Introduction
As an aggressive malignant disease, pancreatic
cancer (PC) is the fourth leading cause of cancer mortality
worldwide (1). Despite a number of
in-depth studies on PC conducted in the past few decades, the
mortality rate of PC patients is nearly equal to its incidence
(2). Since traditional
chemotherapy and radiotherapy are ineffective on this malignancy,
the 5-year survival rate of PC patients remains <5%, and the
survival time following diagnosis is no more than one year in most
patients (1,3).
In recent years, research on the molecular
mechanisms underlying PC has led to the development of new
treatment strategies, such as the inhibition of relevant signaling
pathways, gene therapy and immunotherapy. Proteins of the Hedgehog
(Hh) signaling pathway, an important regulator of human embryonic
development, are highly expressed in pancreatic cancer tissues or
cells, and their expression appears to correlate to the occurrence,
development and biological behavior of tumor cells (4). Hh signaling activation is a common
event in pancreatic cancer (5).
The Hh protein functions by binding to a 12-transmembrane domain
receptor called patched 1 (Ptch1). The binding of Hh to Ptch1
allows Ptch1 to inhibit smoothened (SMO), a 7-transmembrane domain
protein. The activated SMO then relocalizes to the primary cilia,
and initiates an intracellular signaling cascade that eventually
leads to the activation of the Gli-1 transcription factor and the
upregulation of the downstream target genes, which include Ptch1
(6,7).
Resveratrol (Res) is a polyphenolic compound
(trans-3,5,4′-tri-hydroxystilbene) found in grapes and 72
additional species (belonging to 12 families, 32 genera). Its
highest content is reported in the fresh root of the traditional
Chinese medical plant Polygonum cuspidatum (8). Previous studies have shown that
resveratrol has numerous biological properties, including
anti-inflammatory, antioxidant and antitumorigenic effects
(9–11). Our recent study also showed that
Res can suppress PC cell migration, invasion, and the progression
of the epithelial-to-mesenchymal transition through the inhibition
of the PI-3K/Akt/NF-κB signaling pathway (12).
The present study investigated whether Res affects
the proliferation and apoptosis of human MIA PaCa-2 PC cells, and
the expression of proteins of the Hh signaling pathway. This study
may provide a new therapeutic strategy for PC, as well as an
experimental basis for the clinical application of Res.
Materials and methods
Reagents
The human PC cell line MIA PaCa-2 was obtained from
the Peking Union Medical College (Beijing, China). Dulbecco’s
modified Eagle’s medium (DMEM), trypsin and fetal bovine serum
(FBS) were from Hyclone™ (Thermo Fisher Scientific, Waltham, MA,
USA). Res (purity >99.9%), which was obtained from Sigma-Aldrich
(St. Louis, MO, USA), was dissolved in dimethyl sulfoxide (DMSO) to
obtain a 400 μmol/l stock solution. 5-Fluorouracil (5-Fu) was
purchased from Xudong Haipu Pharmaceutical Co., Ltd. (Shanghai,
China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) was purchased from Sigma-Aldrich. The Annexin V-FITC
apoptosis kit was purchased from Zhu hai Jian Kang yuan Co.
(Zhuhai, China). The primary antibodies against Indian hedgehog
(Ihh), Ptch and Smo were obtained from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). The primers were designed and
synthesized by Takara Bio (Dalian, China).
Cell culture and groups
The human PC cell line MIA PaCa-2 was cultured in
DMEM containing 10% dialyzed heat-inactivated FBS, 100 U/ml
penicillin and 100 μg/ml streptomycin in a humidified atmosphere
(95% relative humidity) of 5% CO2 at 37°C. Cells at
70–80% confluence were digested and passaged with 2.5 g/l trypsin.
Cells at the logarithmic growth phase were used in all the
experiments.
Cells were divided into five groups: high
concentration Res (200 μmol/l), medium concentration Res (100
μmol/l), low concentration Res (50 μmol/l), the positive control
group (0.75 mg/ml 5-Fu), and the negative control group (0 μmol/l
Res in DMEM-10% FBS).
MTT assay
Cells were seeded in a 96-well plate at a
1×103 cells/well density, and kept at 37°C overnight.
When the cells had reached 70% confluence, they were divided into
five groups, each including 5 wells, in a final volume of 100 μl.
After 24, 48 and 72 h, respectively, 20 μl of MTT solution (5 g/l)
were added to each well. Following an additional 4-h incubation,
the medium was removed, and 150 μl of DMSO were added to each well,
followed by 10 min shaking. Finally, the optical density (OD) was
measured at 490 nm on a spectra microplate multilabel counter
reader (Victor 2-1420-015; Perkin Elmer, Waltham, MA, USA). The
cell inhibition rate (%) was calculated as = (ODcontrol
- ODsample)/(ODcontrol) × 100.
Colony formation assay
Cells were digested into a single cell suspension,
and 200 cells were seeded in a 24-well plate. Following adherence,
the cells were divided into five groups, with 4 wells in each
group, and were incubated at 37°C in a humidified atmosphere
containing 5% CO2 for 6 days. Cell colonies were then
stained with crystal violet (Sigma-Aldrich), and were manually
counted under a XDS-1B microscope (Nikon, Tokyo, Japan). Only
colonies containing >50 cells were counted. The colony formation
rate (%) was calculated as = (median colony number/number of seeded
cells) × 100.
Annexin V-propidium iodide (PI)
assay
Cells were inoculated in 25-ml flasks and were
divided into five groups following adherence. The cells were
harvested following exposure to Res, 5-Fu and DMEM for 24 h. The
cells were then centrifuged at 1,500 × g for 5 min and collected,
followed by resuspension in binding buffer consisting of 10 mmol/l
HEPES-NaOH (pH 7.4), 140 mmol/l NaCl and 2.5 mmol/l
CaCl2. The samples were then incubated with 5 μl Annexin
V in the dark for 10 min, washed with binding buffer, and
resuspended in binding buffer supplemented with 1% formaldehyde, at
40°C for 30 min. After an additional wash in binding buffer, the
cells were stained with 500 μl PI for 15 min, and were then
analyzed by flow cytometry (BD Biosciences, San Jose, CA, USA). The
percentage of apoptotic cells was determined using the CellQuest
software (BD Biosciences). Early apoptotic cells were identified as
cells that were PI-negative and Annexin V-positive.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the PC cells using the
Fastgen200 RNA isolation system (Voerson Technology Co, Xi’an,
Shaanxi, China) according to the manufacturer’s protocol. Total RNA
was reverse transcribed into cDNA using the RevertAid™ First Strand
cDNA Synthesis kit (MBI Fermentas Inc., Burlington, ON, Canada).
The primer sequences were as follows: Ihh forward (F), 5′-TCG CCC
TGT GGA TGA CTG AG-3′, and reverse (R), 5′-CAG AGT CTT CAG AGA CAG
CCA GGA-3′; Smo F, 5′-TCA GGA TGC GTC CAC CAA GAA G-3′, and R,
5′-TGT GTC CAC GGC GGC AAT C-3′; Ptch F, 5′-TGC GTG TGG AGT ATT TGG
ATG AC-3′, and R, 5′-CAG TGT GAT GAT GGT GAG GAT GG-3′; β-actin F,
5′-GAC TTA GTT GCG TTA CAC CCT TTC T-3′, and R, 5′-GAA CGG TGA AGG
TGA CAG CAG T-3′.
The PCR amplification was performed using the CFX
Manager 2.1 fluorescent quantitative PCR kit (Bio-Rad Laboratories,
Hercules, CA, USA), under the following conditions: 30 sec at 95°C,
followed by 40 cycles at 95°C for 5 sec, at 60°C for 30 sec, and at
72°C for 30 sec. Following qPCR, a dissociation curve analysis was
conducted. Relative gene expression was calculated using the
2−ΔΔCt method (13).
Each measurement was performed in triplicate.
Western blot analysis
Proteins were electrophoretically resolved on a
denaturing sodium dodecyl sulfate polyacrylamide gel and
electrotransferred onto nitrocellulose membranes. The membranes
were initially blocked with 5% nonfat dry milk in Tris-buffered
saline for 2 h, and then incubated with antibodies targeting Ihh,
Ptch, Smo or β-actin. Following incubation with the primary
antibodies at 4°C overnight, the membranes were hybridized with the
horseradish peroxidase-conjugated polyclonal mouse anti-human IgG
secondary antibody (1:2,000; Pierce, Rockford, IL, USA). The
positive bands were visualized with an enhanced chemiluminescence
system (Millipore, Billerica, MA, USA) according to the
manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using the SPSS
software version 12.0 (SPSS Inc., Chicago, IL, USA). Differences
between two groups were analyzed by two-tailed Student’s t-tests,
and among three or more groups by a one-way analysis of variance
(ANOVA). Values were expressed as mean ± SD, and P<0.05 was
considered to indicate statistically significant differences.
Results
Effect of 5-Fu on the proliferation of
MIA PaCa-2PC cells
The cytotoxicity of 5-Fu was first determined using
the MTT assay. MIA PaCa-2 cells were treated with various
concentrations (0, 0.25, 0.5, 0.75 and 1.0 mg/ml) of 5-Fu for 24,
48 and 72 h. The results demonstrated that the proliferative
ability of MIA PaCa-2 cells is decreased in response to 5-Fu
treatment in a time- and dose-dependent manner. The half maximal
inhibitory concentration (IC50) for MIA PaCa-2 cells was
~0.75 mg/ml of 5-Fu (Fig. 1), and
this concentration exhibited no additional cytotoxic effects on the
MIA PaCa-2 cells (data not shown). Therefore, 0.75 mg/ml 5-Fu was
used for the subsequent experiments.
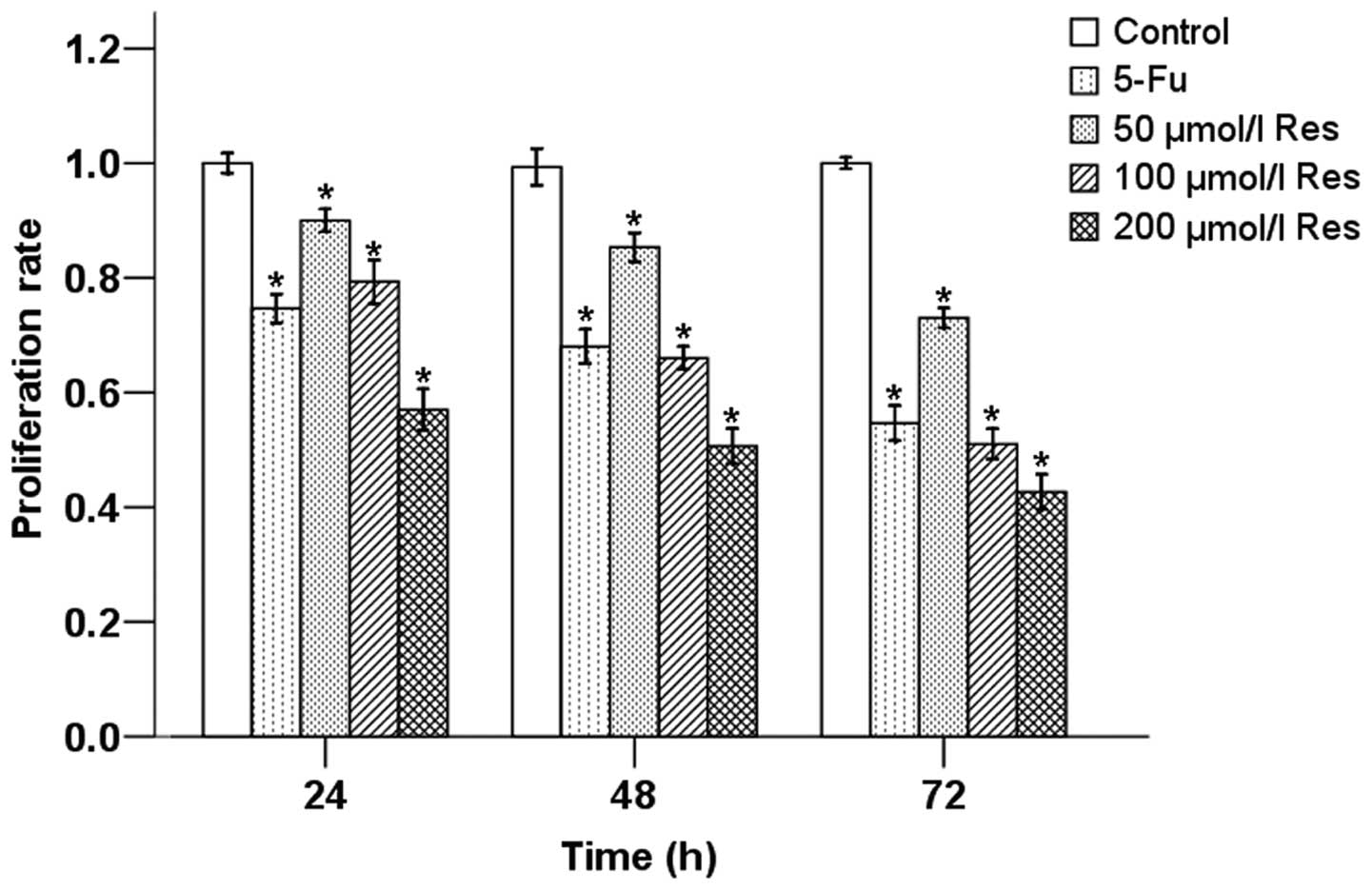
Effect of Res on the proliferation of
pancreatic cancer cells
We next treated MIA PaCa-2 cells with different
concentrations of Res (50, 100 and 200 μmol/l). As shown in
Fig. 2, cell proliferation was
inhibited by the administration of Res in a dose- and
time-dependent manners.
In addition, the number of cell colonies in the
three Res treatment groups and the 5-Fu group was significantly
decreased as compared to the negative control group (Fig. 3). The colonies in the high
concentration Res group were fewer than those in the other Res
groups. The colony number was gradually reduced with the increasing
Res concentration, indicating that Res inhibits the proliferation
of PC cells in a concentration-dependent manner.
Effects of Res and 5-Fu on apoptosis of
cancer cells
Cell apoptosis was assessed by the Annexin V-FITC
and PI double staining method. The results are shown in Fig. 4. The apoptotic rate was
significantly increased in cells treated with 5-Fu and Res, and the
number of apoptotic cells increased with the increasing
concentrations of Res. This result suggested that Res plays an
important role in promoting apoptosis in MIA PaCa-2 PC cells, and
in a concentration-dependent manner.
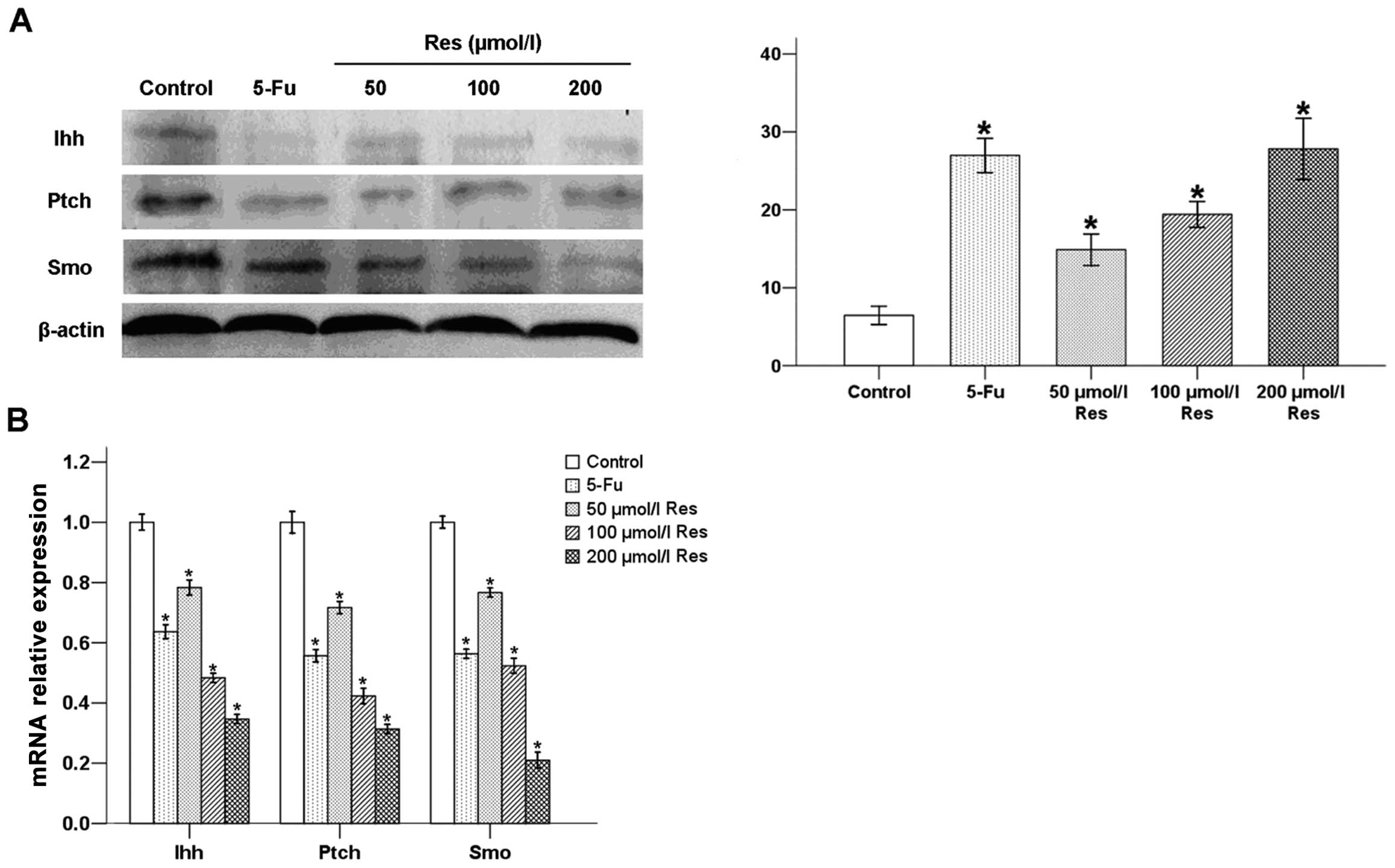
Effects of Res on the protein and mRNA
expression of Ihh, Ptch and Smo
After cells were treated with Res and 5-Fu for 24 h,
the protein and mRNA levels of Ihh, Ptch and Smo were measured by
western blot analysis (Fig. 5A)
and RT-qPCR (Fig. 5B). Compared to
the negative control group, the expression of Ihh, Ptch and Smo was
decreased in all Res groups and the 5-Fu group, at both the protein
and mRNA levels. The effects of Res were dose-dependent.
Discussion
The pathogenesis of pancreatic cancer remains
unknown. Its progresses rapidly with a poor prognosis and a high
mortality rate. It is often diagnosed at a late stage and the
outcome of surgery is commonly unsatisfactory. Currently, the
standard treatments for PC are radiotherapy and chemotherapy, and
there are no effective drugs for this disease (3). A new comprehensive and effective
therapy is thus urgently needed.
Res, or trans-3,5,4-trihydroxy-stilbene
(C14H12O3) has a relative
molecular mass of 228.25, and contains non-flavonoid polyphenol
compounds in its stilbene structure. It has been reported that Res
plays an antagonistic role in numerous tumors, including
gastrointestinal cancer (14,15).
Recently, Yang et al (16)
showed that Res inhibits the growth of gastric cancer cells by
inducing G1 phase arrest and senescence in a Sirt1-dependent
manner. Ji et al (17)
showed that Res inhibits invasion and metastasis of colorectal
cancer cells via MALAT1-mediated inhibition of the Wnt/β-catenin
signaling pathway. A previous study by our group further
demonstrated that Res suppresses PC migration and invasion through
the inhibition of the PI-3K/Akt/NF-κB signaling pathway (12). Based on the above, Res may
constitute a promising new anticancer drug. This study focused on
the inhibitory effect of Res on PC cell growth, as well as on its
effects on the expression of the Hh pathway proteins. We aimed to
clarify the antipancreatic mechanism of Res and further provide
experimental evidence for its clinical application.
The occurrence of tumor is induced by an impairment
in the dynamic equilibrium between cell proliferation and
apoptosis. In this study, following treatment of the human MIA
PaCa-2 cell line with Res at three different concentrations, cell
proliferation was measured by the MTT assay, and cell apoptosis was
detected by flow cytometry. The results showed that Res can inhibit
cell proliferation in a time- and dose-dependent manner, and that
the inhibitory effects of different concentrations of Res are
significantly different. In addition, Res significantly promoted
cell apoptosis. The rate of apoptosis increased with the
resveratrol concentration. In general, the results of our
experiments confirm that Res inhibits the growth of MIA PaCa-2 PC
cells in vitro, by decreasing cell proliferation rates and
promoting cell apoptosis.
The imbalance between cell proliferation and
apoptosis is often due to a genetic defect in cellular control
mechanisms. This imbalance may be induced by the mutation of
specific genes, and mutations in signal transduction genes are of
key importance in this context. The Hh signal transduction pathway
is closely associated with the occurrence and development of PC.
The Hh signaling pathway, which is quiescent in the adult healthy
pancreas, was shown to be active in pancreatic cancer and to relate
to cancer progression (18).
Abnormal expression of the Hh ligand or the downstream signaling
pathway proteins, as well as dysfunctions in these pathway
components, can induce tumorigenesis. The potential mechanisms
include mutations in the Smo gene, loss of function of Ptch,
etc. Yang et al (5)
identified 54 cases of PC using immunohistochemical methods, and
high expression of the proteins Ihh, Ptch and Smo was detected in
these cases. In the corresponding adjacent tissues, the proteins
were found expressed only in the islet cells; no expression was
observed in healthy pancreatic ductal tissues. Our experiments show
that the mRNA and protein expression of Ptch, Smo and Ihh are
significantly reduced upon Res treatment. Their expression levels
gradually decrease as the Res concentration increases, indicating
that Res influences the Ptch, Smo and Ihh expression in a
concentration-dependent manner. Res may thus block the Hh signaling
pathway in PC cells by downregulating the expression of Ihh, Ptch
and Smo.
In conclusion, Res inhibits the growth of human MIA
PaCa-2 cells in vitro by inhibiting cell proliferation and
promoting cell apoptosis. This effect may related to an inhibition
of the Hh signaling pathway through downregulation of Ihh, Ptch and
Smo at the mRNA and protein levels. Res treatment may thus be a
novel option for therapy of PC via the inhibition of the Hh
signaling pathway.
Acknowledgements
We greatly appreciate the technical assistance
offered by staff members of the Biology and Genetics Laboratory, at
the Xi’an Jiaotong University. This study was supported by grants
from the National Natural Science Foundation of China (nos.,
81172360 and 81301846).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Mancuso A, Calabro F and Sternberg CN:
Current therapies and advances in the treatment of pancreatic
cancer. Crit Rev Oncol Hematol. 58:231–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krug S and Michl P: New developments in
pancreatic cancer treatment. Minerva Gastroenterol Dietol.
58:427–443. 2012.PubMed/NCBI
|
|
4
|
Li X, Ma Q, Xu Q, Liu H, Lei J, Duan W,
Bhat K, Wang F, Wu E and Wang Z: SDF-1/CXCR4 signaling induces
pancreatic cancer cell invasion and epithelial-mesenchymal
transition in vitro through non-canonical activation of Hedgehog
pathway. Cancer Lett. 322:169–176. 2012. View Article : Google Scholar
|
|
5
|
Yang Y, Tian X, Xie X, Zhuang Y, Wu W and
Wang W: Expression and regulation of hedgehog signaling pathway in
pancreatic cancer. Langenbecks Arch Surg. 395:515–525. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dosch JS, Pasca di Magliano M and Simeone
DM: Pancreatic cancer and hedgehog pathway signaling: new insights.
Pancreatology. 10:151–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Villavicencio EH, Walterhouse DO and
Iannaccone PM: The sonic hedgehog-patched-gli pathway in human
development and disease. Am J Hum Genet. 67:1047–1054. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giovinazzo G, Ingrosso I, Paradiso A, De
Gara L and Santino A: Resveratrol biosynthesis: plant metabolic
engineering for nutritional improvement of food. Plant Foods Hum
Nutr. 67:191–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fulda S: Resveratrol and derivatives for
the prevention and treatment of cancer. Drug Discov Today.
15:757–765. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jha RK, Ma Q, Sha H and Palikhe M:
Emerging role of resveratrol in the treatment of severe acute
pancreatitis. Front Biosci (Schol Ed). 2:168–175. 2010.PubMed/NCBI
|
|
11
|
Pervaiz S and Holme AL: Resveratrol: its
biologic targets and functional activity. Antioxid Redox Signal.
11:2851–2897. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li W, Ma J, Ma Q, Li B, Han L, Liu J, Xu
Q, Duan W, Yu S, Wang F and Wu E: Resveratrol inhibits the
epithelial-mesenchymal transition of pancreatic cancer cells via
suppression of the PI-3K/Akt/NF-κB pathway. Curr Med Chem.
20:4185–4194. 2013.PubMed/NCBI
|
|
13
|
Livak KJ1 and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh CK, George J and Ahmad N:
Resveratrol-based combinatorial strategies for cancer management.
Ann N Y Acad Sci. 1290:113–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aluyen JK, Ton QN, Tran T, Yang AE,
Gottlieb HB and Bellanger RA: Resveratrol: potential as anticancer
agent. J Diet Suppl. 9:45–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Q, Wang B, Zang W, Wang X, Liu Z, Li
W and Jia J: Resveratrol inhibits the growth of gastric cancer by
inducing G1 phase arrest and senescence in a sirt1-dependent
manner. PLoS One. 8:e706272013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L,
Sun J, Cai J, Qin J, Ren J and Li Q: Resveratrol inhibits invasion
and metastasis of colorectal cancer cells via MALAT1 mediated
Wnt/β-catenin signal pathway. PLoS One. 8:e787002013.PubMed/NCBI
|
|
18
|
Olive KP, Jacobetz MA, Davidson CJ,
Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA,
Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C,
Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara
M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K,
Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH,
Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J
and Tuveson DA: Inhibition of Hedgehog signaling enhances delivery
of chemotherapy in a mouse model of pancreatic cancer. Science.
324:1457–14611. 2009. View Article : Google Scholar : PubMed/NCBI
|