Introduction
Gamma knife surgery (GKS) is the stereotactic
delivery of a single high-radiation dose as an alternative to
neurosurgical open surgery. The frequency of GKS use is expanding
in the treatment of vascular malformations, brain tumors and
functional brain diseases (1–3).
During the course of application of a therapeutic dose of radiation
to the lesion, a certain volume of surrounding normal tissue,
although small, receives a destructive radiation dose. This leads
to the occurrence of a variety of clinical complications, among
which the delayed formation of edema in the radiosurgery bed is the
most common complication associated with normal tissue radiation
injury (4). However, the
mechanisms producing brain edema following Gamma knife irradiation
remain to be elucidated.
Brain edema formation is closely associated with
pathological angiogenesis. Vascular abnormalities are also most
often observed in central nervous system (CNS) damage resulting
from radiosurgery, which includes endothelial cell swelling, vessel
dilation and basement membrane thickening, as well as changes in
vascular permeability (5,6). Increased vascular permeability
resulting from radiation-induced pathological angiogenesis is one
of the main causes of radiation-induced edema (7). Furthermore, damage to the
microvasculature in the CNS is the primary event that is causative
in the subsequent development of late effects, thus the present
study aimed to examine the expression of growth factors associated
with angiogenesis.
Vascular endothelial growth factor (VEGF) is a
member of a family of angiogenesis-associated growth factors
(8). It is a highly conserved
36–46 kD diametric glycoprotein with potent and specific activity
for endothelial cell proliferation and vascular permeability to
water and large-molecular-weight proteins. In pathological states,
VEGF is upregulated in response to increased metabolic demand
(9). The biochemical properties of
VEGF provide potential therapeutic anti-VEGF agents to control
edema (10). There have also been
several studies investigating how VEGF contributes to pathological
angiogenesis and edema formation in brain tumors, stroke and
traumatic brain injury, however, information on GKS is extremely
limited (11). Previous studies
have revealed that radiation-induced increases in VEGF expression
in white matter following conventional radiotherapy were present in
regions of blood-spinal cord barrier disruption and tissue hypoxia
(12). The radiobiological changes
associated with radiosurgery are different to the changes observed
following conventional radiotherapy with a lower dose. Dynamic
alterations in the expression of VEGF following GKS in vivo,
however, have yet to be determined.
In the current study, in order to identify the
effects of VEGF on pathological angiogenesis and brain edema
following GKS, whether VEGF expression is upregulated in normal
tissue by gamma knife irradiation was investigated and subsequently
fluctuations in the expression of VEGF during the course of gamma
knife irradiation was monitored.
Materials and methods
Animal protocol
A total of 96 male Wistar rats weighing between 200
and 240 g were housed in cages (two rats per cage) and maintained
in environmentally controlled rooms (22–24°C) with a 12-h
light/dark cycle. Experiments involving animals were approved by
the Animal Care and Ethics Committee of Tianjin Medical University
(Tianjin, China). A maximum dose of 60 Gy was administered into the
right parietal cortex via the Leksell gamma knife model C (Elekta
Instrument AB, Stockholm, Sweden) by using a 4-mm collimator
(Elekta Instrument AB, Stockholm, Sweden) as described previously
(13). The selection of the
radiation dose was based on previous studies (14,15).
A rat anesthetized with 10% chloraldurat (3 ml/kg) was fixed in a
stereotactic brain frame. Following obtaining high-resolution
magnetic resonance (MR) images, the center of the irradiation area
was calculated with reference to a standard rat stereotactic atlas
(16) and the cerebral structures
visible on the MR images. Leksell Gamma Plan software (Elekta
Instrument AB) was used to attain target localization for the
radiosurgery. The control animals were treated identically but did
not receive any radiation.
Histology and immunohistochemistry
At 4, 8, 12, 16, 20 and 24 weeks post-irradiation,
the rats were perfused transcardially with normal saline followed
by 4% buffered paraformaldehyde (PFA) under intraperitoneal
anesthesia (n=4 per each time point). The brain was removed and the
brains were excised. Following fixation in 4% phosphate-buffered
PFA, the brains were cut transversally into 2 mm-thick slices and
embedded in paraffin. Sections (4 μm thick) were cut and mounted on
positively charged glass slides. The avidin-biotin complex (ABC)
method (Vectastain Elite ABC kit; Vector Laboratories, Burlingame,
CA, USA) was used for the CD31 and VEGF primary antibodies (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The sections were
boiled at 95–100°C in citrate buffer (pH 6.0) for antigen
retrieval. Sections were treated with 1.5% normal horse or goat
serum for 30 min, followed by incubation overnight at 4°C with
mouse polyclonal primary antibodies (1:100). Following incubation
with diluted biotinylated rabbit anti-mouse secondary antibody,
sections were incubated with ABC reagent for 30 min, and 0.015%
hydrogen peroxide and 0.05% diaminobenzidine for 5–10 min. Nuclear
staining was performed with hematoxylin. The CD31+
structure was quantified from 10 random ×200 fields in a blinded
fashion and was converted to square millimeters.
Enzyme-linked immunosorbent assay
(ELISA)
Under deep anesthesia, animals were sacrificed at
designated time points. Following decapitation, the brains were
rapidly removed and the ipsilateral parietal cortex receiving GKS
was dissected freely. The cortical tissue samples were weighed and
homogenized on ice in four volumes of extraction buffer consisting
of 150 mmol/l sodium chloride, 50 mmol/l mm tris aminomethane
hydrochloride and 1% Triton X-100 (pH 7.2) with a proteinase
inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). These were
then agitated for 90 min at 4°C and centrifuged for 15 min at 3,000
× g. The resulting supernatants were aspirated and VEGF
concentrations were determined by ELISA, according to the
manufacturer’s instructions (R&D Systems, Minneapolis, MN,
USA). Cytokine concentrations were standardized against total
protein concentration in the homogenates using a BCA protein assay
BCA (tm) Protein Assay kit (Pierce, Rockford, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
For analysis of mRNA levels, brains between 4 and 24
weeks post-irradiation were removed and the irradiated ipsilateral
cortex was carefully dissected and snap-frozen in liquid nitrogen.
Total RNA was isolated according to the manufacturer’s instructions
of the TRIzol® reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) including DNase treatment. For qPCR, reverse
transcription of RNA samples was performed using a PrimeScript™ RT
reagent (Takara Bio Inc., Shiga, Japan). The relative quantity of
target mRNA was determined using SYBR® Premix Ex Taq™ II
(Takara Bio Inc.) according to the manufacturer’s instructions. The
following PCR primers were used: VEGF, forward
5′-GCTCTCTTGGGTGCACTGGA-3′ and reverse 5′-CACCGCCTTGGCTTGTCACA-3′.
Cycle thresholds (CT) for single reactions were determined using
MyiQ software (Bio-Rad, Hercules, CA, USA) and the target genes
were normalized against GAPDH. The 2−ΔΔCT method was
used to calculate relative changes in gene expression.
Transmission electron microscopy
The rats were perfused with 4% PFA and 0.125%
glutaraldehyde under intraperitoneal anesthesia with 10%
chloraldurat (3 ml/kg). The radiation target was identified by the
rat stereotactic atlas and using a previously described method
(14). The parietal cortical
tissue specimens at the site of radiation were immediately excised
and 1 mm3 tissue blocks were cut out, followed by
fixation in a solution of 2% PFA and 2.5% glutaraldehyde in 0.1
mol/l phosphate buffer. The samples were dehydrated in a series of
ethanol dilutions and transferred to 100% propylene oxide for 15
min, followed by graded resin infiltration and embedding. Ultrathin
sections were prepared on a Leica Ultracut ultramicrotome (Leica,
Bensheim, Germany) using a 45 degree diamond histoknife. Images
were captured using an Hitachi H7600 transmission electron
microscope (Hitachi, Tokyo, Japan).
Evans Blue (EB) extravasation
A 2% (w/v) solution of EB at 4 ml/kg was injected
intravenously and allowed to circulate for 30 min. The rats were
sacrificed and the brains were dissected. Quantification of the
brain EB extravasation was performed using a spectrophotometer
(Tecan, Männedorf, Switzerland). Brains were homogenized by
vortexing in 250 μl phosphate-buffered saline (PBS) for 2 min.
Subsequently, 250 μl of 60% trichloroacetic acid was added and the
samples were vortexed for 2 min. Following cooling for 30 min, the
samples were centrifuged for 5 min at 10,000 g. Absorbance readings
were measured at 620 nm and EB extravasation was expressed as ng of
EB per milligram of brain tissue.
Fluorescent microscopy for EB
extravasation
EB can be visualized as bright orange under a
fluorescent microscope (Leica DMLB; Leica, Wetzlar, Germany). In
order to process fluorescence microscopy, the brains were perfused
via the ascending aorta with 5 ml saline, followed by 5 ml of 4%
PFA in 0.1 m PBS at pH 7.4. The brains were removed and stored
overnight in the same solution. The brains were immersed in 30%
sucrose in 0.1 m PBS for 48 h and then frozen in Optimal Cutting
Temperature medium (Sigma) and stored at −80°C. The EB
extravasation was observed in the cryostat sections (20 μm) using a
fluorescent microscope (Leica DMLB; Leica, Wetzlar, Germany).
Water content analysis by dry/wet weight
measurements
Following anesthesia, rats were euthanized by
cervical dislocation, the brains were collected and the irradiated
cortexes were separated and carefully dissected. Tissues were
weighed immediately and dried in a vacuum for up to 2 weeks. The
weights were collected throughout the drying period until the final
dry weight was established. Water content was calculated as
follows: Water content = (wet weight − dry weight) / wet weight ×
100%.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Comparisons among multiple groups were performed by analysis of
variance with Dunnett’s post hoc tests. Comparisons between the two
groups were performed by Student’s t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
VEGF+ cells in irradiated
brain tissue
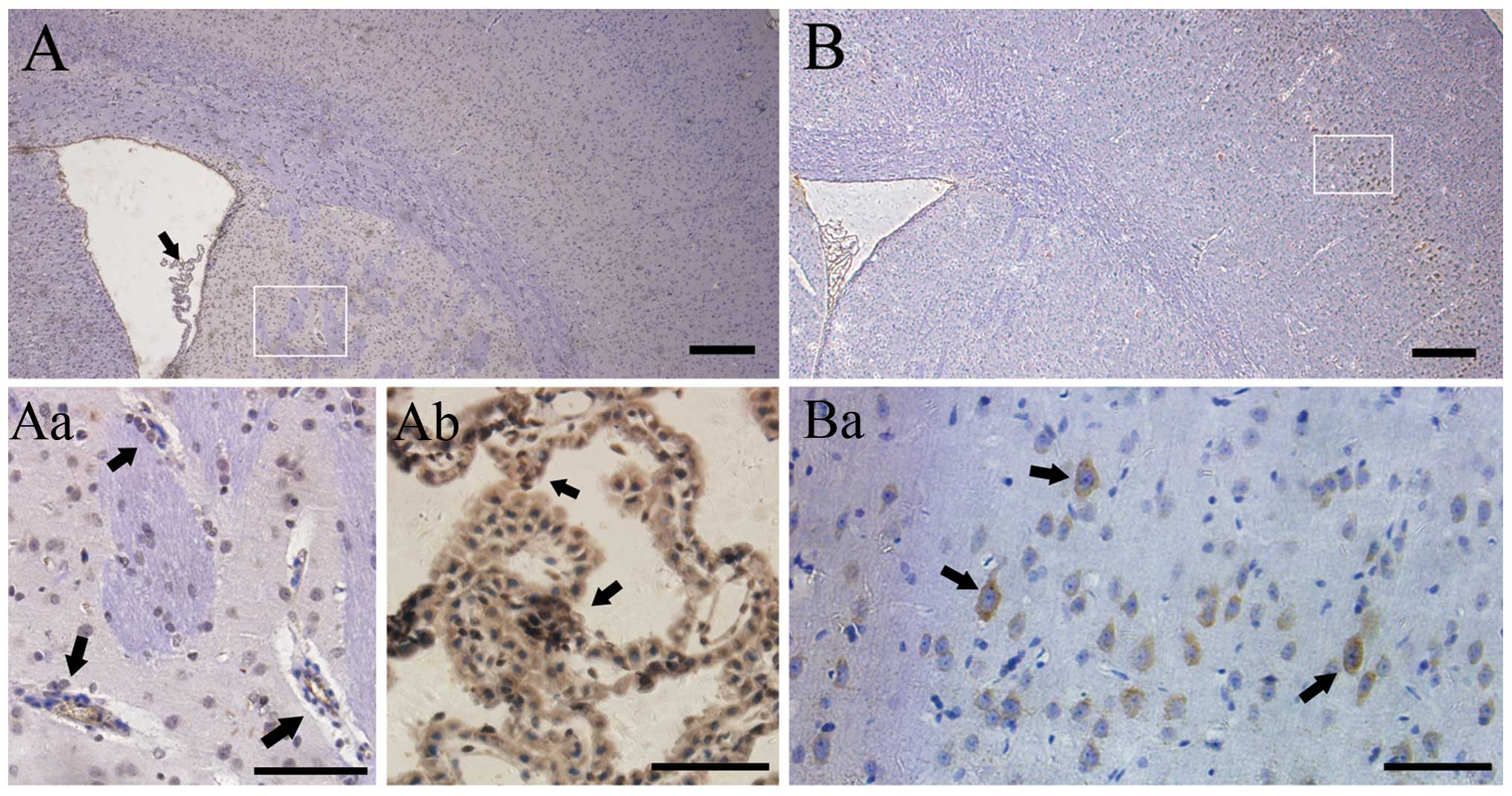
In the control group, positive staining for VEGF was
not observed in the cortex (Fig.
1A), but rather in choroid plexuses (Fig. 1Aa) and endothelium of the
periventricular region (Fig. 1Ab).
In comparison, the prominent VEGF+ cells were detected
in the superficial neuronal layers of the injured ipsilateral
cortex (Fig. 1B and Ba) 8 weeks
after radiation. The results demonstrated a dynamic change in VEGF
expression. There were hardly any immunopositive stained cells in
the irradiated right parietal cortex 4 weeks after GKS. The number
increased rapidly 8 weeks after GKS (25.500.96
cells/mm2; P<0.001; Fig.
3E) and then reached a plateau at ~16 weeks after radiation
(45.00±2.45 cells/mm2; P<0.001; Fig. 1A), prior to decreasing to baseline.
In comparison, VEGF+ cells remained constantly lower in
brain tissue from the control rats, and no significant change was
identified in VEGF+ cell distribution and quantity in
the control group during the same follow-up period (P>0.05).
Quantitative concentration analyses of
VEGF over time following irradiation
The content of VEGF in irradiated brain tissue was
quantified by ELISA. At 4, 8, 12, 16, 20 and 24 weeks post
radiation, the concentration of VEGF in the radiation group was
5.51±0.36, 7.70±1.16, 9.12±0.76, 12.95±2.13, 11.44±1.57 and
6.36±0.97 pg/ml, respectively, as compared with 5.36±0.60,
5.56±0.61, 5.55±0.84, 5.83±0.52, 6.05±0.59 and 5.50±0.87 pg/ml in
the cortex of the right hemisphere of the sham rats. The expression
of VEGF was significantly higher in rats with GKS compared with the
controls at 8, 12, 16 and 20 weeks after GKS (P<0.01; Fig. 2). Over the subsequent 8 weeks,
however, VEGF expression in the rat brain tissue receiving GKS
began to decrease and recovered to levels that were similar to
those recorded in the normal nonirradiated cortex. No difference
was observed between the radiation groups and controls at 4 and 24
weeks after GKS (P=0.82 and 0.14; Fig.
2).
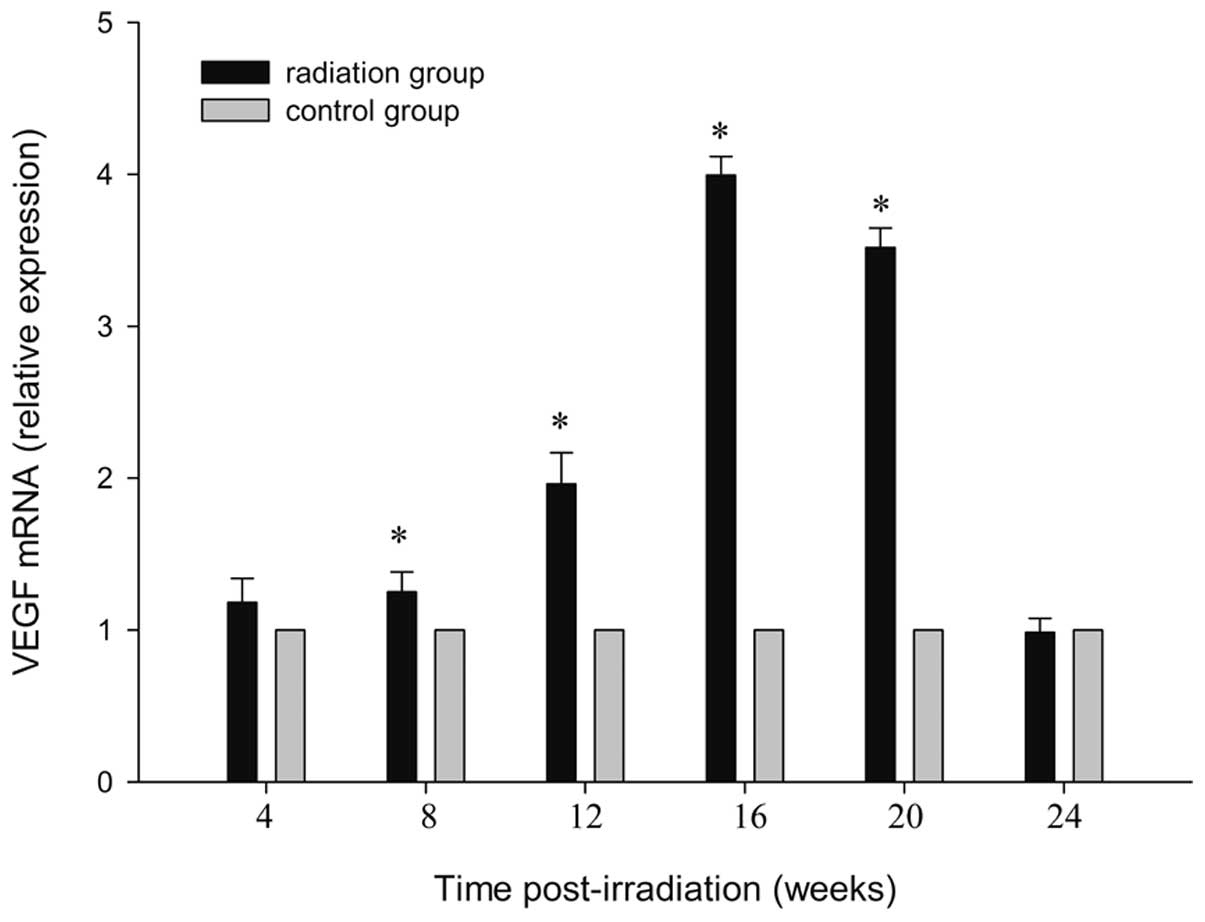
qPCR analysis
Using qPCR analysis, mRNA from irradiated brain
tissue was examined 4, 8, 16, 24, 20 and 24 weeks after irradiation
and compared with the sham-irradiated controls. At 4 weeks after
irradiation, a statistically significant upregulation was detected
(P<0.01). The highest expression of VEGF mRNA was observed at 16
weeks post-irradiation (P<0.001), and returned to the control at
24 weeks post radiation (P=0.71). Data up to 24 weeks after
irradiation are shown in Fig. 3.
By contrast, a few differentially expressed genes in the cortex of
the control rats were observed (P=0.98). The steep response in the
VEGF mRNA essentially paralleled that for protein.
Vessel density
To determine whether the changes in angiogenesis
occurred in the radiation target, CD31+ cell counts were
assessed in the sham and radiation group. Sham animals had an
average vessel density of 12.50±1.29 vessels/mm2 4 weeks
after sham injury (Fig. 4C). The
results in the sham control rats were not significantly different
at any time point following the procedure (P=0.38). As compared
with the control level, a decrease in cell counts was observed 4
weeks after GKS (8.00±0.82 vessels/mm2, P=0.02; Figs. 4D and 5). At a later time, CD31+ cell
counts increased slowly. It was significantly higher in the cortex
of irradiated rats compared with that of the control rats 12 weeks
post-irradiation (13.75±1.50 vessels/mm2; P<0.001;
Fig. 5), reached acme 16 weeks
after GKS (15.75±0.96 vessels/mm2; P<0.001; Figs. 4E and 5) and then gradually decreased to the
control level (12.00±1.16 vessels/mm2; P=0.55; Figs. 4F and 5) 24 weeks after GKS.
Electron microscopy
Ultrastructural changes of Gamma-irradiated
hemispheres of rat brains were detected by transmission electron
microscopy. The abnormal brain endothelial cells and surrounding
tissue were demonstrated in the irradiated cortex 16 weeks post
radiation (Fig. 6). Chromatin
condensation and aggregation were observed at the periphery of the
nucleons. The membranous structures demonstrated destructive
changes, including the thickened basement and the folding of the
plasma membrane. The swelling of astrocytic perivascular processes
were also observed around damaged endothelial cells. The
perivascular cells showed the presence of vacuolar-disturbed
structures in the matrix.
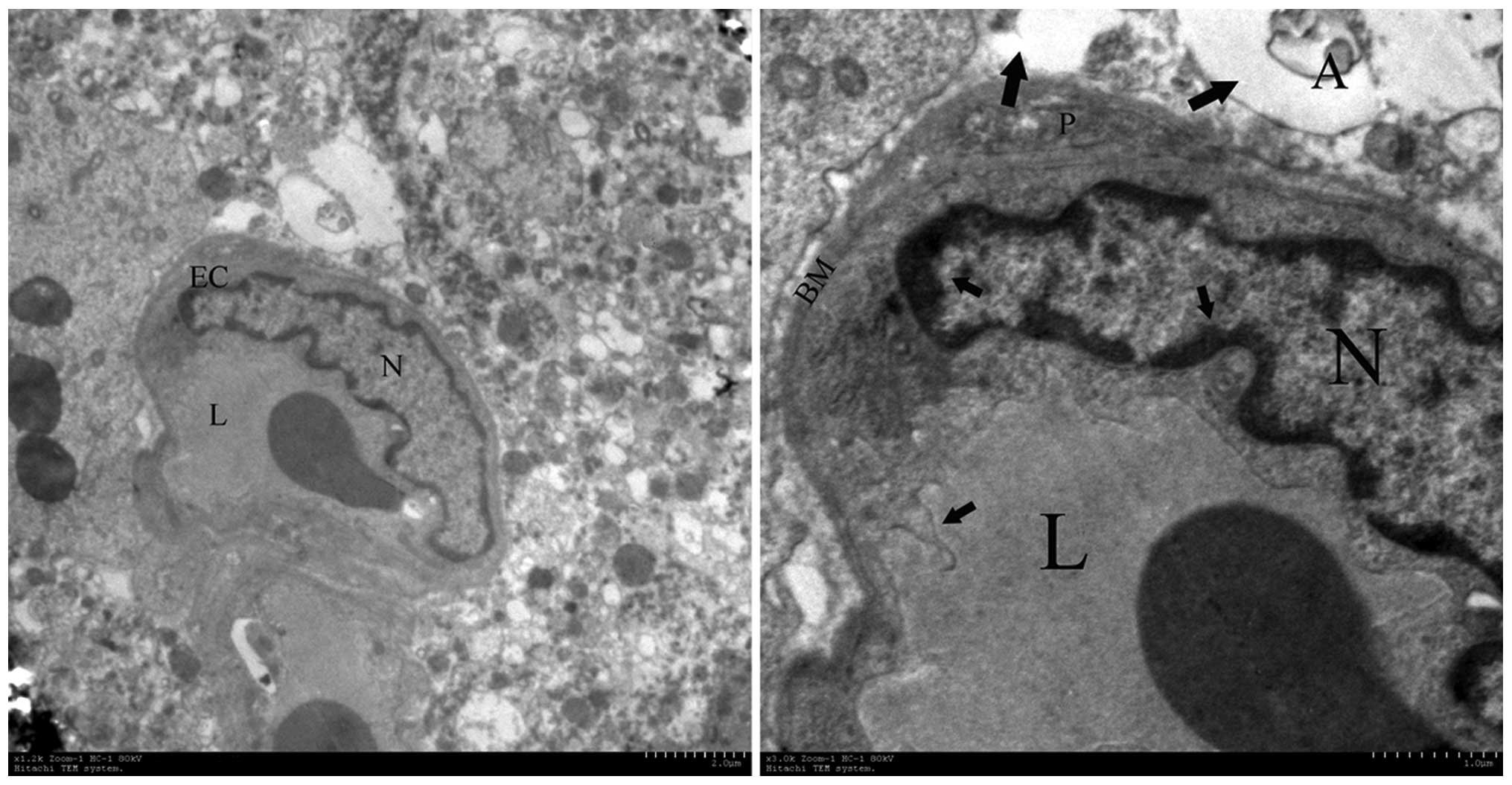 | Figure 6Ultrastructural observations of brain
capillary endothelial cells. The most pronounced alterations were
observed in disturbed vessels and perivascular structures in the
irradiated tissue at week 16, which contained enlargement of
endothelial nuclei, pykno-chromatin (short arrow), thickening of
the capillary basement membrane, folding of the plasma membrane
(short arrow) and astroglial edema (long arrow). A, astrocyte; EC,
endothelial cells; N, nuclei, L, capillary lumen; BM, basement
membrane. Magnification: Left, ×10,000; right, ×50,000. |
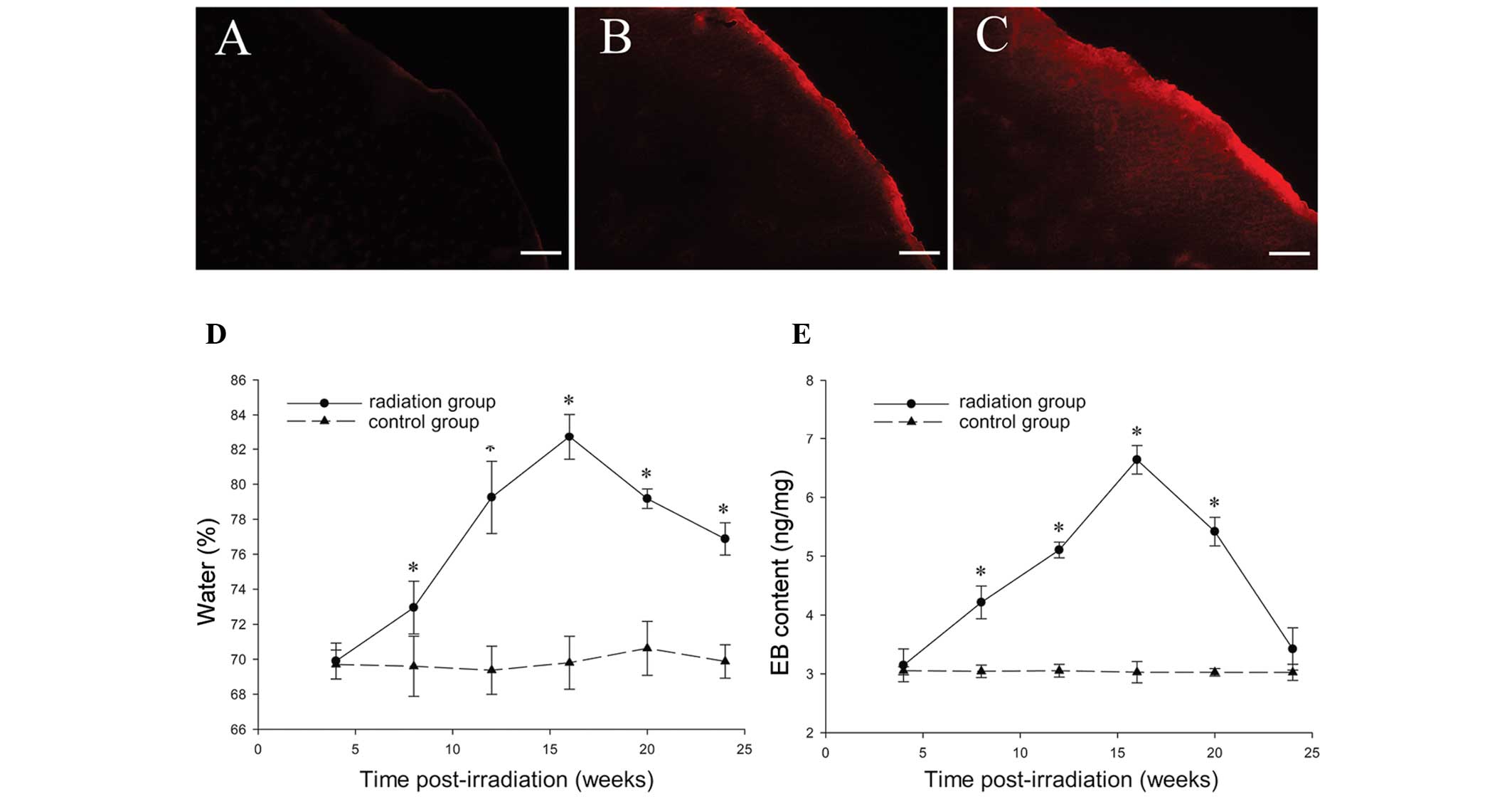
Brain water content
An increase in brain water content was observed in
irradiated tissue (Fig. 7D) 8
weeks post-irradiation as compared with the sham controls (P=0.03).
The highest brain water content was observed 20 weeks
post-radiosurgery in the irradiated tissue (82.73±1.30%;
P<0.001), following which the content gradually decreased;
however, it remained higher than the control levels (P<0.001).
No change in brain water content was detected in any of the
sham-irradiated control animals at various intervals (P=0.85).
EB extravasation experiment
No EB fluorescence was detected in the right
parietal cortex 4 weeks after GKS (Fig. 7A). EB fluorescence was only
observed in the irradiated cortex (Fig. 7B) 8 weeks following GKS, and
appeared to be significantly stronger 16 weeks after GKS (Fig. 7C). The EB extravasation in the
radiation and sham surgery groups was quantified as ng of EB per
milligram of brain tissue at 4, 8, 12, 16, 20 and 24 weeks after
GKS. The EB concentration in the radiation tissue was significantly
higher than in the sham operation group at 8, 12, 16 and 20 weeks
after GKS (P<0.001; Fig. 7E).
Although the content demonstrated a maximum level 16 weeks after
GKS (6.64±0.24 ng/mg; P<0.001; Fig.
7E), there was a significantly steep slope of EB content in the
radiation group between 20 and 24 weeks after GKS. No differences
were identified between the radiation and sham groups 24 weeks
after GKS (P=0.09; Fig. 7E).
Discussion
In the field of neurological surgery, GKS is
becoming an indispensable treatment means of various brain
disorders. The radiation dose that can be administered safely is
nearly always limited by the risk of radiation injury to normal CNS
tissue. An understanding of the mechanisms of normal tissue injury
following GKS may have important implications and applications in
the development of radiation modulators or treatments to reduce the
consequences of a given radiation exposure.
The protein level of VEGF is overexpressed in a
variety of CNS diseases, including tumor, ischemia and traumatic
brain injury (17–19). In intraoperative radiotherapy, the
expression of VEGF in the irradiated cerebral hemispheres was
significantly increased within 8 weeks in the radiation group
(20). Furthermore, in the rat
spinal cord, the induction of the expression of VEGF by a high dose
of 22 Gy X-ray has been previously demonstrated (21). However, alterations in VEGF in
response to GKS remain largely unknown. The present study disclosed
marked VEGF-positivity in the radiated normal brain following GKS
in the subacute stage. In this series, VEGF expression induced by
radiosurgery was time dependent. Its expression in irradiated
regions gradually increased over a period of 8 weeks, reached
maximal expression 16 weeks after radiosurgery and started to
decrease 20 weeks after radiosurgery. The mRNA expression in
irradiated tissue was also observed between 4 and 20 weeks after
GKS, which was similar to that of the protein expression. The early
changes of VEGF following conventional radiotherapy and
radiosurgery are similar, but the increased VEGF following
radiosurgery sustained longer time. Radiosurgery-induced delayed
damage to the normal brain can occur several months following
treatment. This later decrease in VEGF expression was likely
associated with delayed cell loss or necrosis. VEGF is important in
angiogenesis, which is either a protective factor or a response to
injury. VEGF drives the formation of new blood vessels in various
neurological disorders, including tumors, stroke and traumatic
brain injury (22–24). In the current study, this dynamic
change in VEGF was likely attributable to pathological angiogenesis
following GKS.
Several experiments have demonstrated endothelial
cell dynamics in the rat brain following local irradiation. For
example, it has been demonstrated that a decrease in endothelial
cell number was observed within 1 day of irradiation with doses of
25 Gy, an abortive recovery occurred at ~25 weeks, and then
decreased to 50% by the time white matter necrosis became evident
(25). By contrast, a tendency
towards an increase in the average cross-sectional area of the
vessels in irradiated regions occurred 1 month after 75 Gy
radiation, and 1 week after 120 Gy radiation (14). In the current study, the loss of
endothelial cells appeared 4 weeks after GKS compared with the
controls. Subsequently, a slight vessel density increase was
observed 12 weeks after radiation; this effect became clear 16
weeks after GKS and then gradually decreased to the control level.
The change in angiogenesis was consistent with the change in VEGF
expression. This suggested an important role for VEGF in the
angiogenesis of GKS injury; however, the results cannot suggest
that VEGF has a protective effect on GKS injury in rats, as VEGF
may also have detrimental effects, including an increase in
endothelial permeability, which is recognized as a cardinal feature
of pathological angiogenesis. A previous study demonstrated that
there were pathological vascular alterations in the irradiated
field following GKS, which included increased vascularity, edema
and fibrin exudation (26). In the
present study, the swollen endothelial cell, thickened basement
membrane and severely edematous end-feet of glial cells in
irradiated areas could be observed 16 weeks after radiation. This
morphological alteration indicated that there could be pathological
angiogenesis in the radiation-damaged region. In this condition,
the blood brain barrier function was likely altered, resulting in a
subsequent increase in vascular permeability.
VEGF-induced increases in microvessel permeability
have been demonstrated not only in normal brain endothelial cells,
but also in diseased conditions, including in tissue surrounding
brain tumors (27) and the
ischemia lesion area (28). In
addition, the upregulation of VEGF in the irradiated spine was
present in regions where increased permeability was observed
(16). In the present study, the
EB extraction ratio also increased with time, attaining a plateau
value in ~16 weeks and then declining toward control levels.
Increases in brain water content were initially observed 8 weeks
after irradiation, increased sharply to maximal value 16 weeks
after radiosurgery treatment and then declined at 20 weeks. The
maximal expression of VEGF coincided with the peak value of brain
edema and EB extraction ratio. This is also similar to a previous
study where VEGF expression in the spinal cord was present 16–20
weeks after 22 Gy irradiation, when significant blood spinal cord
barrier breakdown is observed (29). By contrast, EB extravasation was
observed in the irradiated cortex with higher VEGF expression, and
no VEGF expression or changes in EB extravasation were detected in
nonirradiated regions in animals receiving radiosurgery and the
cortex in the controls. The temporal and spatial association of
increased VEGF protein and vascular lesions suggests that VEGF
upregulation is associated with increases in vascular permeability
and edema formation resulting from Gamma knife radiation
injury.
In contrast to VEGF and EB extraction, brain water
was at a higher level between 20 and 24 weeks after GKS, although
it began to decrease. Considering the complexity of edema formation
and pathological effects of radiation, the current experiments
cannot fully clarify this result. Previous studies have
demonstrated that histological changes following radiation are time
dependent (14). The vascular
response occurred shortly following radiation, while necrosis and
loss of cells appeared following a more prolonged
post-radiosurgical interval (30).
The delayed tissue injury is likely to be initiated by capillary
modifications leading to microcirculatory disturbance. Although
VEGF was at a lower level 24 weeks following GKS, early VEGF
increases and vascular abnormality were likely associated with late
edema formation.
In conclusion, our data revealed that the expression
of VEGF in the irradiated cortex was significantly dynamic over
time following the subacute period, when abnormal angiogenesis and
alterations in vascular permeability were observed. These results
indicated that the alterations in VEGF expression in the irradiated
tissue may be an important cause of the development of pathological
angiogenesis and cerebral edema following GKS. The time course of
expression of VEGF may provide important dependencies for
therapeutic opportunity of GKS radiation injury. The aim of our
future experiments is to identify crucial molecular mechanisms in
this event that may potentially cause gamma knife radiation
injury.
References
|
1
|
Buis DR, Meijer OW, van den Berg R, et al:
Clinical outcome after repeated radiosurgery for brain
arteriovenous malformations. Radiother Oncol. 95:250–256. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dewan S and Norén G: Retreatment of
vestibular schwannomas with Gamma Knife surgery. J Neurosurg.
109(Suppl): 144–148. 2008.PubMed/NCBI
|
|
3
|
Friehs GM, Park MC, Goldman MA, et al:
Stereotactic radiosurgery for functional disorders. Neurosurg
Focus. 23:E32007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai R, Barnett GH, Novak E, Chao ST and
Suh JH: Principal risk of peritumoral edema after stereotactic
radiosurgery for intracranial meningioma is tumor-brain contact
interface area. Neurosurgery. 66:513–522. 2010. View Article : Google Scholar
|
|
5
|
d‘Avella D, Cicciarello R, Albiero F, et
al: Quantitative study of blood-brain barrier permeability changes
after experimental whole-brain radiation. Neurosurgery. 30:30–34.
1992.PubMed/NCBI
|
|
6
|
Yuan H, Gaber MW, McColgan T, et al:
Radiation-induced permeability and leukocyte adhesion in the rat
blood-brain barrier: modulation with anti-ICAM-1 antibodies. Brain
Res. 969:59–69. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stewart PA, Vinters HV and Wong CS:
Blood-spinal cord barrier function and morphometry after single
doses of x-rays in rat spinal cord. Int J Radiat Oncol Biol Phys.
32:703–711. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mukhopadhyay D, Tsiokas L, Zhou XM, et al:
Hypoxic induction of human vascular endothelial growth factor
expression through c-Src activation. Nature. 375:577–581. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamoun WS, Ley CD, Farrar CT, et al: Edema
control by cediranib, a vascular endothelial growth factor
receptor-targeted kinase inhibitor, prolongs survival despite
persistent brain tumor growth in mice. J Clin Oncol. 27:2542–2552.
2009. View Article : Google Scholar
|
|
11
|
Ma Y, Qu Y and Fei Z: Vascular endothelial
growth factor in cerebral ischemia. J Neurosci Res. 89:969–978.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li YQ, Ballinger JR, Nordal RA, Su ZF and
Wong CS: Hypoxia in radiation-induced blood-spinal cord barrier
breakdown. Cancer Res. 61:3348–3354. 2001.PubMed/NCBI
|
|
13
|
Tokumaru O, Hayashi M, Katayama Y, et al:
Gamma knife radiosurgery targeting protocols for the experiments
with small animals. Stereotact Funct Neurosurg. 85:135–143. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kamiryo T, Kassell NF, Thai QA, et al:
Histological changes in the normal rat brain after gamma
irradiation. Acta Neurochir (Wien). 138:451–459. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hirano M, Rakwal R, Kouyama N, et al:
Gel-based proteomics of unilateral irradiated striatum after gamma
knife surgery. J Proteome Res. 6:2656–2668. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paxinos GWC: The Rat Brain in Stereotaxic
Coordinates. 4th edition. Academic Press; San Diego, CA: pp.
871998
|
|
17
|
Hai J, Li ST, Lin Q, et al: Vascular
endothelial growth factor expression and angiogenesis induced by
chronic cerebral hypoperfusion in rat brain. Neurosurgery.
53:963–970. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plate KH, Breier G, Millauer B, Ullrich A
and Risau W: Up-regulation of vascular endothelial growth factor
and its cognate receptors in a rat glioma model of tumor
angiogenesis. Cancer Res. 53:5822–5827. 1993.PubMed/NCBI
|
|
19
|
Shore PM, Jackson EK, Wisniewski SR, et
al: Vascular endothelial growth factor is increased in
cerebrospinal fluid after traumatic brain injury in infants and
children. Neurosurgery. 54:605–611. 2004. View Article : Google Scholar
|
|
20
|
Kim JH, Chung YG, Kim CY, Kim HK and Lee
HK: Upregulation of VEGF and FGF2 in normal rat brain after
experimental intraoperative radiation therapy. J Korean Med Sci.
19:879–886. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li YQ, Ballinger JR, Nordal RA, Su ZF and
Wong CS: Hypoxia in radiation-induced blood-spinal cord barrier
breakdown. Cancer Res. 61:3348–3354. 2001.PubMed/NCBI
|
|
22
|
Bulnes S and Lafuente JV: VEGF
immunopositivity related to malignancy degree, proliferative
activity and angiogenesis in ENU-induced gliomas. J Mol Neurosci.
33:163–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen F, Fan Y, Su H, et al:
Adeno-associated viral vector-mediated hypoxia-regulated VEGF gene
transfer promotes angiogenesis following focal cerebral ischemia in
mice. Gene Ther. 15:30–39. 2008. View Article : Google Scholar
|
|
24
|
Sköld MK, von GC, Sandberg-Nordqvist AC,
Mathiesen T and Holmin S: VEGF and VEGF receptor expression after
experimental brain contusion in rat. J Neurotrauma. 22:353–367.
2005.PubMed/NCBI
|
|
25
|
Ljubimova NV, Levitman MK, Plotnikova ED
and Eidus LKh: Endothelial cell population dynamics in rat brain
after local irradiation. Br J Radiol. 64:934–940. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schultheiss TE, Kun LE, Ang KK and
Stephens LC: Radiation response of the central nervous system. Int
J Radiat Oncol Biol Phys. 31:1093–1112. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Criscuolo GR: The genesis of peritumoral
vasogenic brain edema and tumor cysts: a hypothetical role for
tumor-derived vascular permeability factor. Yale J Biol Med.
66:277–314. 1993.PubMed/NCBI
|
|
28
|
Zhang ZG, Zhang L, Jiang Q, et al: VEGF
enhances angiogenesis and promotes blood-brain barrier leakage in
the ischemic brain. J Clin Invest. 106:829–838. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsao MN, Li YQ, Lu G, Xu Y and Wong CS:
Upregulation of vascular endothelial growth factor is associated
with radiation-induced blood-spinal cord barrier breakdown. J
Neuropathol Exp Neurol. 58:1051–1060. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kamiryo T, Lopes MB, Kassell NF, Steiner L
and Lee KS: Radiosurgery-induced microvascular alterations precede
necrosis of the brain neuropil. Neurosurgery. 49:409–414.
2001.PubMed/NCBI
|