Introduction
Chronic lower back pain is a common and debilitating
disorder that accounts for societal economic losses. It is
estimated that ~70% of the population will experience lower back
pain during their life (1).
Intervertebral disc (IVD) degeneration is the most prominent cause
of chronic lower back pain (2).
IVD degeneration can progress to disk herniation, spinal canal
stenosis, and, in conjunction with facet joint arthrosis,
degenerative spondylolisthesis. The mechanism of IVD degeneration,
however, has not been fully elucidated. The of IVD degeneration
process is considered to have a biochemical basis, involving
inhibition of nuclear proteoglycan synthesis and enhanced matrix
degradation (3). Previous studies
have suggested that in the process of IVD degeneration, the loss of
IVD cells due to excessive apoptosis is a central factor (4–6). It
is therefore required to identify the causes of IVD cellular
apoptosis. It has been demonstrated that numerous factors can lead
to apoptosis of IVD cells, including abnormal mechanical stresses
(7–9), interleukin-1β (IL-1β) (10) and serum withdrawal (11). If apoptosis of IVD cells can be
inhibited, degradation of the IVD may be decelerated.
IVD tissue has been shown to produce inflammatory
cytokines, including matrix metalloproteinases (MMPs), IL-1β, IL-6,
tumor necrosis factor-alpha (TNF-α), nitric oxide, and
prostaglandin E2 (PGE2) (12,13).
Among these cytokines, IL-1β is a multifunctional inflammatory
cytokine which functions in the progression of IVD cell apoptosis.
IL-1β is known as a proinflammatory cytokine since it has been
shown that IL-1β can increase the synthesis of matrix-degrading
enzymes (MMP-2, MMP-3, MMP-13), decrease the synthesis of
proteoglycan, collagen I and collagen II, and induce the expression
of IL-6, cyclooxygenase-2, and PGE2 (12,13,15,16).
Le Maitre et al (15)
identified that in human IVD degeneration, there was local
production of IL-1β by native disc cells that could induce the
cellular and matrix changes of IVD degeneration. Hoyland et al
(14) showed that enzyme activity
was upregulated by IL-1β and reduced by its inhibitor, therefore
placing IL-1β as a key regulator of matrix enzyme activity in
normal and degenerate IVD cells. It has been additionally reported
that IL-1β could induce apoptosis of AF cells in vitro (10). Previous studies have demonstrated
that IL-1β could enhance the effects of serum deprivation on rat
annular cell apoptosis and IL-1β sensitized rat IVD cells to Fas
ligand-mediated apoptosis in vitro (17,18).
Since serum may alter the effects of IL-1β, culture medium without
fetal bovine serum (FBS) was used in the present study.
Estrogen hormone is considered to be key in the
preservation of quality of life. There is evidence that estrogen
affects multiple tissues and organs in humans, as well as female
sexual features (19,20). Studies have shown that, compared
with males, female subjects have a higher incidence of IVD
degeneration as (21). Compared
with untreated post-menopausal women, estrogen-replete females
maintain higher and therefore healthier IVDs as (22). It has been reported that female
rats have a tendency to develop IVD degeneration following an
ovariectomy, and estrogen supplementation can halt the development
of this ovariectomy-associated disc degeneration (23). Taken together, these studies
demonstrate that estrogen hormone is closely associated with IVD
degeneration. In addition, 17β-estradiol (17β-E2) can protect
pancreatic β-cells, synovial fibroblasts and human chondrocytes
from apoptosis (24–26). Whether 17β-E2 can protect IVD cells
from apoptosis, however, has not been reported.
There is evidence showing that nucleus pulposus (NP)
tissue retains notochordal cells throughout the lifespan (27), and an in vitro study has shown that
notochordal cells in the IVD can interact with NP cells and is able
to affect annulus fibrosus (AF) cells (28). By contrast, cells in the AF share a
similar phenotype. In an effort to maintain the homogeneity of
cells cultured in vitro and exclude interactions between different
cell types, the present study used only the cells from the rat
AF.
The present study examined whether 17β-E2 inhibited
IL-1β-induced apoptosis in rat AF cells, and the dose-response
effect of 17β-E2 on cell apoptosis.
Materials and methods
Materials
Collagenase type II, trypsin, 17β-E2, ICI182, 780
and Dulbecco’s modified Eagle’s medium (DMEM) high glucose medium
were purchased from Sigma-Aldrich (St. Louis, MO, USA). IL-1β was
purchased from PeproTech (Rocky Hill, NJ, USA). Fetal bovine serum
(FBS) and Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12) were
obtained from HyClone (HyClone Laboratories, Logan, UT, USA).
Hank’s Balanced Salt Solution (HBSS) was bought from Gibco-BRL
(Grand Island, NE, USA). The Cell Proliferation kit I (MTT) was
purchased from Solarbio (Beijing Solarbio Science & Technology
Co., Ltd, Beijing, China), the Annexin V/propidium iodide binding
kit was obtained from Multi Sciences Biotech. Co., Ltd. (Hangzhou,
China) and the Caspase-3 activity assay kit was obtained from
Beyotime (Beyotime Institute of Biotechnology, Shanghai,
China).
Primary disc cell isolation
Male Sprague Dawley rats (weighing 180–240 g) were
obtained from the Laboratory Animal Center of Hebei Medical
University (Hebei, China). The Animal Care and Experimental
protocols conformed to the Guide for the Care and Use of Laboratory
Animals, published by the US National Institutes of Health and were
approved by the Animal Ethics Committee of Hebei Medical
University. The rats were sacrificed by intravenous administration
of 150 mg/kg pentobarbital sodium, and the rat lumbar IVD (L1–L5)
were harvested immediately in a sterile environment. The muscle
tissues and ligaments around the IVD and inner NP were removed, and
the outer AF was obtained through an operating microscope to ensure
the identity of the tissue. The AF tissues were then placed into
DMEM/F12 and were cut into small pieces (<1 mm3). To isolate the
cells, the AF tissues in the DMEM/F-12 were digested with 0.25%
collagenase type II for ~1 h in a water bath at 37°C. Subsequently,
the tissue was additionally digested with 0.2% trypsin (including
0.02% EDTA) for ~5 min at 37°C. Following the two-step enzyme
digestion, the suspension was filtered through a 70 μm mesh. The
filtered cells were then washed twice with Hank’s Balanced Salt
Solution. Finally, the AF cells were added to DMEM/F12 media,
supplemented with 15% FBS, 100 U/ml penicillin G, and 100 μg/ml
streptomycin and cultured at 37°C in a humidified atmosphere of 95%
air and 5% CO2. The medium was changed every two days. The AF cells
were passaged three times prior to being collected for use.
Cell culture and drug treatment
When the cell culture became 80–90% confluent, the
cells were digested and subcultured into appropriate culture plates
and cultured as previously described. When the cell confluence in
each well reached 80–90%, the medium was replaced with DMEM-high
glucose medium without FBS, phenol red, penicillin and
streptomycin. Six experimental groups were established, consisting
of one control, one induction and four treatment groups. In the
four treatment groups, 0.1, 1, and 10 μmol/l 17β-E2, and 10 μmol/l
17β-E2+10 μmol/l ICI182, 780 was respectively added to the medium
(without FBS). The cells were cultured for 1 h and then 75 ng/ml
IL-1β was added to the induction group and the four treatment
groups. Cultures without the addition of IL-1β, 17β-E2 and ICI182,
780 acted as the control group. All the groups were cultured in a
humidified atmosphere with 5% CO2 at 37°C for 24 h.
Morphological observation
Cells were sub-cultured in 6-well plates at 2×105
cells/well in complete culture medium. Following 24 h treatment,
coverslips with adherent cells were observed under a fluorescence
inverted microscope (Olympus IX50). Three areas of 200×200 pixels
in one sample were randomly selected from the image to observe the
morphological changes in the apoptotic cells.
Cell viability assay
Cell viability was measured using an MTT assay. The
MTT assay relies on the observation that succinate dehydrogenase in
the mitochondria of the viable cells revert MTT into a visible
dark-blue formazan reaction product, which provides an indirect
measure of cell viability. Cells were distributed into 96-well
plates at 5,000 cells/well and maintained for one day in complete
media with 15% FBS. The next day, the medium was replaced with
DMEM-high glucose medium (200 μl/well, without FBS). After 24 h
treatment, 20 μl of MTT (5 mg/ml), dissolved in phosphate-buffered
saline (PBS), was added to each well. Following a 4 h incubation in
a humidified atmosphere with 5% CO2 at 37°C, the plates were
centrifuged at 1,900 × g for 10 min. The medium was then removed
and replaced with dimethyl sulfoxide (150 μl/well). The plates were
placed onto a shaker for agitation at a low speed for 10 min.
Finally, the solubilized mixture was measured by reading the
absorbance at 490 nm wavelength using a microplate reader.
Apoptosis assay
Cells were sub-cultured in 6-well plates at 2×105
cells/well with complete culture medium. Following 24 h treatment,
cells still attached to the plate and those present in the
supernatant were collected together and resuspended in cold binding
buffer. The apoptotic incidence was detected using an Annexin
V/fluorescein isothiocyanate (FITC) apoptosis detection kit.
Apoptosis was determined by staining cells with both Annexin V/FITC
and propidium iodide (PI), according to the manufacturer’s
instructions. Annexin V/FITC was used to quantitatively determine
the percentage of cells undergoing apoptosis based upon the loss of
membrane asymmetry in the early phases of apoptosis. In apoptotic
cells, the membrane phospholipid phosphatidylserine is translocated
from the inner leaflet of the plasma membrane to the outer leaflet,
thereby exposing phosphatidylserine to the external environment.
Cells that were positively stained with Annexin V/FITC and
negatively stained for PI were therefore considered to be
undergoing apoptosis. Cells that were positively stained for both
Annexin V/FITC and PI were considered to be undergoing necrosis
(29,30). The cells were then stained with 5
μl Annexin V/FITC and 10 μl PI, followed by the addition of 500 μl
binding buffer for 15 min at room temperature in the dark. The
samples were analyzed by flow cytometry (FCM) within 1 h.
Measurement of caspase-3 activity
Caspase-3 activity was assayed using the caspase-3
activity assay kit, based upon the ability of caspase-3 to convert
acetyl-Asp-Glu-Val-Asp p-nitroanilide into the yellow formazan
product, p-nitroaniline. Cells were placed in 6-well plates at
2×105 cells/well and treated as above. The cells were then lysed
with lysis buffer (100 μl/2×106 cells) for 15 min on ice following
washing with cold HBSS. The mixture was composed of 10 μl cell
lysate, 80 μl reaction buffer and 10 μl of 2 mM caspase-3 substrate
and was incubated in 96-well microtiter plates at 37°C.
Statistical analysis
Values are presented as the mean ± standard
deviation. Statistical analyses were performed using the SPSS 13.0
statistical software program (SPSS, Inc., Chicago, IL, USA). The
means of apoptotic incidences among groups, as well as the
absorbances among groups were compared by one-way analysis of
variance, followed by pairwise comparison using the
Student-Newman-Keuls-q test. All statistical tests were two-sided
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Morphological changes of apoptotic AF
cells induced by IL-1β and the protective effects of 17β-E2
Using a fluorescence inverted microscope, apoptotic
cells exhibited plasma membrane blebbing, cell shrinkage and nuclei
condensation. A total of 75 ng/ml IL-1β was used for this
experiment. Few apoptotic cells were observed in the control group.
As compared with the control group, treatment with IL-1β (75 ng/ml)
induced more apoptotic cells. Pretreatment with 17β-E2 (10 μm/l)
resulted in a decrease in this effect, with the number of apoptotic
cells less than those treated with IL-1β (75 ng/l; Fig. 1). Fig.
1D demonstrated that estrogen receptor antagonist ICI182, 780
(10 μm/l) could revert the protective effects of 17β-E2 (10 μm/l).
There was no significant difference between the IL-1β (75 ng/l) and
IL-1β (75 ng/l) + 17β-E2 (10 μm/l) + ICI182, 780 (10 μm/l) groups
(Fig. 1).
 | Figure 1Morphological changes in rat annulus
fibrosus (AF) cells. (A) Phase-contrast photomicrographs of AF
cells cultured in serum-free media. (B–D) Phase-contrast
photomicrographs of AF cells cultured in serum-free media
stimulated with IL-1β, IL-1β + 17β-E2, IL-1β + 17β-E2 + ICI182,
780, respectively. Apoptotic cells were characterized by plasma
membrane blebbing, cell shrinkage and nuclei condensing, as
indicated by the black arrows. Scale bar=200 μm; original
magnification, ×100. 17β-E2, 17β-estradiol; IL-1β, interleukin-1β;
ICI182, 780, estrogen receptor antagonist. |
Effect of 17β-E2 on cell viability in AF
cells damaged by IL-1β
An MTT assay for cell viability was used to
determine whether 17β-E2 could prevent cell death in cells exposed
to IL-1β, and whether 17β-E2 (0.1, 1, 10 μmol/l) pretreatment could
increase cellular proliferation in a concentration-dependent
manner. As shown in Fig. 2A, as
compared with the control group, treatment with IL-1β alone caused
an ~45% decrease in cell viability (P<0.001). The cell viability
in the 17β-E2 + IL-1β group was significantly increased, as
compared with the IL-1β group. There were no significant changes in
cell viability between the IL-1β group and 17β-E2 + IL-1β + ICI182,
780 group. The inhibitory effect of 17β-E2 was abolished by the
application of the estrogen receptor antagonist ICI182, 780. Taken
together, these results showed that 17β-E2 can protect cells from
apoptosis induced by IL-1β. Cell growth was inhibited following
treatment with IL-1β, as compared with the control group
(P<0.001). The cellular proliferation of IL-1β-induced apoptotic
cells was significantly increased after pretreatment of cells for 1
h with 17β-E2 at concentrations of 0.1 μmol/l (0.421±0.021), 1
μmol/l (0.537±0.027) and 10 μmol/l (0.629±0.031; Fig. 2B). Therefore, 17β-E2 pretreatment
increased cellular proliferation in a concentration-dependent
manner.
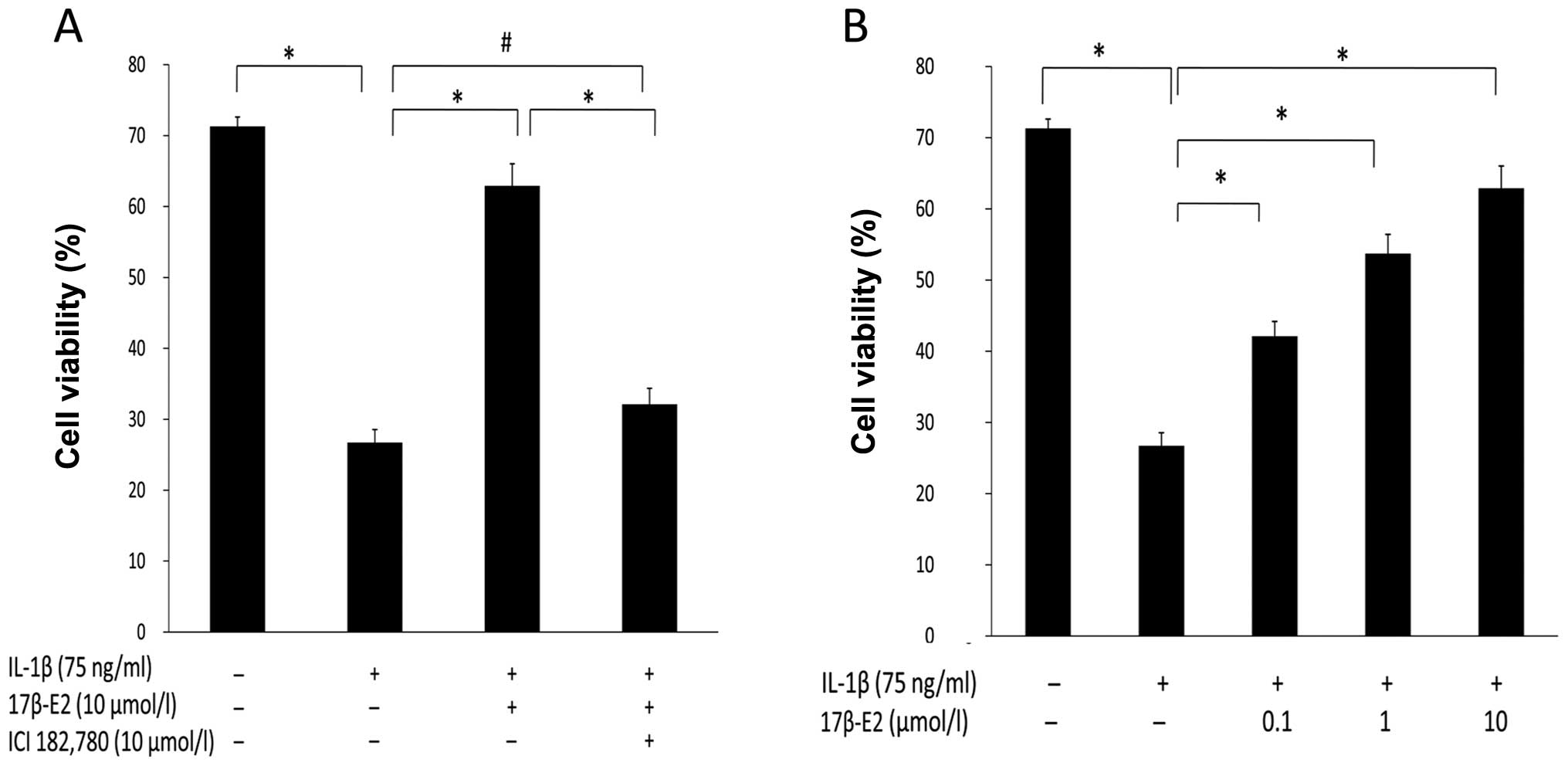 | Figure 2Evaluation of cell viability. The cell
viability was measured by MTT assay. (A) The rat annulus fibrosus
(AF) cells were incubated in serum-free media or stimulated with
IL-1β (75 ng/ml), IL-1β (75 ng/ml) + 17β-E2 (10 μm/l), IL-1β (75
ng/l)+17β-E2 (10 μm/l) + ICI182, 780 (10 μm/l). (B) Cells were
treated with IL-1β (75 ng/l) for 1 h alone or followed by treatment
with differing doses of 17β-E2 (0.1, 1, 10 μmol/l).
*P<0.001; #P>0.05. It was identified
that 17β-E2 could prevent cell death in AF cells exposed to IL-1β,
and 17β-E2 (0.1, 1, 10 μmol/l) pretreatment could increase AF cell
proliferation in a concentration-dependent manner. Error bars
represent the mean ± standard deviation. IL-1β, interleukin 1β;
17β-E2, 17β-estradiol; ICI182, 780, estrogen receptor
antagonist. |
Effect of 17β-E2 on IL-1β-induced AF
cells apoptosis
It was further investigated whether 17β-E2 could
inhibit IL-1β-induced apoptosis in cells and whether 17β-E2 (0.1,
1, 10 μmol/l) pretreatment could decrease apoptosis in a
concentration-dependent manner. The level of apoptotic cells was
determined by double staining with Annexin V/FITC and PI. The
apoptotic ratio of cells was calculated as a percentage of
apoptotic cells/total cells (Table
I). Annexin V/FITC and PI labeled cells were quantified by FCM,
thus allowing for discrimination between viable/intact cells
(Annexin V-PI−), early apoptotic (Annexin V+PI−) and late apoptotic
or necrotic cells (Annexin V+PI+). As is shown in Fig. 3, the percentage of apoptosis
increased in cells following treatment with IL-1β alone (19.9%).
Cells pretreated with 17β-E2 showed a reduced rate of apoptosis
(12.3%). To further elucidate the potential contribution of 17β-E2,
an estrogen receptor antagonist ICI182, 780, was used. When cells
were incubated with both 17β-E2 and ICI182, 780, the protective
effects of 17β-E2 were reduced (18.0%). To investigate the
association between the concentration of 17β-E2 and the protective
effects by 17β-E2, three different concentrations of 17β-E2 (0.1, 1
and 10 μmol/l) were used. Statistical analysis showed that
pretreatment with increasing concentration of 17β-E2 reduced the
rate of apoptosis in a concentration-dependent manner (12.3, 9.5,
5.3%).
 | Table IEffect of 17β-E2 on IL-1β-induced
apoptosis. |
Table I
Effect of 17β-E2 on IL-1β-induced
apoptosis.
| Group |
|---|
|
|
|---|
| Control | IL-1β | 17β-E2a | 17β-E2a | 17β-E2a | 17β-E2a + ICI182,780 |
|---|
| Dose | 75 ng/ml | 0.1 μmol/l | 1 μmol/l | 10 μmol/l | 10 μmol/l | 10 μmol/l |
| Apoptosis (%) | 3.47±0.25 | 18.80±0.99 | 12.97±0.61 | 8.67±0.74 | 6.00±0.61 | 18.57±0.51 |
Effect of 17β-E2 on caspase-3 activity in
AF cells induced by IL-1β
Caspase-3 is a critical mediator of apoptosis. As
shown in Fig. 4A, caspase-3
activity was increased in cells following treatment with IL-1β
alone, as compared with the control cells. However, cells
pretreated with 17β-E2 showed a reduction in this ability. Addition
of estrogen receptor antagonist ICI182, 780 resulted in elimination
of the protective effects of 17β-E2. Fig. 4B shows that cells pretreated with
increasing concentrations of 17β-E2 reduced caspase-3 activity in a
concentration-dependent manner.
Discussion
The present study examined the effects of 17β-E2 on
IL-1β-induced apoptosis in AF cells. The data provide evidence that
17β-E2 attenuated IL-1β-induced apoptosis in AF cells, which may
improve the survival and proliferative capacity of AF cells under
inflammatory stress. These data are in agreement with the study of
Hattori et al (26), in which
similar concentrations of TNF-α were used to induce cell death in
human chondrocytes.
Morphological observations and FCM were used to
identify whether AF cells underwent apoptosis following exposure to
IL-1β. It was observed that the effects of IL-1β on the number of
apoptotic cells were prevented upon pretreatment with 17β-E2. This
protective action of 17β-E2 was in a concentration-dependent
manner. The results of the MTT assay verified that preincubation
with 17β-E2 could increase the viability of AF cells.
Previous studies in human IVD degeneration has
identified that IL-1β expression is derived from native disc cells
(15). The inflammatory cytokine
IL-1β has a crucial function in IVD degeneration including
promotion of apoptosis in IVD cells. Three concentrations of IL-1β
(40, 75, 150 ng/ml) were selected to induce apoptosis in AF cells.
The results of the FCM indicated that IL-1β (40 ng/ml) generated
3.4% apoptosis and there was no significant difference between the
other two groups (20.4, 19.9%). A concentration of 75 ng/ml IL-1β
was subsequently used to induce cellular apoptosis. Serum contains
various growth factors, including insulin-like growth factor-1
(IGF-1). IGF-1 can inhibit IL-1β-induced apoptosis in growth plate
chondrocytes from metatarsal bones and pancreatic β-cells (31,32).
Cells were therefore cultured in media without FBS in order to
eliminate the effects of serum. The results of the MTT assay and
FCM demonstrated that IL-1β could induce cell apoptosis and
suppress proliferation in AF cells.
Estrogen is an essential sex hormone in males and
females. Estrogen has effects on the reproductive system, as well
as neurotransmitter release, bone structure, cognitive function and
blood vessels (33,34). Understanding the importance of
estrogen has significant implications. The beneficial effects of
estrogen have been previously investigated. Huppmann et al
(35) showed that 17β-E2
attenuated hyperoxia-induced apoptosis in astrocytes. Ronda et al
(36) demonstrated that 17β-E2 has
an anti-apoptotic function in skeletal muscle cells. Whether 17β-E2
can prevent apoptosis in AF cells has not been previously
investigated. To the best of our knowledge, the present study is
the first to demonstrate that 17β-E2 can prevent apoptosis induced
by IL-1β in AF cells. Further evidence has suggested that phenol
red, a pH indicator widely used in growth medium, exhibits minor
oestrogenic activity similar to the effect of steroid hormones
(37). Wesierska-Gadek et al
(38) showed that phenol red added
to the culture medium strongly promoted the cell proliferation and
cell cycle progression of human cells expressing the estrogen
receptor. To eliminate the effects of phenol red, medium without
phenol red was used. The results from the present study indicated
that 17β-E2 has the ability of protecting AF cells from apoptosis,
and pretreatment of AF cells with the estrogen receptor antagonist
ICI182, 780 could attenuate the protective effects of 17β-E2.
Higher concentrations of 17β-E2 (0.1–10 μmol/l) exerted a stronger
protective effect. Taken together, these results support our
conclusion that 17β-E2 can protect IL-1β-induced apoptosis in AF
cells.
There are several limitations to the present study.
Firstly, only three concentrations of 17β-E2 were selected, and
additional concentrations are required to further investigate the
dose-response effect of 17β-E2 on cellular apoptosis. Secondly, the
study design only observed the cellular effects after 24 h
treatment. It is necessary to investigate additional timepoints in
order to determine whether 17β-E2 reduces the rate of apoptosis by
IL-1β in a time-dependent manner. Thirdly, rat AF cells were the
only cell type investigated in this study, which cannot fully
represent human AF cells. Finally, additional studies are necessary
to further elucidate the signaling mechanisms which mediate the
anti-apoptotic action of 17β-E2 in AF cells.
In conclusion, the results of the current study have
shown that incubation of rat AF cells with 17β-E2 can counteract
cell damage induced by IL-1β. Pretreatment of cells with 17β-E2
could decrease AF cell apoptosis in a concentration-dependent
manner. The present study demonstrates that the inflammatory
cytokine, IL-1β, could induce AF cell apoptosis and that the
absence of estrogen may be an important mediator of IVD
degeneration in post-menopausal women. This may be of relevance to
develop novel therapies associated with IVD in post-menopausal
women.
Acknowledgements
The authors would like to thank the Laboratory
Animal Center of Hebei Medical University for providing the Sprague
Dawley rats.
References
|
1
|
Macfarlane GJ, Thomas E, Croft PR, et al:
Predictors of early improvement in low back pain amongst consulters
to general practice: the influence of pre-morbid and
episode-related factors. Pain. 80:113–119. 1999. View Article : Google Scholar
|
|
2
|
Freemont AJ: The cellular pathobiology of
the degenerate intervertebral disc and discogenic back pain.
Rheumatology (Oxford). 48:5–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Podichetty VK: The aging spine: the role
of inflammatory mediators in intervertebral disc degeneration. Cell
Mol Biol (Noisy-le-grand). 53:4–18. 2007.PubMed/NCBI
|
|
4
|
Ding F, Shao ZW and Xiong LM: Cell death
in intervertebral disc degeneration. Apoptosis. 18:777–785. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ariga K, Miyamoto S, Nakase T, et al: The
relationship between apoptosis of endplate chondrocytes and aging
and degeneration of the intervertebral disc. Spine (Phila Pa 1976).
26:2414–2420. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rannou F, Lee TS, Zhou RH, et al:
Intervertebral disc degeneration: the role of the mitochondrial
pathway in annulus fibrosus cell apoptosis induced by overload. Am
J Pathol. 164:915–924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang YH, Zhao CQ, Jiang LS, et al: Cyclic
stretch-induced apoptosis in rat annulus fibrosus cells is mediated
in part by endoplasmic reticulum stress through nitric oxide
production. Eur Spine J. 20:1233–1243. 2011. View Article : Google Scholar
|
|
8
|
Xie M, Yang S, Win HL, et al: Rabbit
annulus fibrosus cell apoptosis induced by mechanical overload via
a mitochondrial apoptotic pathway. J Huazhong Univ Sci Technolog
Med Sci. 30:379–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding F, Shao ZW, Yang SH, et al: Role of
mitochondrial pathway in compression-induced apoptosis of nucleus
pulposus cells. Apoptosis. 17:579–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duan DY, Yang SH, Xiong XQ, et al:
Interleukin-6 protects annulus fibrosus cell from apoptosis induced
by interleukin-1 beta in vitro. Chin Med Sci J. 21:107–110.
2006.PubMed/NCBI
|
|
11
|
Risbud MV, Fertala J, Vresilovic EJ, et
al: Nucleus pulposus cells upregulate PI3K/Akt and MEK/ERK
signaling pathways under hypoxic conditions and resist apoptosis
induced by serum withdrawal. Spine (Phila Pa 1976). 30:882–889.
2005. View Article : Google Scholar
|
|
12
|
Kang JD, Georgescu HI, McIntyre-Larkin L,
et al: Herniated lumbar intervertebral discs spontaneously produce
matrix metalloproteinases, nitric oxide, interleukin-6, and
prostaglandin E2. Spine (Phila Pa 1976). 21:271–277. 1996.
View Article : Google Scholar
|
|
13
|
Weiler C, Nerlich AG, Bachmeier BE and
Boos N: Expression and distribution of tumor necrosis factor alpha
in human lumbar intervertebral discs: a study in surgical specimen
and autopsy controls. Spine (Phila Pa 1976). 30:44–53.
2005.PubMed/NCBI
|
|
14
|
Hoyland JA, Le Maitre C and Freemont AJ:
Investigation of the role of IL-1 and TNF in matrix degradation in
the intervertebral disc. Rheumatology (Oxford). 47:809–814. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le Maitre CL, Freemont AJ and Hoyland JA:
The role of interleukin-1 in the pathogenesis of human
intervertebral disc degeneration. Arthritis Res Ther. 7:R732–R745.
2005.
|
|
16
|
Jimbo K, Park JS, Yokosuka K, Sato K and
Nagata K: Positive feedback loop of interleukin-1beta upregulating
production of inflammatory mediators in human intervertebral disc
cells in vitro. J Neurosurg Spine. 2:589–595. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui LY, Liu SL, Ding Y, et al: IL-1beta
sensitizes rat intervertebral disc cells to Fas ligand mediated
apoptosis in vitro. Acta Pharmacol Sin. 28:1671–1676. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao CQ, Liu D, Li H, et al:
Interleukin-1beta enhances the effect of serum deprivation on rat
annular cell apoptosis. Apoptosis. 12:2155–2161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clarke BL and Khosla S: Female
reproductive system and bone. Arch Biochem Biophys. 503:118–128.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia J, Guan D, Zhu W, et al: Estrogen
inhibits Fas-mediated apoptosis in experimental stroke. Exp Neurol.
215:48–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang YX, Griffith JF, Ma HT, et al:
Relationship between gender, bone mineral density, and disc
degeneration in the lumbar spine: a study in elderly subjects using
an eight-level MRI-based disc degeneration grading system.
Osteoporos Int. 22:91–96. 2011. View Article : Google Scholar
|
|
22
|
Baron YM, Brincat MP, Galea R and Calleja
N: Intervertebral disc height in treated and untreated overweight
post-menopausal women. Hum Reprod. 20:3566–3570. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang YX and Griffith JF: Effect of
menopause on lumbar disk degeneration: potential etiology.
Radiology. 257:318–320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ackermann S, Hiller S, Osswald H, et al:
17beta-Estradiol modulates apoptosis in pancreatic beta-cells by
specific involvement of the sulfonylurea receptor (SUR) isoform
SUR1. J Biol Chem. 284:4905–4913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamaguchi A, Nozawa K, Fujishiro M, et al:
Estrogen inhibits apoptosis and promotes CC motif chemokine ligand
13 expression on synovial fibroblasts in rheumatoid arthritis.
Immunopharmacol Immunotoxicol. 34:852–857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hattori Y, Kojima T, Kato D, et al: A
selective estrogen receptor modulator inhibits tumor necrosis
factor-α-induced apoptosis through the ERK1/2 signaling pathway in
human chondrocytes. Biochemical and Biophysical Research
Communications. 421:418–424. 2012.
|
|
27
|
Risbud MV and Shapiro IM: Notochordal
cells in the adult intervertebral disc: new perspective on an old
question. Crit Rev Eukaryot Gene Expr. 21:29–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moon HJ, Joe H, Kwon TH, et al:
Notochordal cells influence gene expression of inflammatory
mediators of annulus fibrosus cells in proinflammatory cytokines
stimulation. J Korean Neurosurg Soc. 48:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vermes I, Haanen C, Steffens-Nakken H and
Reutelingsperger C: A novel assay for apoptosis. Flow cytometric
detection of phosphatidylserine expression on early apoptotic cells
using fluoresceinlabeled Annexin V. J Immunol Methods. 184:39–51.
1995. View Article : Google Scholar
|
|
30
|
Zhang G, Gurtu V, Kain SR and Yan G: Early
detection of apoptosis using a fluorescent conjugate of annexin V.
Biotechniques. 23:525–531. 1997.PubMed/NCBI
|
|
31
|
Martensson K, Chrysis D and Savendahl L:
Interleukin-1beta and TNF-alpha act in synergy to inhibit
longitudinal growth in fetal rat metatarsal bones. J Bone Miner
Res. 19:1805–1812. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Giannoukakis N, Mi Z, Rudert WA, et al:
Prevention of beta cell dysfunction and apoptosis activation in
human islets by adenoviral gene transfer of the insulin-like growth
factor I. Gene Ther. 7:2015–2022. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simpson E, Rubin G, Clyne C, et al: Local
estrogen biosynthesis in males and females. Endocr Relat Cancer.
6:131–137. 1999. View Article : Google Scholar
|
|
34
|
Simpson ER: Sources of estrogen and their
importance. J Steroid Biochem Mol Bio. 86:225–230. 2003. View Article : Google Scholar
|
|
35
|
Huppmann S, Römer S, Altmann R, et al:
17beta-estradiol attenuates hyperoxia-induced apoptosis in mouse
C8-D1A cell line. J Neurosci Res. 86:3420–3426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ronda AC, Vasconsuelo A and Boland R:
Extracellular-regulated kinase and p38 mitogen-activated protein
kinases are involved in the anti-apoptotic action of
17beta-estradiol in skeletal muscle cells. Journal of
Endocrinology. 206:235–246. 2010. View Article : Google Scholar
|
|
37
|
Tsang LL, Chan LN, Liu CQ, et al: Effect
of phenol red and steroid hormones on cystic fibrosis transmembrane
conductance regulator in mouse endometrial epithelial cells. Cell
Biol Int. 25:1021–1024. 2001. View Article : Google Scholar
|
|
38
|
Wesierska-Gadek J, Schreiner T, Maurer M,
et al: Phenol red in the culture medium strongly affects the
susceptibility of human MCF-7 cells to roscovitine. Cell Mol Biol
Lett. 12:280–293. 2007. View Article : Google Scholar : PubMed/NCBI
|


















