expression in an
extended panel of human tumor cell lines from multiple entities and
in human mesenchymal stem cells. Cell Mol Life Sci. 66:495–503.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaltz N, Funari A, Hippauf S, et al: In
vivo osteoprogenitor potency of human stromal cells from different
tissues does not correlate with expression of POU5F1 or its
pseudogenes. Stem Cells. 26:2419–2424. 2008. View Article : Google Scholar
|
|
19
|
Lengner CJ, Camargo FD, Hochedlinger K, et
al: Oct4 expression is not required for mouse somatic stem cell
self-renewal. Cell Stem Cell. 1:403–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carlin R, Davis D, Weiss M, Schultz B and
Troyer D: Expression of early transcription factors Oct-4, Sox-2
and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol
Endocrinol. 4:82006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Can A and Karahuseyinoglu S: Concise
review: human umbilical cord stroma with regard to the source of
fetus-derived stem cells. Stem Cells. 25:2886–2895. 2007.
|
|
22
|
Weinberg MS, Barichievy S, Schaffer L, Han
J and Morris KV: An RNA targeted to the HIV-1 LTR promoter
modulates indiscriminate off-target gene activation. Nucleic Acids
Res. 35:7303–7312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cockrell AS and Kafri T: Gene delivery by
lentivirus vectors. Mol Biotechnol. 36:184–204. 2007. View Article : Google Scholar
|
|
24
|
Peng X, Zhang X and Zeng B: Locally
administered lentivirus-mediated siRNA inhibits wear debris-induced
inflammation in murine air pouch model. Biotechnol Lett.
30:1923–1929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyoshi N, Ishii H, Nagai K, et al:
Defined factors induce reprogramming of gastrointestinal cancer
cells. Proc Natl Acad Sci USA. 107:40–45. 2010. View Article : Google Scholar : PubMed/NCBI
|