Introduction
Host recognition of microbes is primarily mediated
by Toll-like receptors (TLRs), which bind highly conserved
pathogen-associated molecular patterns (PAMPs) typically shared by
groups of microorganisms (1). Each
member of the TLR family has been shown to recognize the PAMPs of
bacteria, fungi and/or viruses (2,3).
TLR2 forms a heterodimer with either TLR1 or TLR6 to recognize
bacterial tri- or diacyl-lipopeptides, while TLR3, 4 and 5
recognize double-stranded RNA, Gram-negative bacterial
lipopolysaccharide (LPS) and bacterial flagellin, respectively.
TLR7 and 8 recognize single-stranded RNA found in certain viruses,
and TLR9 recognizes the hypomethylated CpG motifs of bacterial DNA.
However, the PAMP recognized by TLR10 is unknown. Following
ligation, TLR signaling results in priming of the adaptive immune
system and initiation of inflammatory responses via the induction
of pro-inflammatory cytokines and chemokines (4,5). In
addition, activation of TLRs appears to be involved in the
pathogenesis of autoimmune disorders, as suggested by the induction
or promotion of organ-specific autoimmune lesions observed in
various experimental animal models (6–8).
The expression of various types of TLR molecules in
several types of epithelial tissue, including oral,
gastrointestinal, bronchial and urinary epithelia, supports the
hypothesis that the epithelium serves a critical function as the
defensive front line of the innate immune system (9–11).
TLRs in salivary glands have also been shown to be associated with
the promotion of inflammatory reactions in autoimmune diseases,
such as Sjögren’s syndrome (SS) and autoimmune sialoadinitis
(12,13). Therefore, submandibular gland
epithelial cells (SMGCs) appear to be actively involved in the
induction of tissue reactions against pathogens in inflammatory
autoimmune diseases via TLRs in the salivary glands.
Cytokines have a central role in the regulation of
immunity, but dysregulation of the cytokine network can contribute
to autoimmune disorders in salivary glands (14). Tumor necrosis factor (TNF)-α and
interleukin (IL)-8 have been identified in functionally and
structurally damaged areas of salivary glands, and have also been
implicated in disease pathogenesis (15–17).
Previously, various TLR agonists were shown to cause secretion of
pro-inflammatory cytokines, such as IL-8, and augment
TNF-α-mediated inflammatory responses in oral keratinocytes and
fibroblasts (18). These findings
may suggest that inflammatory cytokines in SMGCs are induced by
PAMPs and implicated in the development of TNF-α-mediated
inflammatory diseases of the salivary glands. In the present study,
IL-8 production from SMGCs in response to various TLR agonists was
examined. The combined effect of TLR agonists and TNF-α on the
production of IL-8 was also investigated.
Materials and methods
Cell lines
Primary cultures of human SMGCs obtained from
patient specimens extracted during submandibular gland extraction
surgery for sialolithiasis (Hiroshima University Hospital,
Hiroshima, Japan) were prepared. Informed consent from all
participants was obtained according to a protocol approved by the
Ethics Committee of Hiroshima University, Japan. SMGCs were
established using an explant outgrowth technique, as previously
described (19). At 70–80%
confluence, each primary culture was trypsinized, then serially
transferred to culture vessels in serum-free keratinocyte basal
medium (KBM; Lonza, Walkersville, MD, USA) as described previously
(19). For molecular analyses,
normal submandibular gland tissues at the time of submandibular
gland extraction surgery, subsequent to obtaining informed consent
and approval from the Institutional Review Board at Hiroshima
University Hospital, were collected and frozen immediately in
liquid nitrogen, and stored at −80°C.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was prepared from SMGCs and whole
submandibular gland tissues using an RNeasy total RNA isolation kit
(Qiagen, Hilden, Germany). Single-stranded cDNA for a PCR template
was synthesized from a First Strand cDNA Synthesis kit (Amersham
Biosciences, Uppsala, Sweden). Target cDNA was amplified by PCR
with an RT-PCR High Plus system (Toyobo, Osaka, Japan), using the
following specific primers: Sense: 5′-AATTGATCTGGGTGGTGAGC-3′ and
antisense: 5′-GCCAACGGTAGCTTGACATT-3′ for amylase; sense:
5′-CCGTGTCCAAGAAAACCAGA-3′ and antisense:
5′-CAGCTGTGTGATGGGAGCTA-3′ for chromogranin B; sense:
5′-GCCAAGCAGACGAGGACTAC-3′ and antisense:
5′-GGAGCACACCATCACACATC-3′ for kallikrein I; and sense:
5′-GGTCAGGCTCTCTTCACTGG-3′ and antisense:
5′-CCCTTCCCCCAGTTGAGTAT-3′ for claudin I. The PCR conditions were
as follows: One cycle at 95°C for 15 min, four cycles of 95°C for 2
min, 59°C for 30 sec and 72°C for 1 min and one cycle at 72°C for 7
min. The products were analyzed on 2% agarose gels. β-actin was
included as an internal control.
TLR agonists
The TLR agonists used in this study were purchased
from Imgenex Corporation (San Diego, CA, USA) and included
Pam3CSK4, a synthetic bacterial lipopeptide (TLR1/2 agonist); poly
I:C, a synthetic virus double-stranded RNA (TLR3 agonist); E.
coli LPS, a synthetic cell wall component of gram-negative
bacteria (TLR4 agonist); flagellin from Salmonella
Typhimurium, a synthetic bacterial flagellin (TLR5 agonist);
macrophage-activating lipopeptide (MALP-2), a synthetic
Mycoplasma lipopeptide (TLR2/6 agonist); imiquimod (R-837),
a synthetic molecule of the Imidazoquinoline family (TLR7 agonist);
synthetic oligodeoxynucleotide (ODN) containing CpG motifs; and a
synthetic bacterial DNA (TLR9 agonist).
Quantification of IL-8 protein
SMGCs were seeded into 96-well cell culture plates
in KBM. To determine the maximal effective TLR agonist
concentration with regard to activating capacity, IL-8 production
was assessed following stimulation with the following agonists at
various doses: 100 ng/ml-5 μg/ml Pam3CSK4, 100 ng/ml-5 μg/ml poly
I:C, 1–20 μg/ml LPS, 10–500 ng/ml flagellin, 10–500 ng/ml MALP-2
and 0.1–10 μg/ml imiquimod. As determined by these results, in
subsequent experiments, cells were exposed to 1 μg/ml Pam3CSK4, 1
μg/ml poly I:C, 10 μg/ml E. coli LPS, 100 ng/ml flagellin,
500 ng/ml MALP-2, 10 μg/ml imiquimod, 10 μg/ml CpG-ODN or GpC
(negative ODN) as a negative control to CpG-ODN. To half these
cells, 10 ng/ml TNF-α was added, and the cells were incubated for
48 h.
The collected medium samples were centrifuged and
the supernatant fluids stored at −80°C prior to performing assays.
The IL-8 protein level in the medium was determined using an ELISA
kit (R&D Systems, Minneapolis, MN, USA), according to the
manufacturer’s instructions.
Statistical analysis
Data were analyzed using Student’s t-test or one-way
analysis of variance using the Bonferroni or Dunn method and the
results are presented as the mean ± standard deviation.
Results
Establishment of SMGCs
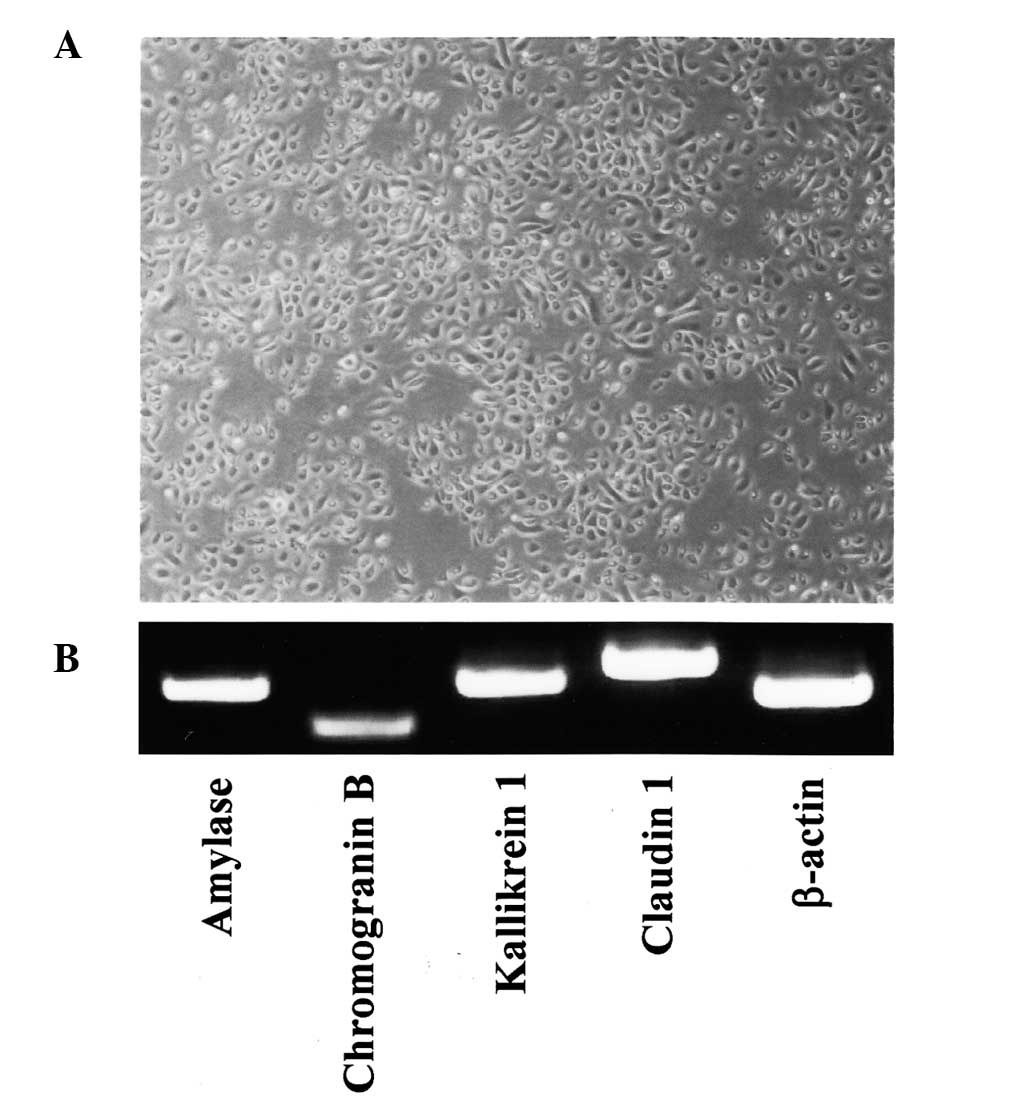
SMGCs were established using an explant outgrowth
method (19). The epithelial
origin of cultured SMGCs was verified by morphology and uniformity
(Fig. 1A), and SMGCs were also
shown to constitutively express salivary gland-associated genes,
such as amylase, chromogranin B, kallikrein 1 and claudin 1
(Fig. 1B) (19, 20).
TLR mRNA expression in SMGCs and whole
submandibular gland tissue specimens
As shown in Fig. 2,
SMGCs and whole submandibular gland tissue samples expressed
TLR1-10 mRNA.
Effects of TLR agonists on IL-8
expression levels in SMGCs
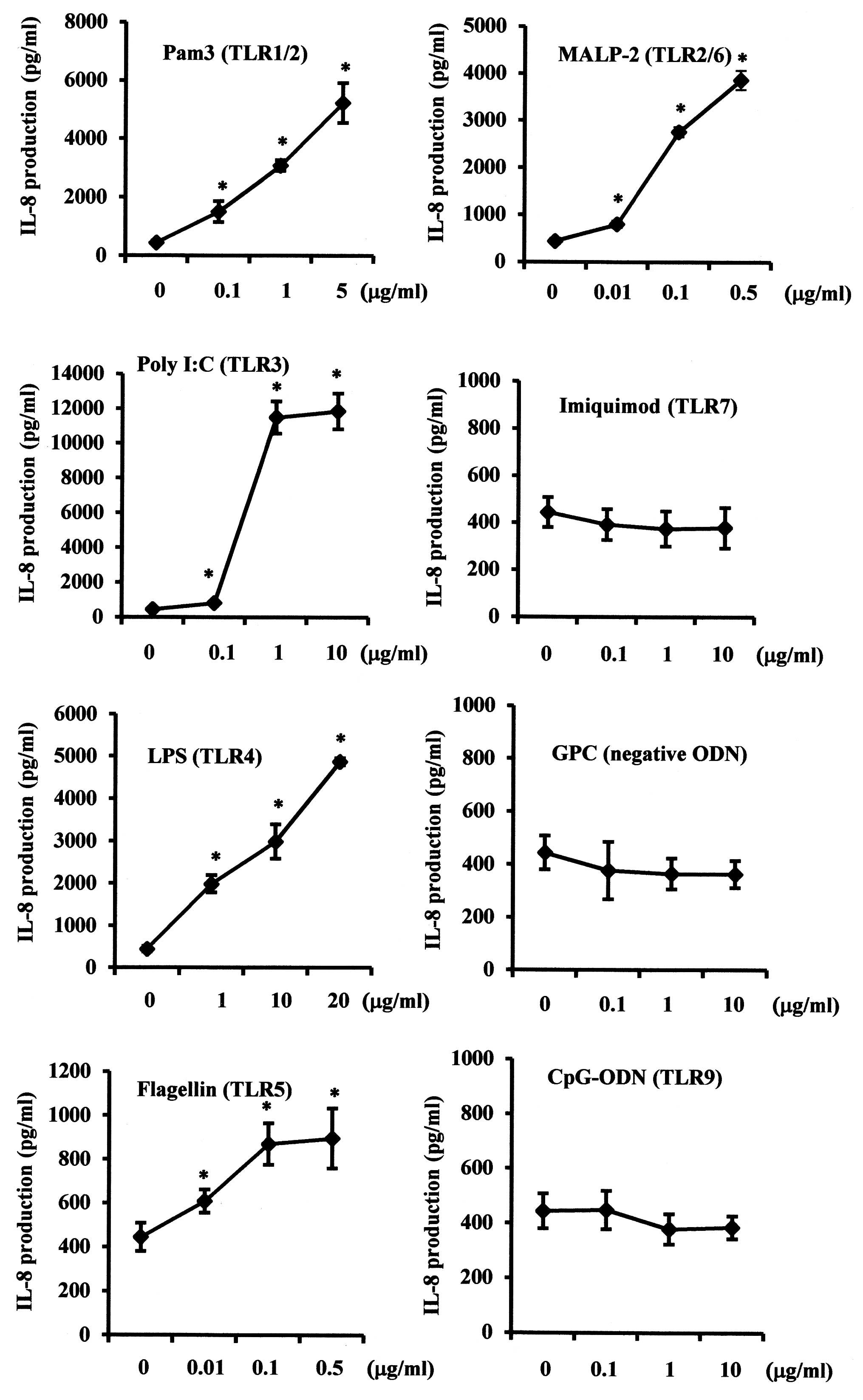
Since functional TLRs induce biological responses,
the effects of various TLR agonists on IL-8 expression levels in
SMGCs were examined. Pam3CSK4 (TLR1/2), poly I:C (TLR3), E.
coli LPS (TLR4), flagellin (TLR5) and MALP-2 (TLR2/6) each
significantly increased IL-8 production in a dose-dependent manner
(P<0.05), whereas no statistically significant effect was
exerted by imiquimod (TLR7) or CpG-ODN (TLR9) (Fig. 3).
Effects of PAMPs on TNF-α-induced IL-8
production in SMGCs
The effects of combinations of TLR agonists and
TNF-α on IL-8 expression levels were also examined. TNF-α
significantly increased IL-8 production in SMGCs in a
dose-dependent manner (P<0.05; Fig.
4A), while Pam3CSK4, poly I:C, LPS, flagellin and MALP-2
significantly enhanced TNF-α-induced IL-8 production in SMGCs, when
compared with either the respective TLR agonist or TNF-α
administered alone (P<0.05; Fig.
4B).
 | Figure 4Effects of various TLR agonists on
TNF-α-induced IL-8 protein expression levels in SMGCs. (A) SMGCs
were stimulated with various concentrations of TNF-α. (B) SMGCs
were stimulated with TLR1-9 agonists and TNF-α, administered alone
or in combination. Cells were cultured, then exposed to 1 μg/ml
Pam3CSK4, 1 μg/ml poly I:C, 10 μg/ml E. coli LPS, 100 ng/ml
flagellin, 500 ng/ml MALP-2, 10 μg/ml imiquimod, 10 μg/ml CpG-ODN
or GpC (negative ODN) as a negative control to CpG-ODN. An
additional 10 ng/ml TNF-α was added to half the cells in each
treatment group, and the cells were incubated for 48 h, subsequent
to which the levels of IL-8 in the culture supernatants were
measured by ELISA. Data are shown as the mean ± standard deviation
of three independent experiments. *P<0.05 compared
with non-treated cells (Student’s t-test). #P<0.05
compared with TNF-α alone (Student’s t-test). $P<0.05
compared with TLR agonists alone (Student’s t-test). TLR, Toll-like
receptor; SMGCs, submandibular gland epithelial cells; TNF, tumor
necrosis factor; LPS, lipopolysaccharide; MALP,
macrophage-activating lipopeptide; ODN, oligodeoxynucleotide. |
Discussion
TLRs are essential in the activation of innate and
adaptive immune responses in salivary gland inflammation (12). mRNA expression of various TLR
family members has been reported in whole human salivary gland
tissues containing heterogeneous cell populations (21,22).
In addition, certain studies have shown that salivary gland
epithelial cells (SGECs) constitutively express particular TLRs.
SGECs derived from SS patients and non-neoplastic SGECs in labial
minor salivary glands were observed to exhibit marked constitutive
expression of TLR1, 2, 3 and 4 mRNA (12). Furthermore, TLR1-5 were found to be
expressed in salivary gland adenocarcinoma cells (23). SMGCs derived from minor salivary
glands were determined to express TLR2, 3 and 7, but not TLR9
(24). In another study, TLR7 and
TLR9 were detected in ductal epithelial cells in parotid gland
biopsy specimens obtained from SS patients and control subjects
(25). In the present study, SMGCs
and whole submandibular gland tissue specimens were shown to
constitutively express TLR1-10 mRNA. Furthermore, imiquimod (TLR7
agonist) and CPG-ODN (TLR9 agonist) were found to exert no effect
on IL-8 induction in SMGCs. It is unknown whether TLR7 and TLR9 in
SMGCs function to recognise PAMPs. Therefore, the roles of TLR7 and
TLR9 in SGECs may remain controversial.
Stimulation of TLR signaling results in the
production and expression of inflammatory mediators, including
IL-6, IL-8 and TNF-α (1–3), which are critically involved in the
disease processes of human inflammatory disorders. Local TLR
expression has been reported in SS and autoimmune sialadenitis
(12,13). Peptidoglycan (PGN; TLR2 agonist),
poly I:C (TLR3 agonist) and LPS (TLR4 agonist) were found to
increase CD54 and IL-6 expression levels in labial salivary gland
cells (26). Furthermore,
flagellin (TLR5 agonist) led to production of IL-6 and IL-8 in
salivary gland adenocarcinoma samples, although Pam3 (TLR1/2
agonist) and LPS (TLR4 agonist) did not exert an effect on cytokine
production (23). Poly I:C (TLR3
agonist) treatment was shown to increase interferon and
inflammatory cytokine expression levels in mice salivary gland
cells (27). In the present study,
IL-8 production from SMGCs was found to be induced by TLR1/2, 3, 4,
5 and 2/6 agonists, of which poly I:C, a TLR3 agonist, markedly
induced the production of IL-8. Thus, the presence of TLR3 suggests
a role of the submandibular gland cells in the antiviral
response.
Pro-inflammatory cytokines, such as TNF-α, have been
reported to be associated with salivary gland loss of function and
destruction. SGECs in SS biopsy samples were observed to produce
TNF-α mRNA in greater quantities than SGECs from individuals with
histologically normal minor salivary glands (15). Also, TNF-α secreted by infiltrating
lymphocytes induced ductal Fas expression and ductal apoptosis in
sialoadenitis associated with SS (28). The present study demonstrated that
the addition of TLR1/2, 3, 4, 5 and 2/6 agonists resulted in an
increase in TNF-α-induced IL-8 production in SMGCs. IL-8 is
important for neutrophil activation and recruitment, and undue
downregulation of this function may compromise the antimicrobial
defense. However, an unduly vigorous or sustained IL-8 response may
result in chronic inflammatory tissue destruction (29). Certain investigators have reported
that increases in various TLR expression levels were identified in
SGECs in the minor salivary gland of patients with SS, as compared
with control subjects (12,26).
Therefore, pro-inflammatory cytokines, including IL-8, induced by
various PAMPs via TLRs may be implicated in the development of
TNF-α-mediated autoimmune inflammatory disease of the submandibular
glands.
With the majority of TLR agonists, the signaling
pathways are mainly mediated by the activation of NF-κB and
mitogen-activated protein kinase (MAPK), although cell-surface TLR4
and intracellular TLRs (TLR3, 7, 8 and 9) also activate cells via
interferon regulatory factor (IRF)-3 and/or IRF-7 (30). It has been reported that PGN, poly
(I:C) and LPS also induced activation of the NF-κB and p38 MAPK
pathways in SGECs in the minor salivary gland (26,31),
while TNF-α activates nuclear factor-κB, which is involved in
inflammatory cytokine signaling pathways, such as IL-8 and IL-6 via
MAPK (32,33). In the present study, TNF-α-induced
IL-8 production was enhanced by almost all TLR ligands, which may
be explained by cooperative signaling among TNF-α and TLR agonists
to induce IL-8 in SMGCs.
In conclusion, the results of the present study
demonstrated that SMGCs expressed TLR1-10 mRNA, that IL-8
production from SMGCs was induced by treatment with various TLR
agonists and that the TLR agonists regulated TNF-α-induced IL-8
production. This suggests that innate immune responses against
microbial components result in the development of TNF-α-mediated
autoimmune inflammatory disease in the submandibular glands.
Acknowledgements
This study was supported by a Grant-in-Aid of
scientific research from the Japan Society for Young Scientists (B)
from the Ministry of Education, Culture, Sports, Science and
Technology of Japan (grant no. 21109871).
References
|
1
|
Takeda K, Kaisho T and Akira S: Toll-like
receptors. Annu Rev Immunol. 21:335–376. 2003. View Article : Google Scholar
|
|
2
|
Midwood KS, Piccinini AM and Sacre S:
Targeting Toll-like receptors in autoimmunity. Curr Drug Targets.
10:1139–1155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar H, Kawai T and Akira S: Toll-like
receptors and innate immunity. Biochem Biophys Res Commun.
388:621–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han DC, Huang GT, Lin LM, et al:
Expression of MHC Class II, CD70, CD80, CD86 and pro-inflammatory
cytokines is differentially regulated in oral epithelial cells
following bacterial challenge. Oral Microbiol Immunol. 18:350–358.
2003. View Article : Google Scholar
|
|
5
|
Kawai T, Takeuchi O, Fujita T, et al:
Lipopolysaccharide stimulates the MyD88-independent pathway and
results in activation of IFN-regulatory factor 3 and the expression
of a subset of lipopolysaccharide-inducible genes. J Immunol.
167:5887–5894. 2001. View Article : Google Scholar
|
|
6
|
Anders HJ, Vielhauer V, Eis V, et al:
Activation of toll-like receptor-9 induces progression of renal
disease in MRL-Fas (lpr) mice. FASEB J. 18:534–536. 2004.PubMed/NCBI
|
|
7
|
Kobayashi Y, Murakami H, Akbar SM, Matsui
H and Onji M: A novel and effective approach of developing
aggressive experimental autoimmune gastritis in neonatal
thymectomized BALB/c mouse by polyinosinic:polycytidylic acid. Clin
Exp Immunol. 136:423–431. 2004. View Article : Google Scholar
|
|
8
|
Qu WM, Miyazaki T, Terada M, et al: A
novel autoimmune pancreatitis model in MRL mice treated with
polyinosinic:polycytidylic acid. Clin Exp Immunol. 129:27–34. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Birchler T, Seibl R, Büchner K, et al:
Human Toll-like receptor 2 mediates induction of the antimicrobial
peptide human beta-defensin 2 in response to bacterial lipoprotein.
Eur J Immunol. 31:3131–3137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Böcker U, Yezerskyy O, Feick P, et al:
Responsiveness of intestinal epithelial cell lines to
lipopolysaccharide is correlated with Toll-like receptor 4 but not
Toll-like receptor 2 or CD14 expression. Int J Colorectal Dis.
18:25–32. 2003.
|
|
11
|
Hertz CJ, Wu Q, Porter EM, et al:
Activation of Toll-like receptor 2 on human tracheobronchial
epithelial cells induces the antimicrobial peptide human beta
defensin-2. J Immunol. 171:6820–6826. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spachidou MP, Bourazopoulou E, Maratheftis
CI, et al: Expression of functional Toll-like receptors by salivary
gland epithelial cells: increased mRNA expression in cells derived
from patients with primary Sjögren’s syndrome. Clin Exp Immunol.
147:497–503. 2007.PubMed/NCBI
|
|
13
|
Shimizu S, Kurashige Y, Nishimura M, et
al: Involvement of toll-like receptors in autoimmune sialoadenitis
of the non-obese diabetic mouse. J Oral Pathol Med. 41:517–523.
2012.PubMed/NCBI
|
|
14
|
Roescher N, Tak PP and Illei GG: Cytokines
in Sjögren’s syndrome. Oral Dis. 15:519–526. 2009.
|
|
15
|
Fox RI, Kang HI, Ando D, Abrams J and Pisa
E: Cytokine mRNA expression in salivary gland biopsies of Sjögren’s
syndrome. J Immunol. 152:5532–5539. 1994.
|
|
16
|
Cuello C, Palladinetti P, Tedla N, et al:
Chemokine expression and leucocyte infiltration in Sjögren’s
syndrome. Br J Rheumatol. 37:779–783. 1998.
|
|
17
|
Pflugfelder SC, Jones D, Ji Z, Afonso A
and Monroy D: Altered cytokine balance in the tear fluid and
conjunctiva of patients with Sjögren’s syndrome
keratoconjunctivitis sicca. Curr Eye Res. 19:201–211.
1999.PubMed/NCBI
|
|
18
|
Ohta K, Shigeishi H, Taki M, et al:
Regulation of CXCL9/10/11 in human oral keratinocytes and
fibroblasts. J Dent Res. 87:1160–1165. 2008. View Article : Google Scholar
|
|
19
|
Dimitriou ID, Kapsogeorgou EK, Abu-Helu
RF, Moutsopoulos HM and Manoussakis MN: Establishment of a
convenient system for the long-term culture and study of
non-neoplastic human salivary gland epithelial cells. Eur J Oral
Sci. 110:21–30. 2002. View Article : Google Scholar
|
|
20
|
Szlávik V, Szabó B, Vicsek T, et al:
Differentiation of primary human submandibular gland cells cultured
on basement membrane extract. Tissue Eng Part A. 14:1915–1926.
2008.PubMed/NCBI
|
|
21
|
Nishimura M and Naito S: Tissue-specific
mRNA expression profiles of human toll-like receptors and related
genes. Biol Pharm Bull. 28:886–892. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zarember KA and Godowski PJ: Tissue
expression of human Toll-like receptors and differential regulation
of Toll-like receptor mRNAs in leukocytes in response to microbes,
their products, and cytokines. J Immunol. 168:554–561. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JH, Yoon HE, Kim DJ, et al: Toll-like
receptor 5 activation promotes migration and invasion of salivary
gland adenocarcinoma. J Oral Pathol Med. 40:187–193. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ittah M, Miceli-Richard C, Gottenberg JE,
et al: Viruses induce high expression of BAFF by salivary gland
epithelial cells through TLR- and type-I IFN-dependent and
-independent pathways. Eur J Immunol. 38:1058–1064. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng L, Zhang Z, Yu C and Yang C:
Expression of Toll-like receptors 7, 8, and 9 in primary Sjögren’s
syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
109:844–850. 2010.PubMed/NCBI
|
|
26
|
Kawakami A, Nakashima K, Tamai M, et al:
Toll-like receptor in salivary glands from patients with Sjögren’s
syndrome: functional analysis by human salivary gland cell line. J
Rheumatol. 34:1019–1026. 2007.
|
|
27
|
Deshmukh US, Nandula SR, Thimmalapura PR,
Scindia YM and Bagavant H: Activation of innate immune responses
through Toll-like receptor 3 causes a rapid loss of salivary gland
function. J Oral Pathol Med. 38:42–47. 2009. View Article : Google Scholar
|
|
28
|
Matsumura R, Umemiya K, Goto T, et al:
Interferon gamma and tumor necrosis factor alpha induce Fas
expression and anti-Fas mediated apoptosis in a salivary ductal
cell line. Clin Exp Rheumatol. 18:311–318. 2000.PubMed/NCBI
|
|
29
|
Hajnická V, Kocáková P, Sláviková M, et
al: Anti-interleukin-8 activity of tick salivary gland extracts.
Parasite Immunol. 23:483–489. 2001.PubMed/NCBI
|
|
30
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar
|
|
31
|
Ittah M, Miceli-Richard C, Gottenberg JE,
et al: B-cell-activating factor expressions in salivary epithelial
cells after dsRNA virus infection depends on RNA-activated protein
kinase activation. Eur J Immunol. 39:1271–1279. 2009. View Article : Google Scholar
|
|
32
|
Lisi S, Sisto M, Lofrumento DD and D’Amore
M: Sjögren’s syndrome autoantibodies provoke changes in gene
expression profiles of inflammatory cytokines triggering a pathway
involving TACE/NF-κB. Lab Invest. 92:615–624. 2012.
|
|
33
|
Li J, Kartha S, Iasvovskaia S, et al:
Regulation of human airway epithelial cell IL-8 expression by MAP
kinases. Am J Physiol Lung Cell Mol Physiol. 283:L690–L699.
2002.PubMed/NCBI
|