Introduction
The assessment of mediastinal adenopathy by
non-invasive imaging modalities, such as computed tomography (CT)
and positron emission tomography (PET), is a clinically useful
technique. However the accuracy of these imaging modalities is
usually insufficient to distinguish between benign and malignant
lymph nodes for the purpose of determining subsequent treatment
options. The sensitivity of CT and PET is between 60 and 85%; while
the specificity is between 79 and 90% (1,2). In
current practice, pathological examination is necessary for
PET-positive lymph nodes for mediastinal staging of lung cancer.
Endobronchial ultrasound-guided transbronchial needle aspiration
(EBUS-TBNA) is a minimally invasive procedure that was approved by
the US Food and Drug Administration in March 2006. The
effectiveness of EBUS-TBNA in mediastinal staging of lung carcinoma
and in diagnosing central and peripheral lung masses, mediastinal
involvement of lymphoma and isolated mediastinal lymphadenopathy
has been previously reported (1–3). In
addition to impressive sensitivity and specificity, EBUS-TBNA may
be performed in the ambulatory care setting under sedation, the
procedure is cost-effective, accessible to more lymph node
stations, with less post-procedural complications (2,3).
Previous studies emphasized the importance of close communication
between bronchoscopists, radiologists and pathologists for the
success of EBUS-TBNA procedure (4,5).
However, the majority of these published data are from large
tertiary medical centers with advanced stages of disease and
greatly enlarged lymph nodes (6,7).
Conclusions from these studies may not represent the typical
patient population in general practice.
The present retrospective study reviewed our initial
experience with EBUS-TBNA since its first introduction into the
Newark Beth Israel Medical Center (Newark, NJ, USA), covering a
period of 16 months. This study additionally discussed the
efficient use of immunostaining with the limited material in cell
blocks
Materials and methods
Patients
All patients with mediastinal or hilar
lymphadenopathy or a mediastinal mass referred for EBUS-TBNA in the
Newark Beth Israel Medical Center between January 2011 and April
2012 were enrolled in this study. This study was approved by the
institutional Ethics Committee, and followed international and
national regulations. All patients provided informed consent. The
primary indications for TBNA were the sampling and diagnosis of
enlarged lymph nodes of unknown origin and lung cancer staging. A
chest radiograph was routinely performed in these patients, and
selected patients also underwent a CT scan of the chest (plain
and/or contrast enhanced).
EBUS-TBNA
Conventional flexible bronchoscopy (BF-7160
bronchoscope; Olympus, Ltd., Tokyo, Japan) was first performed by
standard methodology to examine the tracheobronchial tree, followed
by EBUS-TBNA using the new ultrasound biopsy bronchoscope
(XBF-UC260F-OL8; Olympus, Ltd.). Both bronchoscopy procedures were
performed by the same operator. Endobronchial ultrasound was
performed using a prototype linear array ultrasonic bronchoscope.
The instrument, similar to a standard bronchoscopic videoscope, had
an outer diameter of 6.9 mm, a 2.0-mm instrument channel and 30°
oblique forward viewing optics. An electronic convex array
ultrasound transducer was mounted at the distal tip and was covered
by a water inflatable balloon sheath. Scanning was performed at a
frequency of 7.5 MHz and with a penetration of 50 mm. The angle of
view was 90° and the direction of view was 30° forward oblique.
A dedicated 21/22-gauge needle (XNA-202C; Olympus,
Ltd.) was developed to perform the TBNA. The needle was also
equipped with a stylet that was withdrawn subsequent to passing the
bronchial wall, avoiding contamination during the TBNA. The needle
was mounted at the biopsy channel inlet of the endoscope prior to
puncture and the needle exited the outer covering of the insertion
tube at 20°. The needle was visualized through the optics and on
the ultrasound image.
Biopsy procedure
The probe was introduced through the mouth and vocal
cords into the main carina. The balloon was partially inflated
(0.3–0.5 ml water) and the regional lymph node stations of the
middle mediastinum and hilar regions (stations 2, 3, 4, 7, 10 and
11) were systematically imaged and measured (short-axis diameter)
during the slow withdrawal and rotation of the transducer. Fine
needle aspiration (FNA) was performed by passing the dedicated
prototype 21/22-gauge needle through the airway wall and into the
lymph nodes under real-time ultrasound guidance. Needle punctures
were performed using the jabbing method. Integrated power Doppler
ultrasound was used to visualize and avoid potentially intervening
vessels immediately prior to needle puncture.
One to four mediastinal lymph nodes were sampled per
patient, with a maximum of seven passes/node. EBUS-TBNA aspirates
were expelled from the needle by either blowing air through a 20-ml
syringe or by reinsertion of the stylet. A portion of the aspirates
was smeared on glass slides, air-dried and immediately stained with
a Diff-Quick (DQ) staining kit (NC9943455; Fisher Scientific,
Pittsburgh, PA, USA). Another portion of the aspirates was also
smeared on glass slides and immediately placed into 95% ethanol for
Papanicolaou (Pap) staining. The remaining specimen, collected
subsequent to rinsing with Cytorich Red (05NG-00003; BD
Diagnostics, Burlington, NC, USA), was collected in a
Cytorich-containing cup and processed for ThinPrep (BD PrepStain
Slide Processor; BD Diagnostics) and cell block evaluation. The
last two to five aspirates of EBUS-TBNA specimens/site were
collected and placed directly into Cytorich Red for additional
studies, including cell block preparations. These were not examined
on-site. The sediment obtained from the Cytorich Red cup was
processed in paraffin blocks, and the histological slides were
stained with hematoxylin-eosin. Up to five passes/lymph node were
collected, smeared and examined on-site. However, the procedure was
terminated earlier if a tumor/granuloma or other specific diagnoses
were reached.
Acid-Fast Bacilli (AFB) and Gomori-Grocott’s
methenamine silver stains were performed in the cell block slides
of all samples with granuloma. In certain cases, culture of
Mycobacterium tuberculosis was also conducted.
Immunostaining was conducted at the discretion of the attending
pathologists.
Final diagnosis
Lymph node aspirates obtained by EBUS-TBNA were
considered to be representative if two lymphohistiocytic aggregates
or five groups of lymphocytes (>20 cells/group) were identified.
Aspirates containing other cellular material that resulted in a
specific diagnosis (carcinoma, thymoma, granulomas or lymphoma)
were also considered representative (8).
The diagnosis of tumors from EBUS-TBNA was
considered final. When a granuloma was identified, a diagnosis of
mycobacteria infection was explored by AFB stain and/or bacterial
culture. The final diagnosis of sarcoidosis was based on clinical
and radiological suspicion, tissue confirmation of noncaseating
granulomas and a follow-up period, subsequent to which similarly
presenting diseases, such as lung cancer, lymphoma or tuberculosis,
could be excluded.
Additional tissue examination of a particular
patient was based wholly on clinical suspicion/treatment decisions.
Whenever possible, the tissue diagnosis was compared with that
derived from the EBUS-TBNA aspirates. Reactive lymph nodes
diagnosed by biopsy were considered true negative results; tumors
(including lymphoma) and sarcoidosis diagnosed by biopsy/clinical
follow-up were considered true positive results.
Statistical methods
The primary end-point of the study was the
percentage of biopsy specimens obtained that contained
lymphocytes/lymphohistiocytic aggregates. The secondary end-point
was the percentage of confirmed diagnoses made possible with
EBUS-TBNA. Diagnostic sensitivity, specificity and accuracy were
calculated using the standard definitions: The proportion of true
positive results, true negative results and all correct results,
respectively. Reactive lymph nodes diagnosed by biopsy were
considered true negative results; tumors (including lymphoma) and
sarcoidosis diagnosed by biopsy/clinical follow-up were considered
true positive results. The unit of analysis was the patient.
Results
Patient demographics
EBUS-TBNA was performed in all 51 patients; the
majority of the patients (29 patients, 57%) were of of
African-American descent. The average age of the patients was 57.1
years. On-site smear preparation and interpretation, as well as
cell block preparation, were attempted in all patients.
Learning analysis
The retrospective analysis showed that, in the first
8 months, tumor/granuloma diagnoses were made in only 9.1% of cases
[three nodes/masses, one lymphoma, one thymoma and one squamous
cell carcinoma (SCC)] while specimens that were negative for
malignant cells comprised 84.9% of cases. By contrast,
tumor/granuloma diagnoses were achieved in 27% of cases in the
second 8 months (21 nodes, 10 pulmonary adenocarcinoma, two SCCs,
one non-classified poorly differentiated carcinoma and eight
granulomas) (P=0.045 versus the first 8 months) (Table I).
 | Table IComparison of sample adequacy and
diagnostic accuracy over time. |
Table I
Comparison of sample adequacy and
diagnostic accuracy over time.
| Parameters | First 8 months,
n/total n (%) | Second 8 months,
n/total n (%) | P-value |
|---|
| Cell block | 14/33 (42) | 71/99 (90) | <0.001 |
| Tumor/granuloma | 3/33 (9) | 21/79 (27) | 0.045 |
| Non-diagnostic
smears | 2/33 (6) | 3/79 (4) | 0.460 |
| FNA versus tissue
correlation for tumor/granuloma | 1/3 (33) | 12/12 (100) | 0.029 |
In the first 8 months, cell blocks could be made in
only 14 out of 33 lymph nodes/masses (42.4%) due to poor
cellularity in the remaining cases. By contrast, cell blocks were
available in 71 out of 79 cases in the second 8 months (90.0%)
(P<0.001 versus the first 8 months). Smear preparations
(including DQ, Pap smear and thin preparations) were deemed as
non-diagnostic samples in two out of 33 nodes/masses (6.1%) in the
first 8 months of the EBUS-TBNA procedure; in the second 8 months
the node-diagnostic smear samples decreased to three out of 79
nodes (3.8%) (P=0.46 versus the first 8 months) (Table I).
Correlation between EBUS-TBNA and
biopsy
In the first 8 months, tissue diagnoses were
available for 17 nodes/masses. Fourteen of the diagnoses were
benign/reactive lymph nodes, and the corresponding FNA diagnoses
were 100% matched with the tissue diagnoses. There were three tumor
diagnoses by tissue examination: SCC, thymoma and B-cell lymphoma.
The corresponding FNA diagnoses were SCC, atypical lymphoid tissue
and suspicious for thymoma, respectively. In the second 8 months,
tissue diagnoses were available for 23 nodes/masses, including
three adenocarcinomas, two SCCs and seven granulomas, representing
33.0, 100 and 88.0% of the total EBUS-TBNA case numbers in their
respective category, respectively. The eight benign/reactive
diagnoses of the biopsy showed 100% concordance with the FNA
diagnoses. There were 12 tumor/noncaseating granuloma diagnoses by
tissue examination, three adenocarcinomas, two SCCs and eight
noncaseating granulomas. The corresponding FNA diagnoses were 100%
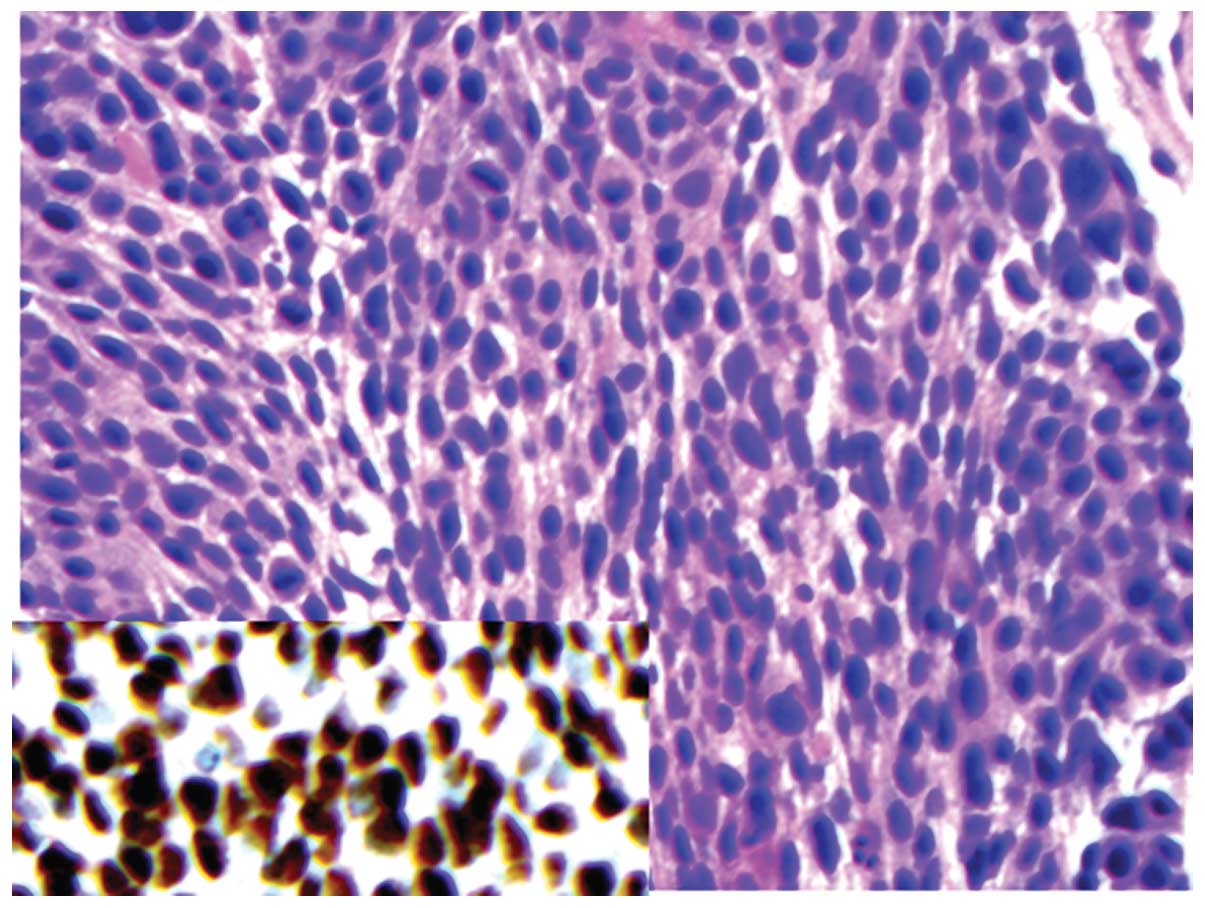
matched with the tissue diagnoses (Figs. 1 and 2). The sensitivity, specificity and
accuracy in the first 8 months were 33.0, 100 and 88.0%,
respectively. The sensitivity, specificity and accuracy in the
second 8 months were all 100% (Fig.
3).
Immunohistochemical and genetic
prognostic marker analysis
Immunohistochemical stains were performed in cell
blocks of 10 tumor samples: Seven adenocarcinomas and three SCCs.
All of the adenocarcinomas were thyroid transcription factor-1
(TTF-1)-positive with negative or very focal p63 stains; all of the
SCCs were TTF-1-negative and p63- and cytokeratin 5/6
(CK5/6)-positive (Figs. 1 and
2).
EGFR/KRAS mutation analysis was conducted in the
cell blocks of five adenocarcinomas upon the request of the
treating physicians. All of the cell blocks contained sufficient
material for analysis; one case showed a mutation in exons 18–21 of
EGRF, two cases revealed KRAS mutations at codons 12 and 13 and two
cases exhibited no EGFR or KRAS mutation.
Complications
No sedative drugs were administered to any patients
during the procedure. No major complications, such as pneumothorax,
mediastinal emphysema or bleeding from ruptured major vessels in
the mediastinum, were noted during the procedure or in the
follow-up period.
Discussion
This study is not the first to report a learning
curve following the initial application of EBUS-TBNA in a medical
center (7). Groth et al
(9) suggested that the learning
curve for EBUS-FNA for thoracic surgeons is ~10 procedures. Sun
et al (10) reported that,
following the initial five procedures, the sensitivity of EBUS-TBNA
for diagnosing lung cancer should be ≥90% for pulmonologists
experienced in bronchoscopy. However, the majority of these reports
are from tertiary medical centers with a selected patient
population. At least one recent study has suggested that EBUS-TBNA
results from these centers may not be entirely representative in a
more general patient population in routine care (11). The present study was conducted in
an urban community medical center, with the patient population
including 57% African-Americans and a significant number of
patients who were human immunodeficiency virus (HIV)-positive. In
this medical center, diagnoses of 13 out of the first 16 patients
were benign (no evidence of malignant cells). Four of these 13
patients underwent biopsy/resection and 100% agreement between TBNA
and the histological diagnoses was achieved. The first EBUS-TBNA
diagnosis of metastatic pulmonary carcinoma was made in the 16th
patient, which was confirmed later by an evaluation of a
histological biopsy sample. The two misclassified tumors (one
thymoma and one B-cell lymphoma) occurred in the third and eighth
patients, respectively. It is important to note that both false
positive and false negative results have been reported for
EBUS-TBNA, even in centers experienced in this procedure (12). Limited cellularity is clearly a
contributing factor to the difficulty in classification, as the
aspirated material from one of three tumors was not sufficient to
make a cell block (the thymoma case); the material from another
patient (the lymphoma case) was not sufficient to run flow
cytometry. In the first 8 months of this study, cell blocks were
made in only 42% of cases due to poor cellularity. Rapid on-site
evaluation (ROSE) and effective communication with the
bronchoscopists are therefore critical to guarantee sufficient
cellularity and achieve a correct diagnosis. As suggested by Monaco
et al (13), the presence
of cytologists also allows the appropriate triage of material for
ancillary studies, including tissue culture, flow cytometry,
immunostaining and molecular studies. At the initial stage of the
EBUS-TBNA procedure, a lack of standardization of the ROSE
procedure and the different expections from bronchoscopists,
pathologists and radiologists may also contribute to the suboptimal
early results.
The tumor/granuloma diagnoses were significantly
increased to 27% in the second 8 months of the study. Sufficient
diagnostic material in the majority of cases (cell blocks in 90% of
cases) and effective communication with bronchoscopists (ROSE) were
necessary for this improvement. Other factors, including accurate
positioning of the probe into the lesion following further practice
(14), may also have influenced
the improvement. The pattern of the learning curve for this
EBUS-TBNA is similar to that of previous reports (5,8,9,15).
However, significant differences between our results and previous
reports were also noted. Firstly, the tumor diagnosis rate in the
cohort in the second 8 months was 16%, less than that of previous
reports (~45%) (2,5). Secondly, the rate of sarcoidosis was
10%, which was higher than that reported in previous studies (~5%).
In addition, the malignancy rate in the second 8 months was 71%,
which was higher than that reported in previous studies (~35%). The
majority of these differences may be attributed to different
patient populations due to the following reasons: i) There was no
significant increase in the tumor/granuloma diagnostic rate in the
center, even during the most recent months (after the second 8
months); ii) a recent study using a population under routine care
also achieved a similar tumor/sarcoidosis diagnostic rate of ~27%
(11); iii) a significant number
of patients in our medical center immunosuppressive (patients who
had undergone heart, lung or renal transplantations and patients
with HIV) (16). Rates of
infection and associated mediastinal reactive lymphadenopathy were
therefore expected to be higher than those in previously reported
patient populations.
With the advances in personalized chemotherapy, the
subclassification of non-small cell lung carcinomas has become
necessary. The EGFR inhibitors erlotinib and gefitinib are most
efficacious in tumors with EGFR mutation, which are predominantly
adenocarcinomas (17). Similarly,
the folate inhibitor pemetrexed is currently the most effective
drug treatment for adenocarcinoma, but not SCC (18). An inhibitor of anaplastic lymphoma
kinase (ALK), crizotinib, targets tumors with EML4-ALK fusion,
which occurs predominantly in adenocarcinoma (19). One caveat of the EBUS-TBNA specimen
is the limited diagnostic material, which requires the strategic
use of antibodies rather than screening with a panel of antibodies.
It is noteworthy that in the present analyzed cohort, only two or
three antibodies were required to differentiate the non-small cell
carcinomas of the pulmonary origin. In this study,
immunohistochemical stains were performed in the cell blocks of
seven metastatic carcinomas of the pulmonary origin (four
adenocarcinomas and three SCCs). All the adenocarcinomas were
TTF-1-positive with negative or very focal p63 stains and the SCCs
were TTF-1-negative and extensively positive for p63 and CK5/6.
These results closely correlated with the conclusions by
Mukhopadhyay (20) from studies of
small histology biopsy samples. The analysis of genetic mutations
to EGFR, Ras and ALK is requied for the effective treatment of
pulmonary adenocarcinoma, either for the selection of chemotherapy
or due to the selection criteria for clinical trials. In all the
cases of pulmonary adenocarcinoma, EGFR and KRAS mutation analyses
were successfully performed.
The findings from this study indicated a significant
difference between the cytological results from the early and late
case groups, suggesting that a steep learning curve should be
expected when EBUS-TBNA is first adopted in a medical center. Every
attempt should be made to prepare cell blocks, which are critical
for the subclassification of tumors and possibly for the assessment
of therapeutic and prognostic markers. TTF-1, p63 and possibly
CK5/6 are usually sufficient to differentiate SCC from
adenocarcinoma of the lung.
References
|
1
|
Varela-Lema L, Fernández-Villar A and
Ruano-Ravina A: Effectiveness and safety of endobronchial
ultrasound-transbronchial needle aspiration: a systematic review.
Eur Respir J. 33:1156–1164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Navani N, Lawrence DR, Kolvekar S, Hayward
M, McAsey D, Kocjan G, Falzon M, Capitanio A, Shaw P, Morris S,
Omar RZ and Janes SM: REMEDY Trial Investigators: Endobronchial
ultrasound-guided transbronchial needle aspiration prevents
mediastinoscopies in the diagnosis of isolated mediastinal
lymphadenopathy: a prospective trial. Am J Respir Crit Care Med.
186:255–260. 2012. View Article : Google Scholar
|
|
3
|
Oki M, Saka H, Kitagawa C, Kogure Y,
Murata N, Ichihara S and Moritani S: Prospective study of
endobronchial ultrasound-guided transbronchial needle aspiration of
lymph nodes versus transbronchial lung biopsy of lung tissue for
diagnosis of sarcoidosis. J Thorac Cardiovasc Surg. 143:1324–1329.
2012. View Article : Google Scholar
|
|
4
|
Gurioli C, Ravaglia C, Romagnoli M, Casoni
G, Tomassetti S, Nanni O and Poletti V: EBUS-TBNA in
mediastinal/hilar lymphadenopathies and/or masses: an Italian case
series. Clin Respir J. 6:3–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Delattre C, Fournier C, Bouchindhomme B,
Renaud F, Escande F, Ramon P and Copin MC: Endoscopic ultrasound
guided transbronchial fine needle aspiration: a French Department
of Pathology’s 4-year experience. J Clin Pathol. 64:1117–1122.
2011.PubMed/NCBI
|
|
6
|
Herth FJ, Eberhardt R, Vilmann P, Krasnik
M and Ernst A: Real-time endobronchial ultrasound guided
transbronchial needle aspiration for sampling mediastinal lymph
nodes. Thorax. 61:795–798. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wallace MB, Pascual JM, Raimondo M,
Woodward TA, McComb BL, Crook JE, Johnson MM, Al-Haddad MA, Gross
SA, Pungpapong S, Hardee JN and Odell JA: Minimally invasive
endoscopic staging of suspected lung cancer. JAMA. 299:540–546.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nayak A, Sugrue C, Koenig S, Wasserman PG,
Hoda S and Morgenstern NJ: Endobronchial ultrasound-guided
transbronchial needle aspirate (EBUS-TBNA): a proposal for on-site
adequacy criteria. Diagn Cytopathol. 40:128–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Groth SS, Whitson BA, D’Cunha J, Maddaus
MA, Alsharif M and Andrade RS: Endobronchial ultrasound-guided
fine-needle aspiration of mediastinal lymph nodes: a single
institution’s early learning curve. Ann Thorac Surg. 86:1104–1110.
2008.
|
|
10
|
Sun JY, Zhao H, Zhang J, Wang XD and Han
BH: First 30 endobronchial ultrasound-guided transbronchial needle
aspirations: a single institution’s early experience. Chin Med J
(Engl). 124:1818–1823. 2011.PubMed/NCBI
|
|
11
|
Lange TJ, Kunzendorf F, Pfeifer M, Arzt M
and Schulz C: Endobronchial ultrasound-guided transbronchial needle
aspiration in routine care - plenty of benign results and follow-up
tests. Int J Clin Pract. 66:438–445. 2012. View Article : Google Scholar
|
|
12
|
Sanz-Santos J, Andreo F, Serra P, Llatjós
M, Castellà E, Astudillo J, Monsó E and Ruiz-Manzano J: False
positive endobronchial ultrasound-guided real-time transbronchial
needle aspiration secondary to bronchial carcinoma in situ at the
point of puncture: a case report. J Cardiothorac Surg. 7:742012.
View Article : Google Scholar
|
|
13
|
Monaco SE, Pantanowitz L and Khalbuss WE:
Comparing endobronchial ultrasound-guided fine needle aspiration
specimens with and without rapid on-site evaluation. Cytojournal.
9:22012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang CT, Tsai YJ, Liao WY, Wu PC, Ho CC,
Yu CJ and Yang PC: Endobronchial ultrasound-guided transbronchial
biopsy of peripheral pulmonary lesions: how many specimens are
necessary? Respiration. 84:128–134. 2012. View Article : Google Scholar
|
|
15
|
Lee BE, Kletsman E, Rutledge JR and Korst
RJ: Utility of endobronchial ultrasound-guided mediastinal lymph
node biopsy in patients with non-small cell lung cancer. J Thorac
Cardiovasc Surg. 143:585–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vaughn AC: The impact of substance abuse
on the care of African Americans and Latinos with HIV/AIDS. J Natl
Med Assoc. 96(2 Suppl): 21S–23S. 2004.PubMed/NCBI
|
|
17
|
Shigematsu H, Lin L, Takahashi T, Nomura
M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N,
Fujisawa T, Feng Z, Roth JA, Herz J, Minna JD and Gazdar AF:
Clinical and biological features associated with epidermal growth
factor receptor gene mutations in lung cancers. J Natl Cancer Inst.
97:339–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S,
Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP and Gandara
D: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naive patients with
advanced-stage non-small-cell lung cancer. J Clin Oncol.
26:3543–3551. 2008. View Article : Google Scholar
|
|
19
|
Kwak EL, Bang YJ, Camidge DR, Shaw AT,
Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB,
Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman
JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve
SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K,
Salgia R, Shapiro GI, Clark JW and Iafrate AJ: Anaplastic lymphoma
kinase inhibition in non-small-cell lung cancer. N Engl J Med.
363:1693–1703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mukhopadhyay S: Utility of small biopsies
for diagnosis of lung nodules: doing more with less. Mod Pathol.
25(Suppl 1): S43–S57. 2012. View Article : Google Scholar : PubMed/NCBI
|