Introduction
In recent years, there has been a consistent
increase in the number of antibiotic-resistant bacteria due to the
extensive use of antimicrobial agents in domestic animals, which
are subsequently transmitted to humans through the food chain. The
virulence and pathogenicity of a bacterium increases with the
increase in its antibiotic resistance. Therefore, it is important
to identify alternative drugs that can replace traditional
antibiotics, thereby reducing the development and spread of
resistance. Additionally, elucidaqting the mechanism of action of
these alternative compounds and the resistance of bacterium to
these compounds provides essential information for basic
microbiological research.
Fraxetin, a major constituent of the traditional
medicinal plant Fraxinus rhynchophylla, has been found to
possess multiple bioactivities, including scavenging reactive
oxygen species and inhibiting lipid peroxidation in the rat brain
(1,2). Previous studies have demonstrated
that fraxetin has antibacterial activities against
Staphylococcus aureus, however, its inhibitory mechanism
remains to be elucidated (3–5).
Fraxetin is widely available and relatively cheap, and is known to
have few side effects, and low resistance as well as other
beneficial properties. In the present study, S. aureus was
used and the antibacterial mechanism of fraxetin was examined
through studies on the permeability of the cell membrane and
changes in the content of nucleic acid and soluble proteins in
order to provide a theoretical basis for the development of
antibacterial drugs with high efficacy and low toxicity.
Materials and methods
Materials
S. aureus (ATCC26112) was obtained from the
Chinese Medicine Bacterial Preservation Centre (Beijing, China).
Fraxetin, at 99% purity, was purchased from Nuowei Xin (Dalian,
China). The fraxetin was dissolved in absolute ethanol and
concentrated solutions were added to the bacterial cultures to
maintain the lowest possible concentration of ethanol in the
cultures. The restriction enzyme, pBR322, was purchased from Takara
Bio, Inc. (Shiga, Japan). DAPI was purchased from Beyotime
Institute of Biotechnology (Shanghai, China). Common chemicals
(ethanol, NaCl, KCl, KH2PO4,
K2HPO4, beef extract, peptone) were purchased
from Tianjin Kemiou Chemical Reagent Co, Ltd. (Tianjin, China).
SDS, Tris-base, bovine serum albumin, adenosine triphosphate and
proteinase K were purchased from Sangon Biotech Co., Ltd.
(Shanghai, China). All assays were performed according to the
manufacturer’s instructions.
Determination of the electrical
conductivity of the culture medium
S. aureus was cultured to logarithmic phase,
subpackaged in a test tube and treated with 0.05 mg/ml fraxetin at
37°C for 0, 1, 2, 3, 4, 5, 6, 7 and 8 h. The electrical
conductivity of the culture medium was then determined, with
ethanol used as a control group. Each experiment was repeated three
times (6).
Measurement of the quantity of DNA and
RNA
S. aureus was obtained by centrifugation
(3,000 × g for 10 min) and washed twice with phosphate-buffered
saline (PBS; 135 mM NaCl, 2.7 mM KCl, 1.5 mM
KH2PO4 and 8 mM K2HPO4;
pH 7.0). The cells were suspended in PBS and treated with 0.05
mg/ml fraxetin for 0, 1, 2, 3, 4, 5, 6, 7 and 8 h. Following the
reaction, the supernatant fluid was measured by ultraviolet-visible
spectrophotometry (UV1100 model; Shanghai Tianmei Scientific
Instruments Co., Ltd., Shanghai, China) to analyze the content of
DNA and RNA, with ethanol used as a control group. Each experiment
was repeated three times (7).
Determination of the S. aureus soluble
protein content
S. aureus was inoculated into 50 ml beef
extract peptone medium (containing 3 g beef extract, 10 g peptone,
5 g NaCl and 1 litre distilled water; pH 7.0) with fraxetin (final
concentration 0.05 mg/ml, with ethanol as a control group) and
cultured in a rotary shaker (120 rpm) at 37°C for 16 h. Cells were
collected and 0.5 mg of cells were suspended in 40 μl double
evaporated water and 160 μl loading buffer, incubated in boiling
water for 8 min and centrifuged to obtain supernatant. The
supernatant was loaded in SDS-PAGE to quantitatively analyze the
change in soluble protein content in S. aureus with ethanol
as a control group.
Determination of the DNA and RNA content
of S. aureus
S. aureus was inoculated into beef
extract-peptone medium with fraxetin (final concentration 0.05
mg/ml, ethanol as a control) and cultured for 12, 16, 20 and 24 h.
The cells were then collected and resuspended in aquae sterilisata.
Triple volume of 4′,6-diamidino-2-phenylindole (DAPI; 1:3 diluent,
quarter-strength ringer’s solution) was added to obtain the
resuspended bacterial culture. The cell samples were then placed
onto a microslide and placed in the dark for 10 min. The
fluorescence of DAPI in cells was observed using an inverted
fluorescence microscope (Olympus IX71; Olympus, Tokyo, Japan). Each
experiment was repeated three times (8).
Enzyme preparation
DNA Topo I and TopoII were extracted from
S.aureus. Topoisomerase activity was examined using the DNA
relaxation reaction. One unit of topoisomerase activity was defined
as the quantity of enzyme required to fully relax 0.5 μg
supercoiled pBR322 DNA (9).
Effects of fraxetin on DNA topoisomerase
activity
DNA relaxation assays were based on the following
procedure: 0.5 μg pBR322, 1 U of Topo I/II and 2 μl fraxetin (final
concentrations 0.02, 0.05 and 0.08 mg/ml) or 100% ethanol (control)
was added to 20 μl reaction buffer containing 10 mM
Tris-hydrochloride (Tris-HCL, pH 7.5), 50 mM potassium chloride, 50
mM sodium chloride, 5 mM magnesium chloride, 0.1 mM EDTA, 15 mg/ml
bovine serum albumin and 1 mM adenosine triphosphate (ATP; omitted
in the Topo I-mediated DNA relaxation). The reaction was performed
at 37°C for 30 min and inhibited by the addition of 1 μl Proteinase
K (10 mg/ml) and 1 μl 10% sodium dodecyl sulfate (SDS) at 37°C for
30 min. The DNA samples were loaded onto a 1% agarose gel and
visualized using a transilluminator (10,11).
Effects of fraxetin on DNA
Various concentrations of fraxetin (final
concentrations 0.01, 0.03 and 0.05 mg/ml) and 0.5 μg pBR322 were
added to 2.5 μl helicase buffer I or helicase buffer II (as
mentioned above), with the final reaction volume made up to 20 μl
with distilled water. The reaction was incubated at 37°C for 30
min. Following incubation, 1 μl proteinase K (10 mg/ml) and 2 μl
10% SDS were added and incubated at 37°C for an additional 30 min
to inhibit enzyme activity. Ethanol was used as a control group.
The samples were loaded onto a 1% agarose gel and visualized using
a transilluminator (12,13).
Fraxetin (final concentration 0.02 mg/ml) and pBR322
(final concentration 0, 0.25, 0.35, 0.45 and 0.55 mg/ml) were
dissolved in 1.5 ml Tris-HCl (pH 7.2). Following incubation at 37°C
for 30 min, the samples were examined using a UV-1100 ultraviolet
spectrophotometer at 300–450 nm (14).
Effects of fraxetin on DNA restriction
enzyme digestion
Fraxetin (0.05 mg/ml) and pBR322 (1 μg) were
dissolved in 0.5 μl Tris-HCl and incubated at 37°C for 30 min. The
digestive reaction was performed using 1 μl TaqI,
EcoRI, EcoRII, HindIII, BamHI and
SalI, respectively and 2 μl loading buffer at 37°C for 30
min, with the final reaction volume made up to 20 μl. The samples
were then loaded onto a 1% agarose gel and visualized using a
transilluminator.
Results
Effect of fraxetin on the cell membrane
of S. aureus
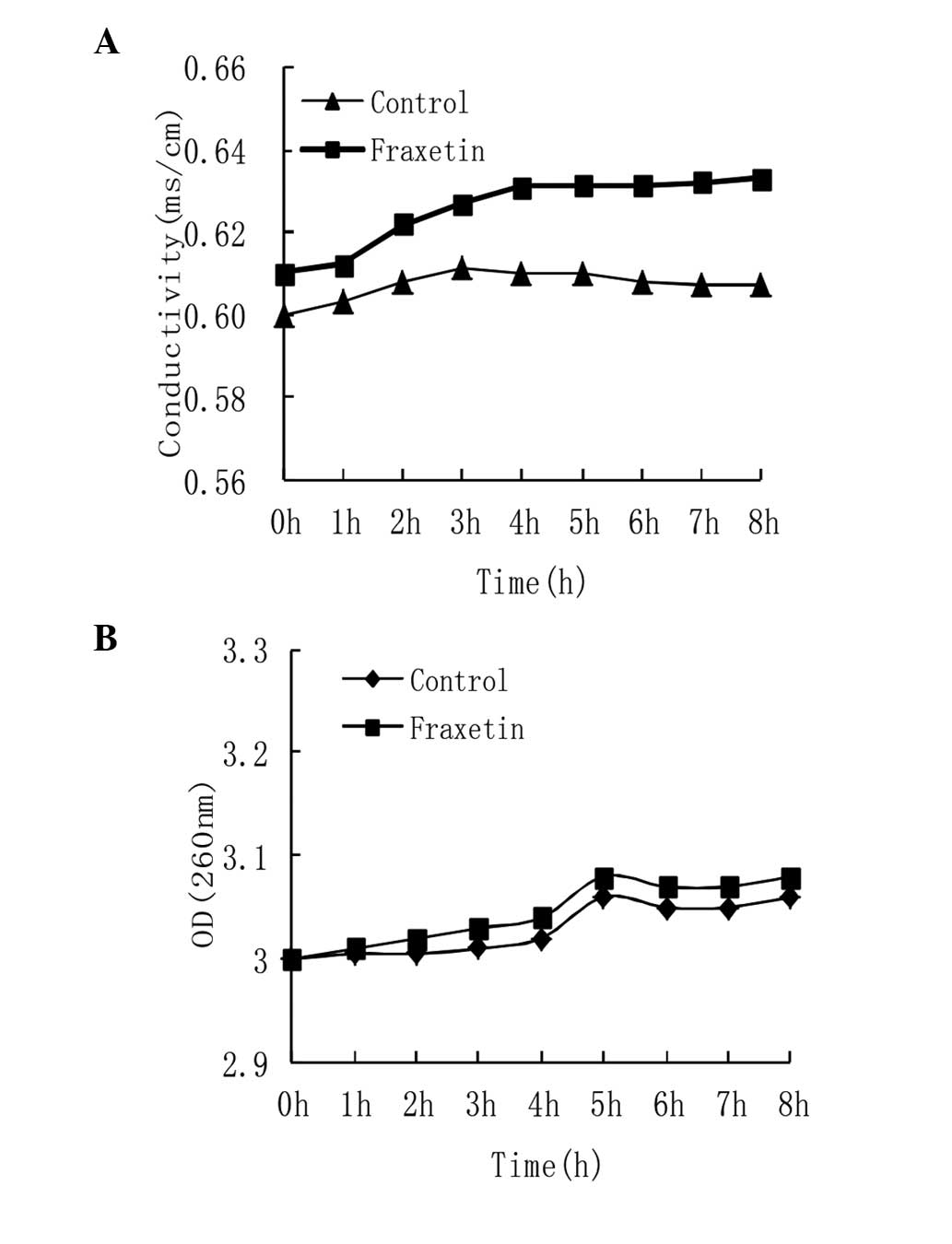
The cell membrane integrity can be conjectured by
measuring the alterations in electrical conductivity and
macromolecules, including DNA and RNA in culture medium following
the addition of drugs. The results indicated that the electrical
conductivity increased as the incubation time with fraxetin
increased. Following treatment of S. aureus with fraxetin
for 8 h, conductivity was increased by 5% (P<0.05), compared
with the control group (Fig. 1A),
which demonstrated that fraxetin had an effect on the integrity of
the membrane.
The release of macromolecular material, including
DNA and RNA can further demonstrate the effect of fraxetin on cell
membrane integrity. The result revealed little change in the
content of DNA and RNA in culture medium following treatment with
fraxetin, compared with the control group (P>0.01; Fig. 1B), which demonstrated that the
fraxetin did not disintegrate the cell membrane but did cause a
small degree of damage.
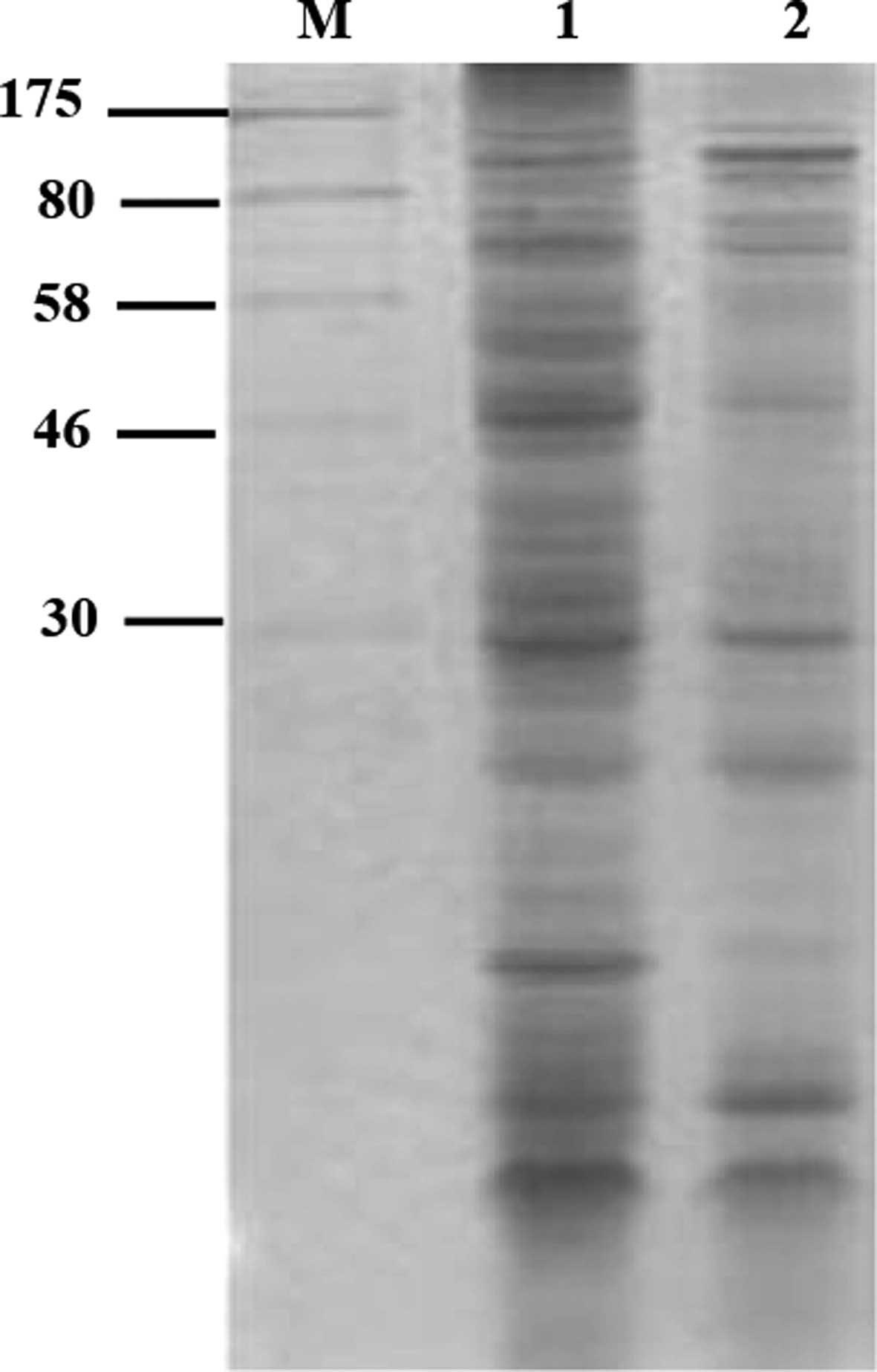
Effect of fraxetin on S. aureus soluble
protein synthesis
The result of SDS-PAGE indicated that soluble
protein synthesis was significantly reduced by 55.74% (P<0.01)
following treatment of S. aureus with fraxetin for 16 h
(Fig. 2), compared with the
control group. This may be due to fraxetin inhibiting nucleic acid
synthesis and the expression of associated genes.
Effect of fraxetin on S. aureus nucleic
acid synthesis
DAPI is a fluoresecent dye that binds DNA and RNA.
The dye increases in its fluorescence with increases in the
quantity of nucleic acids. In the S. aureus cells treated
with fraxetin for 16 h, the fluorescence spectrophotometry
measurements demonstrated that DNA synthesis was significantly
reduced up to 33.86% and that RNA synthesis was significantly
reduced up to 48.96% compared with the control group, indicating
that fraxetin had an adverse effect on nucleic acid synthesis.
However, after 16 h, the nucleic acid content of the cells
demonstrated an increasing trend, which may have been due to the
increase in incubation time, the effectiveness of the drug
gradually being reduced or the cells repairing the damaged DNA
(Fig. 3).
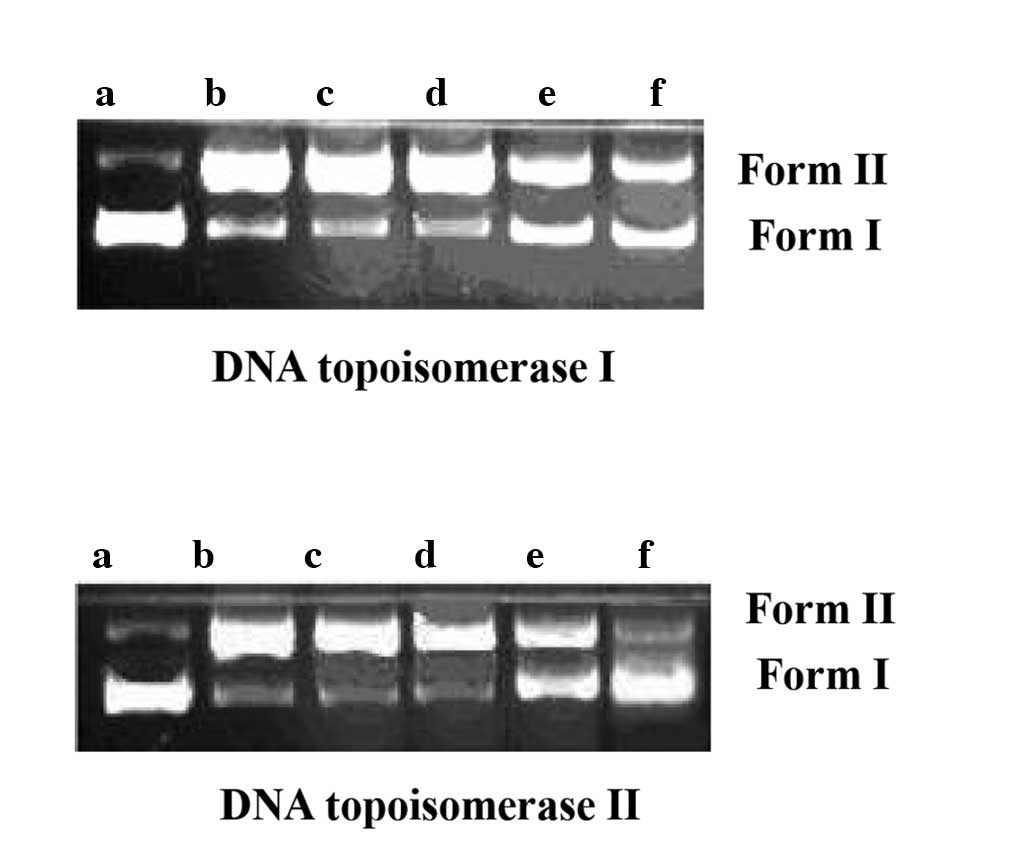
Effect of fraxetin on DNA topoisomerase
activity
The effect of fraxetin on the strand passage
activity of topoisomerase was determined by enzyme-mediated
negatively supercoiled pBR322 relaxation. As shown in Fig. 4, the activity of topoisomerase I
and II was significantly inhibited from a fraxetin concentration of
0.05 mg/ml. These results suggested that fraxetin acted on
topoisomerase I and II, thereby affecting nucleic acid synthesis
and inhibiting bacterial growth.
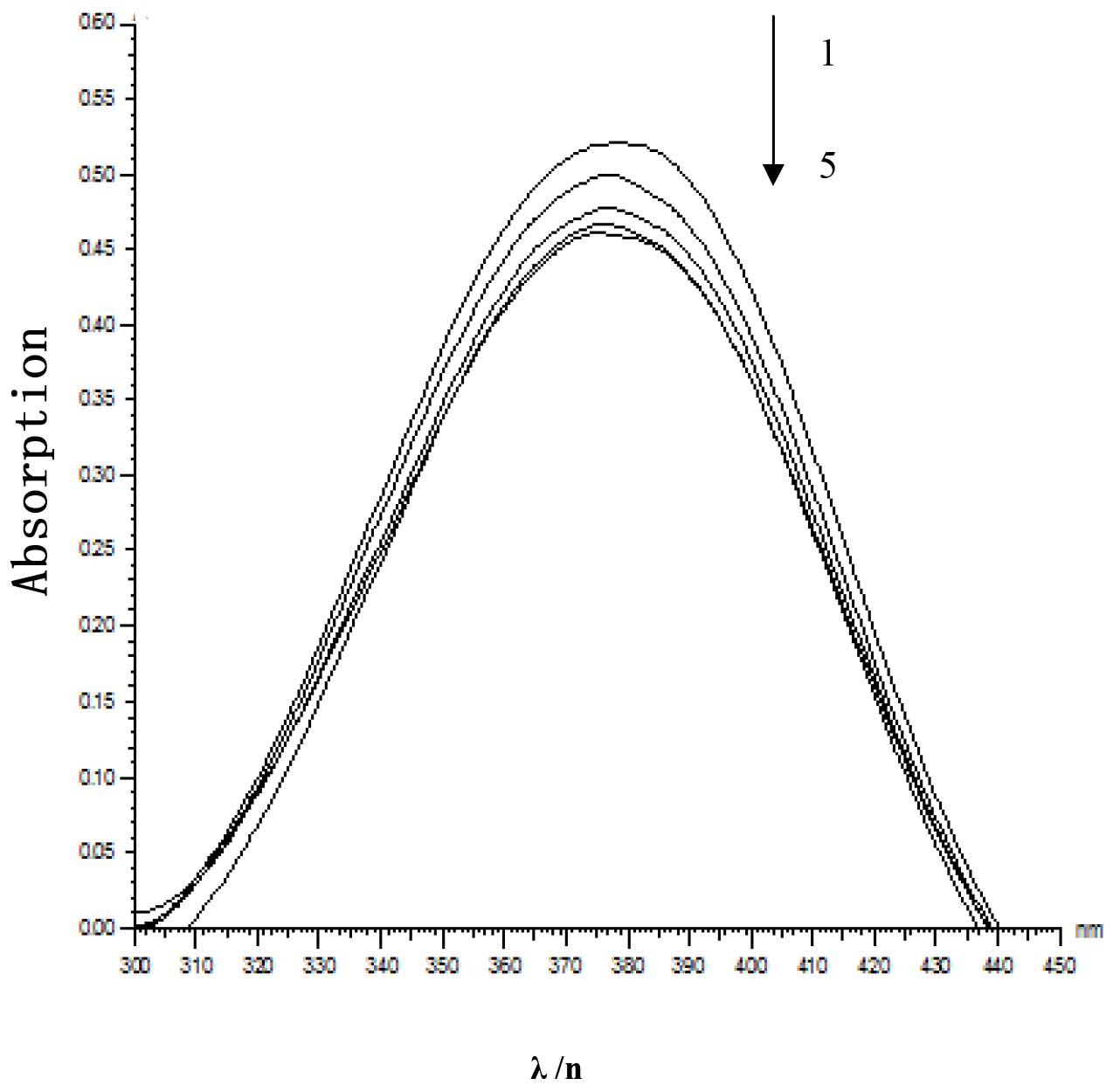
Interaction of fraxetin and DNA
In order to investigate the direct cleavage effect
of fraxetin on DNA, pBR322 DNA was incubated with different
concentrations of fraxetin. With increasing concentrations of
fraxetin, the quantity of supercoiled DNA (Form I) decreased, while
open circular DNA and linear DNA (Form II) increased, indicating
that fraxetin was able to interact with DNA and cleave it (Fig. 5). UV-visible spectrophotometric
determinations of fraxetin demonstrated characteristic blue shift,
hypochromism and isosbestic points with increases in DNA
concentration (Fig. 6). The
results indicated that the binding parameters of fraxetin with DNA
were 3.6±1 (Fig. 7).
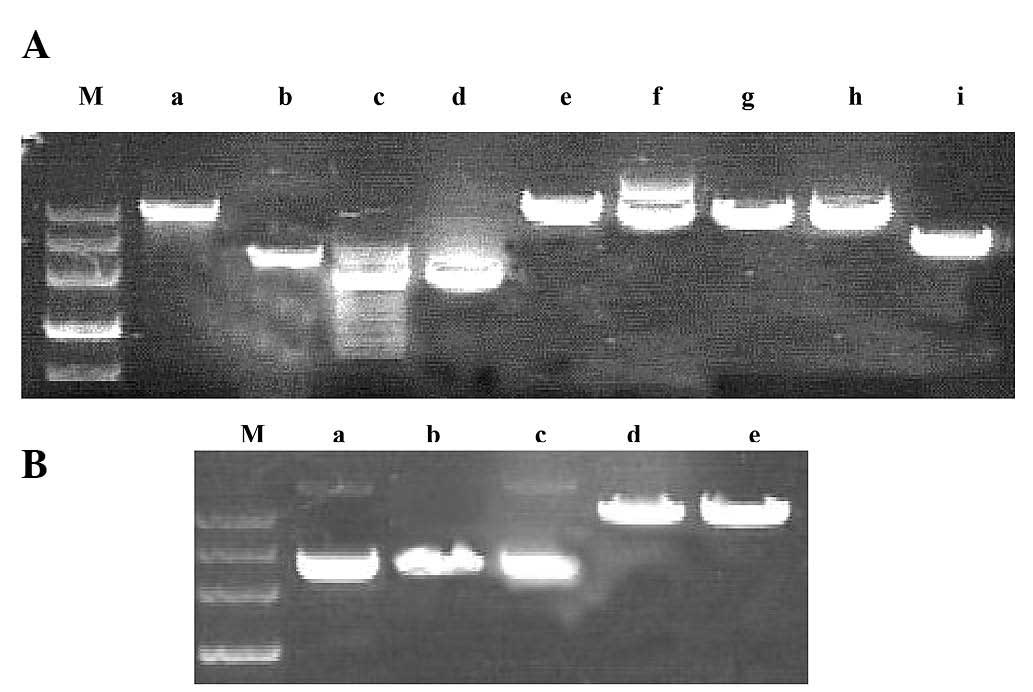
Effect of restriction enzymes
The results mentioned above indicated that fraxetin
interacted intercalatively with DNA. A total of six DNA restriction
enzymes (TaqI, EcoRI, EcoRII, HindIII,
SalI and BamHI) with different cut sites were used to
predict the binding points between fraxetin and DNA through
observing a digestion map (Fig. 8A and
B). The observation indicated that fraxetin specifically
inhibited the digestion of DNA by TaqI and HindIII,
which recognizes the T/CGA and A/AGCT sites, and thus may be the
result of fraxetin binding to the T/CGA, A/AGCT sites or a similar
site.
Discussion
The general mechanisms of antibacterial drugs
include damaging the integrity of the cell wall and the cell
membrane and inhibiting the synthesis of proteins and nucleic acids
(15). The electrical conductivity
results indicated that fraxetin did not cause destruction of the
integrity of the cell wall or cell membrane and that the target of
bacteriostasis was intracellular. Protein band quantitative
analysis demonstrated that fraxetin affects protein synthesis, as
the protein content of solution reduced by 55.74% (P<0.01)
following treatment with fraxetin for 16 h. Incubating S.
aureus with fraxetin for 16 h significantly reduced the
quantities of DNA and RNA by 33.86 (P<0.01) and 48.96%
(P<0.01), respectively, compared with the control group. This
confirmed that there was a change in the genetic material, which
may explain the change in protein content. The results from our
previous experiments indicated that the change in nucleic acid
content was associated with the drug inhibiting DNA topoisomerase
(16). DNA topoisomerase is
necessary for DNA replication, facilitating the short-term
separation of single-stranded or double-stranded DNA (17,18).
The results demonstrated that fraxetin significantly inhibited the
activity of DNA topoisomerase I and II, with inhibition of
topoisomerase I and II activity at 0.05 mg/ml fraxetin and complete
inhibition at 0.08 mg/ml fraxetin. Therefore, from this
observation, it was inferred that DNA topoisomerase could be one of
the direct targets for the antibacterial action of fraxetin.
Previous studies have demonstrated that the mechanism of
topoisomerase inhibitors was to produce drug-topoisomerase-DNA
cleavable complexes or to interfere with the binding of
topoisomerase to DNA (19,20). Results from the present study
revealed that the UV-visible absorption spectrum altered as the
concentration of DNA changed and exhibited hypochromism and a blue
shift. According to the criteria proposed by Long and Barton
(14), through the observation of
hypochromism and blue shifts following treatment of small molecules
with DNA, it can be inferred that the drug had an intercalative
interaction with DNA. Therefore, the change in absorption
characteristics was due to the electronic interaction between the
intercalator and the DNA bases.
In the present study, different DNA restriction
enzymes were used and the digestion maps were observed to determine
the binding sites following treatment with fraxetin (19). Among the four tests, fraxetin
specifically inhibited the digestion of DNA by TaqI and
HindIII, which recognize the T/CGA and A/AGCT sites. This
was due to the drug binding to the T/CGA, A/AGCT or similar sites,
however, the specific mechanism of interaction requires further
investigation.
In conclusion, it was hypothesized that the
inhibitory mechanism of fraxetin on S. aureus may be
associated with the intercalative interaction between the drug and
DNA. This results in the loss of topoisomerase activity and has
effects on the replication and transcription of DNA and the
synthesis of proteins, which in turn prevents the division of
bacterial cells.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of Liaoning Province (no. L2013412) and the
Science and technology Program of Dalian Municipality, China (no.
2013E13SF108).
References
|
1
|
Wu CR, Huang MY, Lin YT, Ju HY and Ching
H: Antioxidant properties of Cortex Fraxini and its simple
coumarins. Food Chem. 104:1464–1471. 2007. View Article : Google Scholar
|
|
2
|
Martín-Aragón S, Benedí JM and Villar AM:
Modifications on antioxidant capacity and lipid peroxidation in
mice under fraxetin treatment. J Pharm Pharmacol. 49:49–52.
1997.PubMed/NCBI
|
|
3
|
Yang T, Ge X and Wang XN: Antibacterial
action of cortex fraxini. Xibei Guofang Yi Xue Za Zhi. 5:387–388.
2003.(In Chinese).
|
|
4
|
Fang LH, Lv Y and Du GH: Progress in the
study of pharmacological effect of Cortex Franxini. Zhongguo Zhong
Yao Za Zhi. 33:2732–2736. 2008.(In Chinese).
|
|
5
|
Feng LS and Liu ML: The advance about
bacterial topoisomerase inhibitor. World Notes on Antibiotics.
30:13–18. 2009.(In Chinese).
|
|
6
|
Lee HJ, Choi GJ and Cho KY: Correlation of
lipid peroxidation in Botrytis cinerea caused by
dicarboximide fungicides with their fungicidal activity. J Agric
Food Chem. 46:737–741. 1998.
|
|
7
|
Chen CZ and Cooper SL: Interactions
between dendrimer biocides and bacterial membranes. Biomaterials.
23:3359–3368. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Q and Xie MJ: Antibacterial mechanism
of Luteolin on Staphylococcus aureus. Wei Sheng Wu Xue Bao.
50:1180–1184. 2010.(In Chinese).
|
|
9
|
Mandraju RK, Kannapiran P and Kondapi AK:
Distinct roles of Topoisomerase II isoforms: DNA damage
accelerating alpha, double strand break repair promoting beta. Arch
Biochem Biophys. 470:27–34. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sulivan DM, Glisson BS, Hodges PK, et al:
Proliferation dependence of topoisomerase II mediated drug action.
Biochemistry. 25:2248–2256. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin J and Wang X: The synergistic
antitumor effects of berberine alpha-hydroxy-beta-decanoylethyl
sulfonate with hydroxycamptothecine and its effect on
topoisomerase. Acta Pharm Sin. 46:390–394. 2011.(In Chinese).
|
|
12
|
Satyanarayana S, Dabrowiak JC and Chaires
JB: Tris (phenanthroline) ruthenium (II) enantiomer interactions
with DNA: mode and specificity of binding. Biochemistry.
32:2573–2584. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng LH, Jiang C, Ding J, et al:
Inhibition of DNA topoisomerases and direct DNA breakage by extract
of Spirulina plantensis geitl. Chin J Cancer. 19:768–771.
2000.(In Chinese).
|
|
14
|
Long EC and Barton JK: On demonstrating
DNA intercalation. Acc Chem Res. 23:271–273. 1990. View Article : Google Scholar
|
|
15
|
Tenover FC: Mechanisms of antimicrobial
resistance in bacteria. Am J Med. 119:S3–S10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yun BY, Zhou L, Xie KP, Wang YJ and Xie
MJ: Antibacterial activity and mechanism of baicalein. Acta Pharm
Sin. 47:1587–1592. 2012.PubMed/NCBI
|
|
17
|
Pan XS, Ambler J, Mehtar S and Fisher LM:
Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin
targets in Streptococcus pneumoniae. Antimicrob Agents
Chemother. 40:2321–2326. 1996.PubMed/NCBI
|
|
18
|
Snyder RD and Gillies PJ: Evaluation of
the clastogenic, DNA intercalative, and topoisomerase
II-interactive properties of bioflavonoids in Chinese hamster V79
cells. Environ Mol Mutagen. 40:266–276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Corneo G, Pogliani E, Biassoni D and
Tripputi P: Inhibition of DNA restriction enzyme digestion by
anthracyclines. Ric Clin Lab. 18:19–22. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Antonini I, Cola D, Polucci P,
Bontemps-Gracz M, Borowski E and Martelli S: Synthesis of
(dialkylamino) alkyl-disubstituted pyrimido [5,6,1-de] acridines, a
novel group of anticancer agents active on a multidrug resistant
cell line. J Med Chem. 38:3282–3286. 1995.PubMed/NCBI
|