Introduction
Hepatocellular carcinoma (HCC) is a predominant
cause of cancer-associated mortality in numerous countries,
particularly in Central and West Africa, and East and Southeast
Asia (1). The disease is commonly
diagnosed at an advanced stage and recurrence rates are high,
typically 30–40% within five years (2). Patients with advanced HCC have a
median survival time of 6–8 months and treatment brings few
benefits for these patients (2).
Conventional chemotherapeutic and radiotherapeutic treatments are
not effective in HCC (3).
Recently, patients with advanced HCC have been treated with a
comprehensive series of vascular interventional therapeutic agents,
but median life expectancies have not been markedly prolonged.
Thus, novel therapeutic strategies are required to improve the
clinical outcomes for HCC patients. Certain Chinese Traditional
Medicines were found to be effective in cancer therapy; drugs
including bufalin, berberine and tetrandrine were reported to
inhibit the proliferation and induce apoptosis in HCC cells
(4–6).
Puerarin,
7-hydroxy-3-(4-hydroxyphenyl)-1-benzopyran-4-one
8-β-d-glucopyranoside (C12H20C9),
is a predominant iso-flavonoid compound extracted from the Chinese
medicinal herb Radix Puerariae. This compound has been suggested to
be useful in the management of various disorders, including
endothelial dysfunction, liver fibrosis, neurotoxicity and bone
injury (7–10). Recently, the anticancer properties
of puerarin have attracted attention; one study suggested that
Pueraria mirifica possesses an estrogenic effect and may
inhibit the growth of breast cancer cells at high concentrations,
similar to other flavonoids (11).
Another two studies reported that pueraria induced apoptosis in
HT-29 colon cancer cells, and that a novel puerarin nanosuspension
exhibited anticancer activity against colon cancer in vitro
and in vivo, with high efficacy and low toxicity (12,13).
However, the anticancer effect of puerarin in HCC has, to the best
of our knowledge, not been analyzed. Thus, the anticancer effects
of puerarin were determined in the SMMC-7721 HCC cell line.
Materials and methods
Cell line
The SMMC-7721 human HCC cell line was purchased from
the the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China) and cultured in RPMI 1640 medium (Gibco, Carlsbad, CA, USA)
containing 10% fetal bovine serum (Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd., Hangzhou, China) and ampicillin
and streptomycin (Beyotime, Shanghai, China) at 37°C in a
humidified atmosphere of 95% air and 5% CO2.
Cell viability assay
The standard MTT assay was used to assess cell
viability. Briefly, cells (5×103 cells/well) were seeded
in 96-well microtiter plates. Following exposure to various
concentrations of puerarin for 48 h (99% pure; Sigma-Aldrich, St.
Louis, MO, USA), 50 ml MTT solution [2 mg/ml in phosphate-buffered
saline (PBS); Sigma-Aldrich] was added to each well and the plates
were incubated for additional 4 h at 37°C. The MTT solution in the
medium was aspirated off. To solubilize the formazan crystals
formed in viable cells, 200 ml dimethylsulfoxide (DMSO) was added
to each well. The absorbance was measured at 490 nm on a automatic
microwell plate reader, using DMSO alone as a blank. All assays
were performed in quintuplicate and repeated at least three
times.
Cell apoptosis analysis
The cells were seeded at a density of
5×105 cells per well in six-well plates, cultured
overnight, and then treated with 0, 500, 1,000 or 1,500 μg/ml
puerarin for either 12 or 24 h. The cells were then harvested and
washed with ice-cold PBS. An Annexin V-fluorescein isothiocyanate
apoptosis detection kit (KeyGEN Biotech, Nanjing, China) was used
to detect cell apoptosis, measured with a FACScan instrument
(Becton-Dickinson, Mountain View, CA, USA).
Hoechst staining
The cells were seeded on coverslips on a six-well
plate and treated with 0, 500, 1,000 or 1,500 μg/ml puerarin for 12
or 24 h. The attached cells were washed with PBS and fixed in
freshly prepared 4% paraformaldehyde for 30 min, then washed with
PBS and incubated with 10 μg/ml Hoechst 33258 staining solution
(Sigma-Aldrich) for 10 min. Following treatment, the cells were
washed with PBS and Antifade Mounting Medium (Beyotime) was added.
Apoptosis, indicated by condensed and fragmented nuclei, was
observed under a Leica DM 500B fluorescence microscope (Leica
Microsystems, Wetzlar, Germany).
Mitochondrial membrane potential
(MMP)
Rhodamine 123 dye (Rho-123; Sigma-Aldrich) was used
to detect the changes in the MMP. Cells (5×104
cells/well) were cultured in a 24-well plate. Following 24 h
exposure to various concentrations of puerarin (0, 500, 1,000 and
1,500 μg/ml), the cells were washed with PBS, incubated with 10
mg/ml Rho-123 and subsequently subjected to flow cytometric
analysis using a BD FACScan instrument (Becton Dickinson, Mountain
View, CA, USA).
Detection of reactive oxygen species
(ROS)
Detection of ROS was performed by flow cytometric
analysis as described previously (14). In brief, 5×104
cells/well were cultured in a 24-well plate. Following 12 h
exposure to puerarin (0, 500, 1,000 and 1,500 μg/ml), the cells
were washed with PBS and resuspended in complete medium followed by
incubation with 0.5 μM dihydrorhodamine 123 (Sigma-Aldrich) for 30
min at 37°C. ROS fluorescence intensity was determined by flow
cytometry with excitation at 490 nm and emission at 520 nm.
Quantitative polymerase chain reaction
(qPCR)
Cells were seeded at a density of 5×105
cells per well in six-well plates, cultured overnight and then
treated with 0, 30 (IC25), 500 (IC50) or
2,000 μg/ml (IC75) puerarin for 12 h. Total RNA from
drug-treated cells was isolated using TRIzol reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA). The reverse transcription
reaction was conducted using 2 μg total RNA with a first strand
cDNA kit (Takara Bio, Inc., Shiga, Japan), according to the
manufacturer’s instructions. PCR amplification was performed for 10
min at 95°C, followed by 40 cycles at 95°C for 15 sec and
annealing/extension at 60°C for 45 s in an ABI 7300 Thermocycler
(Applied Biosystems, Foster City, CA, USA), using the SYBR Premix
Ex Taq kit (Takara Bio, Inc.). The specific primer sequences for
each gene were as follows: Caspase-3, 5′-AACTGGACTGTGGCATTGAG-3′
and 5′-ACAAAGCGACTGGATGAACC-3′ (product size, 161 bp); caspase-8,
5′-CTGGGAGAAGGAAAGTTG-3′ and 5′-TTGGAGAGTCCGAGATTG-3′ (product
size, 184 bp); caspase-9, 5′-GGAAGAGGGACAGATGAATG-3′ and
5′-TTGTTTGGCACCACTCAG-3′ (product size, 242 bp); apoptosis-inducing
factor (AIF), 5′-GCTACAAGCACGCTCTAACATC-3′ and
5′-CAGCCAATCTTCCACTCACAAC-3′ (product size, 119 bp); GAPDH,
5′-CACCCACTCCTCCACCTTTG-3′ and 5′-CCACCACCCTGTTGCTGTAG-3′ (product
size, 110 bp). Data analysis was conducted using the
2−ΔΔCT method for relative quantification and all sample
expression levels were normalized to those of GAPDH, which served
as an endogenous control.
Western blot analysis
Cells were seeded at a density of 5×105
cells per well in six-well plates, cultured overnight and then
treated with 500 μg/ml puerarin for 1, 3 and 6 h. Cell lysates were
made with standard methods, then 20 μg protein samples were
separated by 10% SDS-PAGE, and transferred to polyvinylidene
fluoride membranes (PVDF; Roche Diagnostics, Manheim, Germany).
After blocking with a buffer containing 5% low fat milk and 0.1%
Tween-20 in Tris-buffered saline (TBST), the membrane was incubated
with mouse monoclonal anti-human primary antibodies against AKT1,
phosphorylated (p-)AKT, P38, p-P38, extracellular signal-regulated
kinase 1 (ERK1), p-RK1, c-Jun N-terminal kinase (JNK), p-JNK, AIF
and caspase-3,8 and 9 (Univ-Bio Inc., Shanghai, China) and then
incubated with secondary bovine anti-mouse IgG antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Finally, results were
photographed with an enhanced chemiluminescence substrate
(horseradish peroxidase, cat no. WBKLS0100; Millipore, Billerica,
MA, USA). Protein loading was estimated using mouse anti-GAPDH
monoclonal antibody. Lab Works Image Acquisition and Analysis
Software version 4.5 (UVP, Upland, CA, USA) was used to quantify
band intensities.
Statistical analysis
The SPSS 16.0 software system (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. Data are expressed as
the mean ± standard error. The differences between groups were
analyzed using Student’s t-test for two group comparisons or
one-way analysis of variance when more than two groups were
compared. All tests performed were two-sided. P<0.05 was
considered to indicate a statistically significant difference.
Results
SMMC-7721 cell growth following puerarin
treatment
The effects of 12, 24 and 48 h puerarin treatment on
the viability of SMMC-7721 cells were assessed by MTT assay. As
shown in Fig. 1, high
concentrations of puerarin (500, 1,000, 1,500 and 2,000 μg/ml)
inhibited the proliferation of SMMC-7721 cells.
 | Figure 1Puerarin inhibits the proliferation of
SMMC-7721 human hepatocellular carcinoma cells. The effects of
various concentrations of puerarin (0, 50, 100, 250, 500, 1,000,
1,500 and 2,000 μg/ml) on the viability of SMMC-7721 cells for 12,
24 and 48 h were assessed by MTT assay. Data are presented as the
mean. |
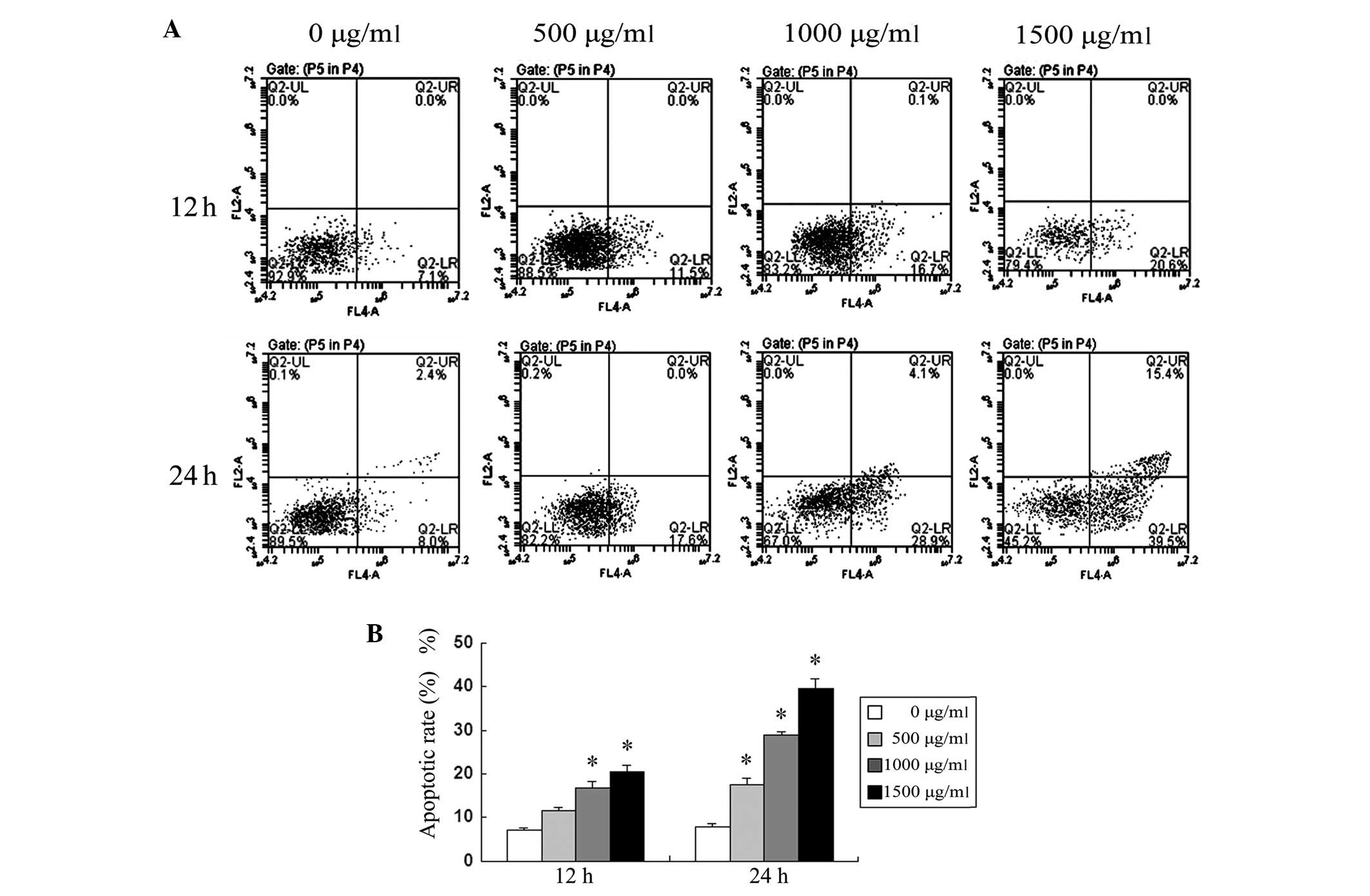
Apoptotic rate of SMMC-7721 cells treated
with puerarin
The treatment of SMMC-7721 cells with puerarin
resulted in a significant increase in apoptotic rates in a time-
and dose-dependent manner, quantified by Annexin V analysis
(Fig. 2). In concordance with the
cell viability rate results, depicted in Fig. 1, these results suggested that the
induction of apoptosis may be the main mechanism of the
antiproliferative effect of puerarin in SMMC-7721 cells.
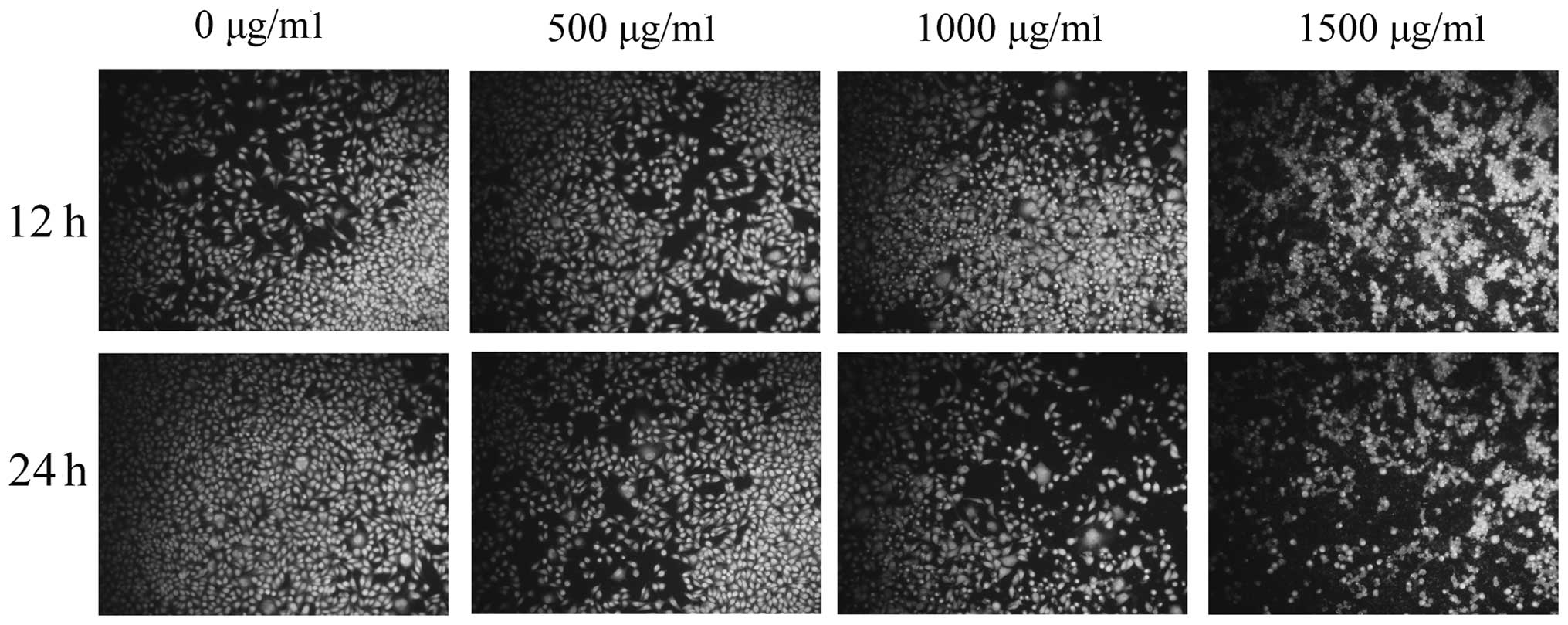
Morphological changes of SMMC-7721 cells
treated with puerarin
To verify puerarin-induced apoptosis, the
morphological changes in SMMC-7721 cells were observed using
Hoechst 33258 staining (Fig. 3).
Following puerarin treatment, the blue emission became markedly
brighter than that in the control cells, indicating a higher rate
of apoptosis. Condensed chromatin was also identified in a number
of puerarin-treated cells and apoptotic body-like structures were
formed in the treated cells.
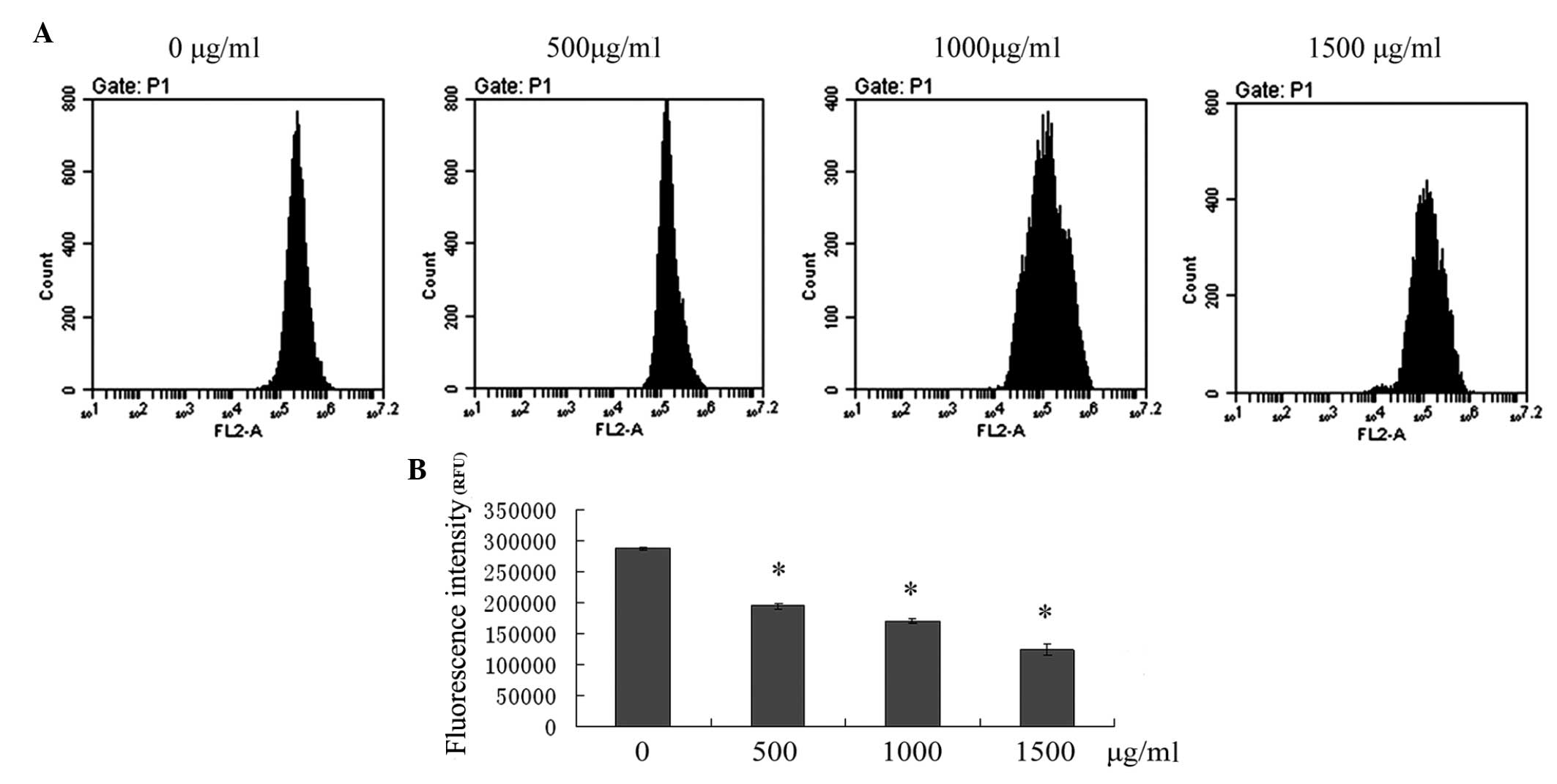
Induction of apoptosis via the
mitochondrial pathway following puerarin treatment
The loss of MMP is associated with activation of the
mitochondrial apoptotic pathway. To assess the effect of puerarin
on the changes of MMP in SMMC-7721 cells, flow cytometric analysis
was performed to detect the fluorescence intensity of Rho-123. As
shown in Fig. 4, treatment of
SMMC-7721 cells with 500, 1,000 and 1,500 μg/ml puerarin for 24 h
resulted in a significant depolarization of MMP in a dose-dependent
manner (P<0.05).
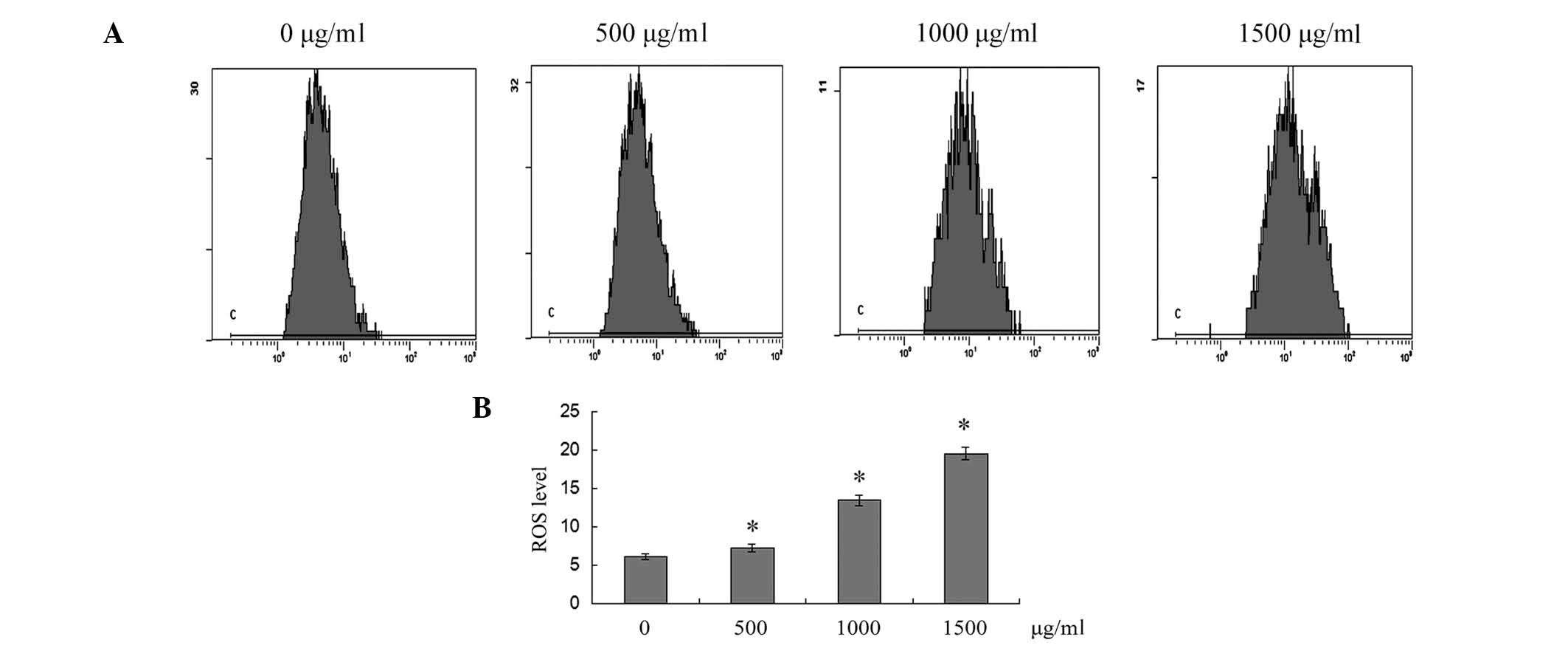
ROS generation is also associated with mitochondria.
The dichloro-dihydro-fluorescein diacetate fluorescence probe was
used to determine the levels of ROS production in SMMC-7721 cells.
As shown in Fig. 5, cells exposed
to 500, 1,000 and 1,500 μg/ml puerarin for 12 h exhibited a
significant increase in the intracellular accumulation of ROS in a
dose-dependent manner (P<0.05).
Expression levels of apoptosis-associated
genes
To clarify the mechanism of SMMC-7721 cell apoptosis
induced by puerarin, the mRNA expression levels of
apoptosis-associated molecules were determined by qPCR. As shown in
Fig. 6A, the expression levels of
caspase-3,8,9 and AIF mRNA, particularly those of caspase-9, were
increased in a dose-dependent manner following puerarin treatment
for 12 h.
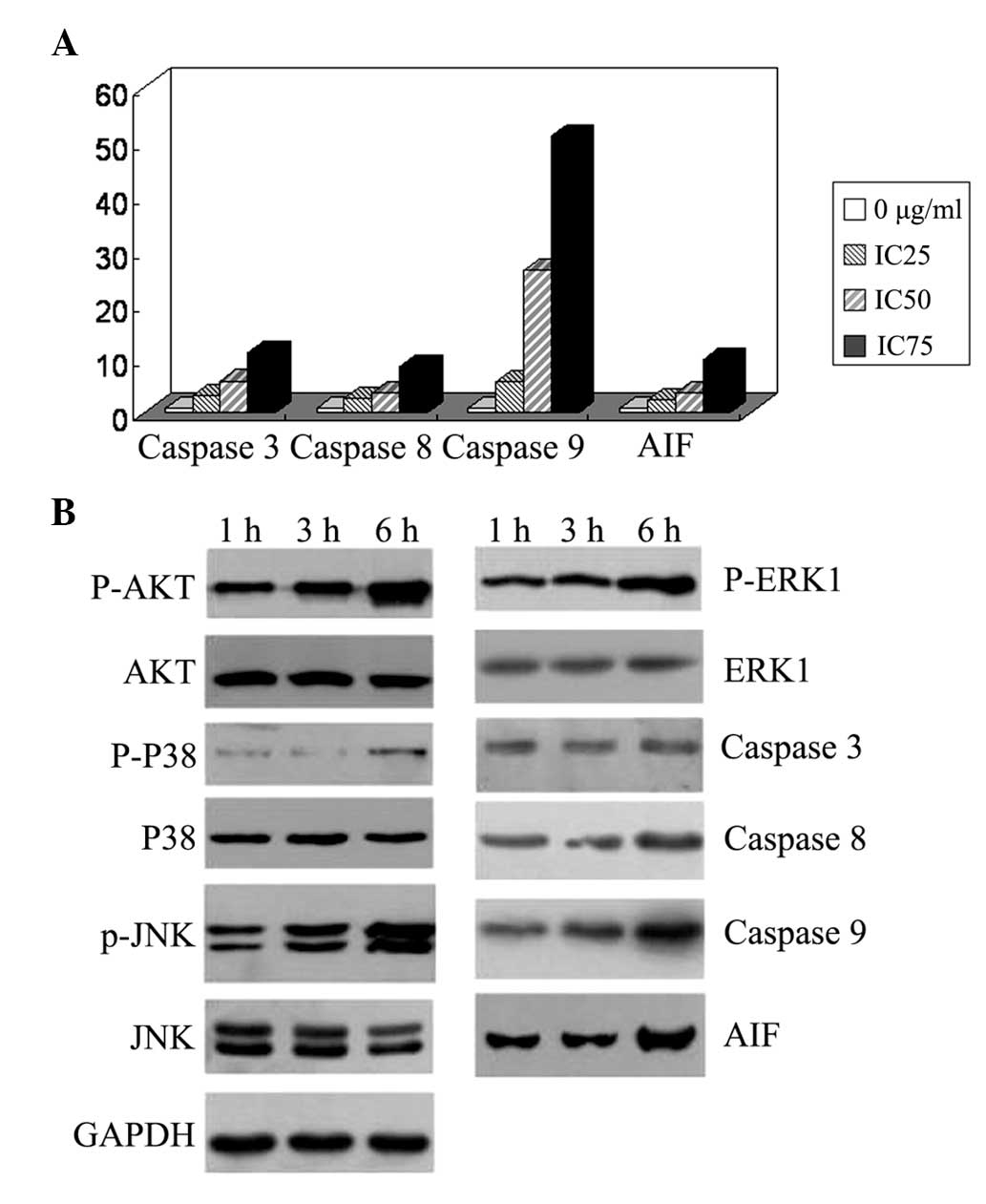 | Figure 6Expression levels of
apoptosis-associated genes in SMMC-7721 human hepatocellular
carcinoma cells. (A) Cells were incubated for 12 h with 0, 30
(IC25), 500 (IC50) and 2,000 μg/ml
(IC75) puerarin. Reverse transcription polymerase chain
reaction revealed that the mRNA expression levels of caspase-3,8,9
and AIF, particularly caspase-9, were increased in a dose-dependent
manner. (B) Cells were treated with 500 μg/ml puerarin for 1, 3 and
6 h. The AKT, P38, and JNK phosphorylation levels were increased,
and caspase-9 and AIF protein expression levels were upregulated.
AIF, apoptosis-inducing factor; p-ERK1, phosphorylated
extracellular signal-regulated kinase 1; JNK, c-Jun N-terminal
kinase. |
Subsequently, western blot analysis was used to
detect the expression levels of apoptosis-associated proteins and
the phosphorylation levels of kinases. As shown in Fig. 6B, treatment with 500 μg/ml puerarin
for 3 or 6 h increased AKT, P38, ERK1 and JNK phosphorylation in a
time-dependent manner. caspase-9 and AIF protein expression was
upregulated.
Discussion
Radix Puerariae, the root of Pueraria lobata,
is extensively used in Chinese Medicine and may also serve as a
food in oriental countries. Radix Puerariae contain a large
quantity of isoflavones, including puerarin, daidzein, daidzin and
genistein. These flavonoids possess various beneficial biological
properties, including antiallergic, anti-inflammatory, antiviral,
antioxidant and antitumor activities (15). One isoflavone, puerarin, has been
shown to exhibit beneficial effects in liver diseases, including
cirrhosis and alcohol-induced liver injury (8,16).
Furthermore, numerous studies have demonstrated the anticancer
activity of puerarin in animal models, as well as proliferation
inhibition and apoptosis induction in a variety of cancer cell
lines in vitro. One study found that puerarin treatment
resulted in a dose-dependent inhibition of cell growth in HS578T,
MDA-MB-231 and MCF-7 breast cancer cell lines (11). Results from cell cycle distribution
and apoptosis assay studies revealed that puerarin induced cell
apoptosis through a caspase-3-dependent pathway and mediated cell
cycle arrest in the G2/M phase (11). Similarly, c-jun was found to be
downregulated in RL95–2 endometrial carcinoma cells following
low-dose puerarin treatment, resulting in reduced aromatase P450
expression levels and substantially decreased growth of endometrial
cancers with P450 overexpression (17). In addition, puerarin reduced cell
proliferation in acute myeloid leukemia and promyelocytic leukemia
cells through cell cycle arrest in S phase or induction of
apoptosis (18,19). Recently, newly developed puerarin
nanosuspensions exhibited an enhanced antiproliferative effect by
reducing cell viability and inducing morphological changes. The
puerarin nanosuspensions inhibited HT-29 human colon cancer cell
growth by the induction of early apoptosis. In vivo, the
puerarin nanosuspensions were better tolerated and induced
significantly higher anticancer efficacy than the puerarin-free
solution (13). Furthermore, the
inhibitory effects of puerarin on the invasive and metastatic
abilities of tumor cells have also been demonstrated (20,21).
In accordance with previous reports, the present
study confirmed that high concentrations of puerarin significantly
inhibited proliferation of SMMC-7721 cells in a time- and
dose-dependent manner. Simultaneously, apoptotic rates were
increased and cell morphology was changed following puerarin
treatment, which suggested that induction of apoptosis may be the
main mechanism of the antiproliferative effect of puerarin in
SMMC-7721 cells.
Apoptosis, programed cell death, is the predominant
mechanism for targeted chemotherapies that induce cancer cell death
or sensitize the cells to established cytotoxic drugs or radiation
treatment. Two signaling pathways that result in apoptosis have
been identified: The extrinsic cell death pathway (cell death
receptor pathway) and the intrinsic cell death pathway (the
mitochondria-initiated pathway) (22). The breakdown of the MMP occurs at
an early stage of the apoptotic process and precedes nuclear
disintegration disruption. During MMP breakdown, mitochondrial
membrane pores are opened, resulting in the loss of MMP (23). This results in an increase in the
permeability of the mitochondrial membrane, followed by the release
of proapoptotic molecules, such as cytochrome c, released from the
mitochondria. Cytochrome c interacts with adenosine triphosphate,
apoptotic protease activating factor 1 and caspase-9, and
subsequently activates caspase-3, which consequently elicits
caspase-dependent apoptotic cell death (24). In the present study, caspase-3, -8
and -9 as well as AIF mRNA expression levels were significantly
increased following puerarin treatment for 12 h, while caspase-9
and AIF protein expression was also upregulated following puerarin
treatment for 6 h.
ROS production and consequent oxidative stress have
long been implicated in cell apoptosis (25). A previous study found that ROS are
predominantly generated in the mitochondria (26). Theoretically, as a consequence of
excessive ROS generation in cells, mitochondrial dysfunction may
occur (27). As expected, in the
present study, the intracellular ROS were significantly increased
when SMMC-7721 cells were exposed to puerarin. Therefore, the
mitochondria-dependent pathway is important in puerarin-induced
apoptosis in SMMC-7721 cells.
Accumulating evidence indicated that activation of
mitogen-activated protein kinases (MAPKs) is associated with cell
cycle arrest and the induction of apoptosis (28,29).
Three predominant parallel MAPK family signaling pathways have been
identified: ERK, JNK and P38. Activation of the ERK signaling
pathway by puerarin in human osteoblasts has been reported
(30). In the present study, the
phosphorylation levels of ERK, JNK and P38 in SMMC-7721 cells were
all increased following puerarin treatment. AKT is a master
regulator involved in transcriptional regulation of the
antiapoptotic protein B-cell lymphoma 2, which is critical in the
prevention of cell death. Activation of the phosphoinositide
3-kinase/AKT signaling pathway was observed to be involved in the
protective effect of puerarin against iodide-induced SH-SY5Y
neuroblastoma cell death (31). In
the present study, the AKT phosphorylation levels were increased,
which may be an adaptive response. Furthermore, in a human breast
cancer multidrug-resistant cell line, nuclear factor kappa-B
activity and IkappaB degradation were inhibited by puerarin
(32). Puerarin stimulated
AMP-activated protein kinase, acetyl-CoA carboxylase and glycogen
synthase kinase-3beta phosphorylation, but reduced cAMP-responsive
element-binding protein phosphorylation (32). Thus, the anticancer mechanisms of
puerarin may be multi-target and therefore require further
analysis.
In conclusion, in the present study, puerarin
inhibited proliferation and induced apoptosis in SMMC-7721 cells
via the mitochondria-dependent pathway, which may provide a novel,
safe and effective option for the treatment of HCC in the
future.
References
|
1
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2013. View Article : Google Scholar
|
|
2
|
Li C, Wen TF, Liao ZX, et al: Recurrence
of hepatocellular carcinoma after liver transplantation: recurrence
characteristics and risk factors. Hepatogastroenterology.
57:567–570. 2010.
|
|
3
|
Gu W, Fang FF, Li B, Cheng BB and Ling CQ:
Characterization and resistance mechanisms of a
5-fluorouracil-resistant hepatocellular carcinoma cell line. Asian
Pac J Cancer Prev. 13:4807–4814. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hou Q, Tang X, Liu H, et al: Berberine
induces cell death in human hepatoma cells in vitro by
downregulating CD147. Cancer Sci. 102:1287–1292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu C, Gong K, Mao X and Li W: Tetrandrine
induces apoptosis by activating reactive oxygen species and
repressing Akt activity in human hepatocellular carcinoma. Int J
Cancer. 129:1519–1531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qiu DZ, Zhang ZJ, Wu WZ and Yang YK:
Bufalin, a component in Chansu, inhibits proliferation and invasion
of hepatocellular carcinoma cells. BMC Complement Altern Med.
13:1852013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang F, Liu K, Du H, Kou J and Liu B:
Puerarin attenuates endothelial insulin resistance through
inhibition of inflammatory response in an IKKβ/IRS-1-dependent
manner. Biochimie. 94:1143–1150. 2012.PubMed/NCBI
|
|
8
|
Li R, Liang T, He Q, et al: Puerarin,
isolated from Kudzu root (Willd.), attenuates hepatocellular
cytotoxicity and regulates the GSK-3β/NF-κB pathway for exerting
the hepatoprotection against chronic alcohol-induced liver injury
in rats. Int Immunopharmacol. 17:71–78. 2013.PubMed/NCBI
|
|
9
|
Li R, Liang T, Xu L, et al: Puerarin
attenuates neuronal degeneration in the substantia nigra of
6-OHDA-lesioned rats through regulating BDNF expression and
activating the Nrf2/ARE signaling pathway. Brain Res. 1523:1–9.
2013. View Article : Google Scholar
|
|
10
|
Zhang MY, Qiang H, Yang HQ, Dang XQ and
Wang KZ: In vitro and in vivo effects of puerarin on promotion of
osteoblast bone formation. Chin J Integr Med. 18:276–282. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin YJ, Hou YC, Lin CH, et al: Puerariae
radix isoflavones and their metabolites inhibit growth and induce
apoptosis in breast cancer cells. Biochem Biophys Res Commun.
378:683–688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu Z and Li W: Induction of apoptosis by
puerarin in colon cancer HT-29 cells. Cancer Lett. 238:53–60. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Ma Y, Zheng Y, et al: In vitro and
in vivo anticancer activity of a novel puerarin nanosuspension
against colon cancer, with high efficacy and low toxicity. Int J
Pharm. 441:728–735. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang CP, Ding H, Shi DH, et al:
Pro-apoptotic effects of tectorigenin on human hepatocellular
carcinoma HepG2 cells. World J Gastroenterol. 18:1753–1764. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Z, Lam TN and Zuo Z: Radix
Puerariae: an overview of its chemistry, pharmacology,
pharmacokinetics, and clinical use. J Clin Pharmacol. 53:787–811.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo C, Xu L, He Q, et al: Anti-fibrotic
effects of puerarin on CCl4-induced hepatic fibrosis in rats
possibly through the regulation of PPAR-γ expression and inhibition
of PI3K/Akt pathway. Food Chem Toxicol. 56:436–442. 2013.PubMed/NCBI
|
|
17
|
Yu C, Li Y, Chen H, Yang S and Xie G:
Decreased expression of aromatase in the Ishikawa and RL95-2 cells
by the isoflavone, puerarin, is associated with inhibition of c-jun
expression and AP-1 activity. Food Chem Toxicol. 46:3671–3676.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shao HM, Tang YH, Jiang PJ, et al:
Inhibitory effect of flavonoids of puerarin on proliferation of
different human acute myeloid leukemia cell lines in vitro.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 18:296–299. 2010.(In
Chinese).
|
|
19
|
Ji O, Shen Q and Si YJ: Effect of
flavonoids of puerarin on the proliferation and apoptosis of
retinoic acid resistant acute promyelocytic leukemia cell line
NB4-R1 cells. Zhonghua Xue Ye Xue Za Zhi. 34:455–457. 2013.(In
Chinese).
|
|
20
|
Wang D, Liu Y, Han J, et al: Puerarin
suppresses invasion and vascularization of endometriosis tissue
stimulated by 17β-estradiol. PLoS One. 6:e250112011.PubMed/NCBI
|
|
21
|
Han J, Yu CQ and Shen W: Inhibitory
effects of puerarin on invasion and metastasis of oophoroma cells
HO-8910. Zhongguo Zhong Xi Yi Jie He Za Zhi. 29:632–635. 2009.(In
Chinese).
|
|
22
|
Xiong Y, Lu QJ, Zhao J and Wu GY:
Metformin inhibits growth of hepatocellular carcinoma cells by
inducing apoptosis via mitochondrion-mediated pathway. Asian Pac J
Cancer Prev. 13:3275–3279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zamzami N, Marchetti P, Castedo M, et al:
Inhibitors of permeability transition interfere with the disruption
of the mitochondrial transmembrane potential during apoptosis. FEBS
Lett. 384:53–57. 1996. View Article : Google Scholar
|
|
24
|
Yan SL, Huang CY, Wu ST and Yin MC:
Oleanolic acid and ursolic acid induce apoptosis in four human
liver cancer cell lines. Toxicol In Vitro. 24:842–848. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang L, Wang P, Wang H, et al: Fucoidan
derived from Undaria pinnatifida induces apoptosis in human
hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated
mitochondrial pathway. Mar Drugs. 11:1961–1976. 2013. View Article : Google Scholar
|
|
26
|
Götz ME, Künig G, Riederer P and Youdim
MB: Oxidative stress: free radical production in neural
degeneration. Pharmacol Ther. 63:37–122. 1994.PubMed/NCBI
|
|
27
|
Ling YH, Liebes L, Zou Y and Perez-Soler
R: Reactive oxygen species generation and mitochondrial dysfunction
in the apoptotic response to Bortezomib, a novel proteasome
inhibitor, in human H460 non-small cell lung cancer cells. J Biol
Chem. 278:33714–33723. 2003. View Article : Google Scholar
|
|
28
|
Cagnol S and Chambard JC: ERK and cell
death: mechanisms of ERK-induced cell death-apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kralova J, Dvorak M, Koc M and Kral V: p38
MAPK plays an essential role in apoptosis induced by
photoactivation of a novel ethylene glycol porphyrin derivative.
Oncogene. 27:3010–3020. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu LJ, Liu LQ, Bo T, et al: Puerarin
Suppress Apoptosis of Human Osteoblasts via ERK Signaling Pathway.
Int J Endocrinol. 2013:7865742013.PubMed/NCBI
|
|
31
|
Zhu G, Wang X, Wu S and Li Q: Involvement
of activation of PI3K/Akt pathway in the protective effects of
puerarin against MPP+induced human neuroblastoma SH-SY5Y cell
death. Neurochem Int. 60:400–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hien TT, Kim HG, Han EH, Kang KW and Jeong
HG: Molecular mechanism of suppression of MDR1 by puerarin from
Pueraria lobata via NF-kappaB pathway and cAMP-responsive element
transcriptional activity-dependent up-regulation of AMP-activated
protein kinase in breast cancer MCF-7/adr cells. Mol Nutr Food Res.
54:918–928. 2010. View Article : Google Scholar
|