Introduction
Atherosclerosis is a chronic, multifactorial
disease, in which abnormalities in the structure and function of
endothelial cells (ECs) have an initial role in its development
(1). Numerous risk factors for
atherosclerosis can result in endothelial injury to the lumen of
blood vessels, but it has been recently demonstrated that exposure
to regional reactive oxygen species (ROS) is one of the major
stimuli for EC dysfunction and damage (2,3). ROS
production can induce oxidative damage to lipids, proteins and
enzymes in ECs, resulting in damage to cellular function and
apoptosis of severely damaged ECs (4,5).
Hydrogen peroxide (H2O2) is one of the most
crucial ROS, that can easily penetrate the plasma membrane and
cause injury to neighboring cells, as well as
H2O2-producing cells (6). In vitro models of
H2O2-induced endothelial cell damage has been
extensively applied to mimic oxidative endothelial injury during
atherogenesis (7). Previous
studies have reported that H2O2 could lead to
the dysfunction of ECs and apoptosis (8,9).
Therefore, the upregulation of the anti-apoptotic pathways in ECs,
which are inhibited by H2O2, has been
considered as an attractive therapeutic strategy in
atherosclerosis.
Radix salviae miltiorrhizae, commonly known as
‘Danshen’, is an herbal supplement that has been used by medical
practitioners of traditional Chinese medicine for decades in the
therapy of a variety of cardiovascular diseases (10). Tanshinol
[3(3,4dihydroxyphenyl)2hydroxypropionic acid], is the main
effective component of Danshen, which has previously been shown to
improve microcirculation, inhibit the production of ROS, restrain
platelet adhesion and aggregation, and protect the myocardium from
ischemia (11–13). In addition, tanshinol has been
shown to have protective effects on ECs suffering from injury
caused by hyperhomocysteinemia (14) and inflammation (15).
There have been few studies performed to ascertain
the regulatory role of tanshinol in preserving antioxidant defenses
and mitochondrial function in ECs. In the present study, the
protective effects of tanshinol in ECs were studied in response to
H2O2-induced oxidative stress. The main focus
was to determine whether tanshinol can protect ECs from
H2O2-induced cytotoxicity by increasing the
expression of antioxidant enzymes such as superoxide dismutase
(SOD). SOD can detoxify free radicals and preserve mitochondrial
function through the upregulation of Bcl-2 expression, which
inhibits the cytochrome c-caspase-3 pathway. The presented
outcomes provide further support for a functional role of tanshinol
in ECs in the prevention of atherosclerosis.
Materials and methods
Reagents
H2O2 was purchased from
Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China).
Dimethylsulfoxide (DMSO), MTT and streptomycin were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Penicillin and glutamine were
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA),
trypsin and medium-199 (M199) were purchased from Gibco-BRL (Grand
Island, NY, USA). Fetal bovine serum (FBS) was purchased from PAA
Co. (PAA Laboratories GmbH, Piscotaway, NJ, USA), β-endothelial
cell growth factor (β-ECGF) was purchased from R&D Systems
(Minneapolis, MN, USA), and heparin was purchased from Sinopharm
Chemical Reagent Co. Ltd., (Shanghai, China). The SOD Activity
Assay kit was purchased from Nanjing Institute of Jiancheng
Bioengineering (Nanjing, China), and the Annexin V-fluorescein
isothiocyanate (FITC) Apoptosis Detection kit was obtained from
Nanjing KeyGen Biotech Co. (Nanjing, China). Bicinchoninic acid
(BCA) Protein Assay and Lipid Peroxidation Malondialdehyde (MDA)
Assay kits were purchased from Beyotime Institute of Biotechnology
(Jiangsu, China).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
isolated and cultured as previously described (16). This study was approved by the
Ethics Board of the Renji Hospital, Shanghai Jiaotong University
School of Medicine, Shanghai, China. Briefly, HUVECs were removed
from human umbilical veins (Renji Hospital) by digestion with
0.125% trypsin, and were cultured in M199 containing 20% FBS,
penicillin (100 U/ml), streptomycin (100 U/ml), and heparin (50
U/ml) and supplemented with L-glutamine (2 mM), sodium pyruvate (1
mM), and β-ECGF (5 ng/ml). The cells were incubated at 37°C in 5%
CO2 on 0.1% gelatin-coated culture flasks. HUVECs were
identified by their morphology, which appears as a ‘cobblestone’
mosaic appearance after reaching confluence, and the presence of
von Willebrand factor (detected by immunofluorescence). HUVECs were
used at passages 3–6 for experiments.
Treatment protocols
For all experiments, HUVECs were cultured to
confluence in 96-well plates or 35 mm2 dishes. The cells
were pre-treated with tanshinol (25, 50, 100, 200 μM) for 24 h
before exposure to H2O2 (600 μM). Following
exposure to H2O2, the cells were harvested
for further analysis.
Cell viability assay
The MTT assay was performed to evaluate the
viability of the HUVECs. Briefly, HUVECs were seeded onto 96-well
plates in M199 media and maintained for 24 h. The cells were
subjected to different concentrations of tanshinol (25, 50, 100,
200 μM) for 24 h followed by stimulation with or without 600 μM
H2O2 for 6 h. Following the
H2O2 incubation, MTT (10 μl/well) reagent was
added to the 100 μl medium, and then incubated at 37°C for 4 h
prior to the detection of the number of viable cells. The MTT
solution was removed and DMSO was added in order to solubilize the
formazan crystals. The absorbance of the medium was measured at 570
nm using a BioTek ELx-800 (BioTek Instruments, Inc., Winooksi, VT,
USA) plate reader. The extent of cell death was estimated by
measuring the activity of lactate dehydrogenase (LDH). The amount
of LDH released from the cells in the supernatant was detected
using an LDH assay kit (Beyotime Institute of Biotechnology,
Shanghai, China), according to the manufacturer’s instructions. The
absorbance was measured using a microplate reader at 490 nm. The
data from each treatment group is expressed as a percentage of the
control group.
Lipid peroxidation assay
The level of MDA was detected using the Lipid
Peroxidation MDA Assay kit, according to the manufacturer’s
instructions. Briefly, HUVECs were lysed using
radio-immunoprecipitation assay buffer, followed by centrifugation
at 1600 × g for 10 min to discard the cellular debris. The
supernatant was used for the MDA assay and the protein
concentration was measured using a BCA assay. The MDA levels were
standardized to milligram protein.
Measurement of intracellular SOD
activities
The culture medium from the HUVECs seeded in the
96-well plates was removed and the cells were washed twice with
phosphate-buffered saline (PBS), followed by cell lysis using the
freeze-thaw method three times. The SOD activity in the cell lysate
was detected using commercial kits according to the manufacturer’s
instructions (Beyotime Institute of Biotechnology, Shanghai,
China).
Flow cytometric evaluation of
apoptosis
Following tanshinol and H2O2
treatments, the HUVECs were double-stained using an Annexin V-FITC
apoptosis detection kit, according to the manufacturer’s
instructions. Samples stained with Annexin V and propidium iodide
(PI) were quantitatively analyzed at 488 nm emission and 570 nm
excitation using flow cytometry (BD FACScalibu; BD Biosciences, San
Jose, CA, USA).
ROS detection
The 2′,7′-dichlorofluorescin diacetate (DCFH-DA)
assay was used to detect ROS in the HUVECs as previously described
(17). To examine the effects of
tanshinol on ROS production, the HUVECs were pretreated with 100 μM
tanshinol for 24 h, followed by stimulation with 600 μM
H2O2 for 4 h, after which the cells were
incubated with DCFH-DA (10 μM) in M199 for 30 min at 37°C in 35-mm
dishes or 96-well black-bottomed plates. The fluorescence intensity
of the dishes was measured at 485 nm excitation and 538 nm emission
by laser-scanning confocal microscopy (FV500, Olympus Optical CO.
Ltd., Tokyo, Japan). The fluorescence intensity of the 96-well
plates was quantified with an Infinite F500 Microplate Reader
(Tecan Group, Ltd., Männedorf, Switzerland) and was standardized to
the total milligram protein.
Western blot analysis
The HUVECs were lyzed in protein lysis buffer
containing a protease inhibitor cocktail. The protein
concentrations of the cell lysates was quantified by BCA assay and
equal amounts of protein were separated by SDS-PAGE and then
transferred onto a polyvinylidene fluoride (PVDF) membrane
(Millipore, Billerica, MA, USA). The membranes were blocked in 5%
non-fat dry milk diluted with Tris-buffered saline with
Tween® 20 (TBST) (Tris-HCl 20 mM, NaCl 150 mM, pH 7.5,
0.1% Tween 20) at room temperature for 1 h and probed overnight at
4°C with either monoclonal rabbit cytochrome c, Bcl-2, SOD-2
or caspase-3 antibodies, or a polyclonal rabbit SOD-1 antibody
(1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
followed by incubation for 1 h with a goat anti-rabbit IgG antibody
conjugated to horseradish peroxidase (Cell Signaling Technology,
Inc.). Incubation with either a monoclonal mouse α-tubulin antibody
(1:1,000 dilution; Sigma-Aldrich) or a polyclonal mouse β-actin
antibody (1:1,000 dilution; Cell Signaling Technology, Inc.) was
performed as the loading sample control. The proteins were
visualized using Enhanced Chemiluminescence Western Blotting
Detection reagents (Amersham Biosciences Corp., Piscataway, NJ,
USA). The densitometry of the bands was quantified using Image J
1.38X software (National Institutes of Health, Bethesda, MD,
USA).
Cytochrome c release assay
HUVEC cells were harvested by centrifugation at
1,000 × g for 3 min at room temperature. The mitochondrial
and cytosol extractions were separated using a Mitochondrial
Isolation kit (Pierce Biotechnology, Inc., Rockford, IL, USA)
according to the manufacturer’s instructions. The presence of
cytochrome c was determined from both the mitochondrial and
cytosol extractions by western blotting, using a monoclonal rabbit
cytochrome c antibody (1:1,000, Cell Signaling Technology,
Inc.).
Statistical analysis
All of the experiments were repeated at least three
times. The values are expressed as the means ± standard error of
the mean. The data was evaluated for statistical significance using
a one-way analysis of variance. A P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using GraphPad Prism® 5.0
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
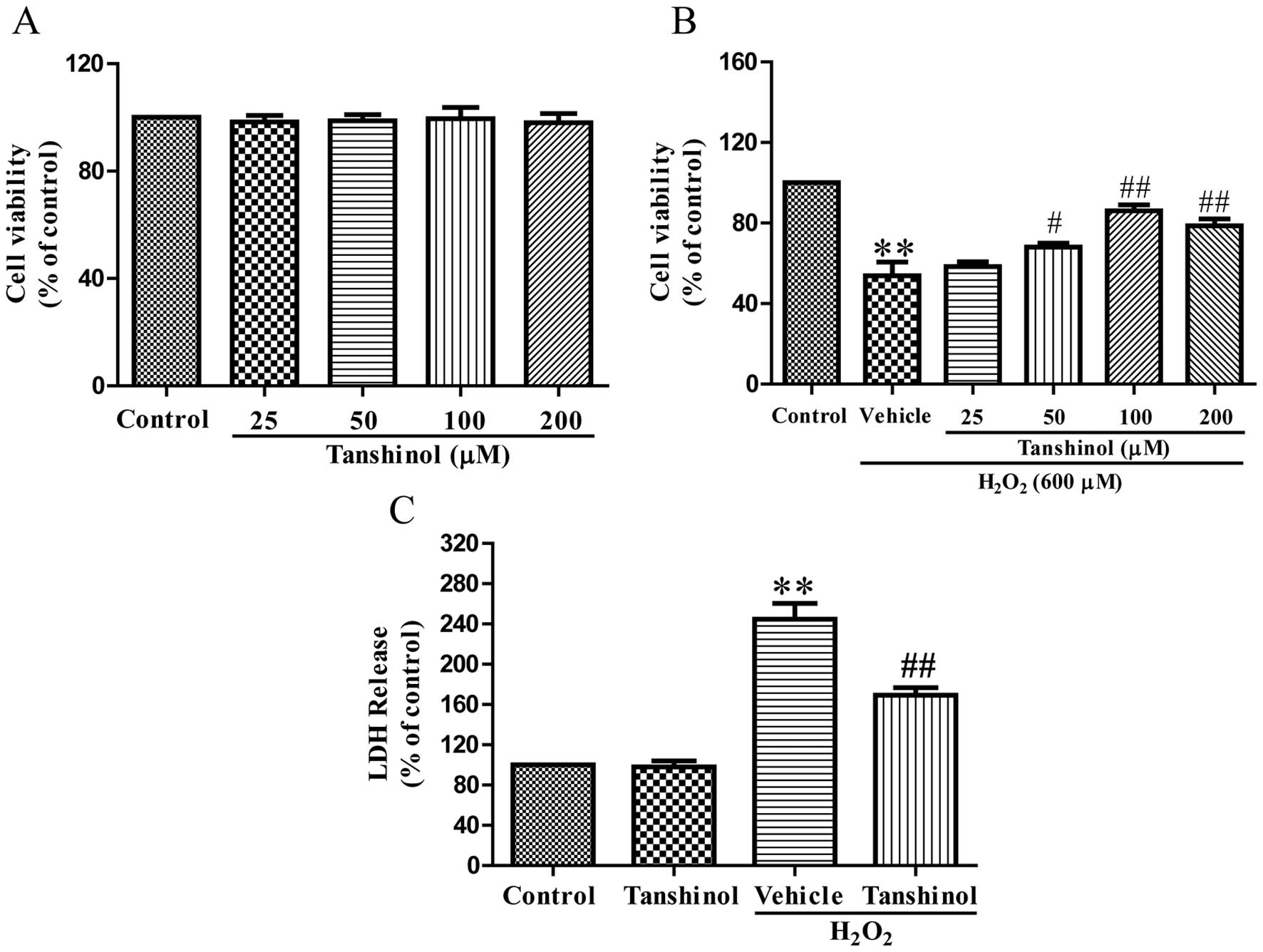
HUVECs exhibit no toxicity to tanshinol
administration
Tanshinol was analyzed by MTT assay to determine its
toxic effects on HUVECs. Tanshinol, at a concentration range
between 25 and 200 μM alone, did not show any toxic effects on
HUVECs (Fig. 1A). MTT assay showed
that HUVEC viability was significantly decreased following a 600 μM
H2O2 challenge for 4 h, as compared with the
control (P<0.01). Tanshinol significantly attenuated this
H2O2-induced decrease in cellular viability
in a concentration-dependent manner (Fig. 1B). Tanshinol demonstrated the
ability to markedly enhance HUVEC cell viability at 100 μM. An LDH
release assay supported the results of the MTT assay (Fig. 1C). As compared with the control,
the vehicle plus H2O2-treated cells exhibited
an induced LDH release (244.8±15.57%, P<0.01), while
pretreatment with tanshinol significantly decreased LDH release
(169.1±7.60%, P<0.01). These results demonstrated the ability of
tanshinol to reduce H2O2 cytotoxicity.
Tanshinol inhibits
H2O2-induced HUVECs apoptosis
To quantitatively determine the anti-apoptotic
effects of tanshinol in H2O2-induced HUVECs,
the total apoptotic rate of the HUVECs was measured by Annexin-V/PI
staining, following treatment with 600 μM
H2O2 for 4 h. As shown in Fig. 2A and B, the rate of apoptosis
increased from 4.12±0.56 to 21.40±1.92%, as compared with the
control group (P<0.01). By contrast, tanshinol at 100 μM
significantly attenuated the apoptotic rate of the HUVECs exposed
to H2O2, as compared with the vehicle plus
H2O2 group (6.64±0.87%, P<0.01).
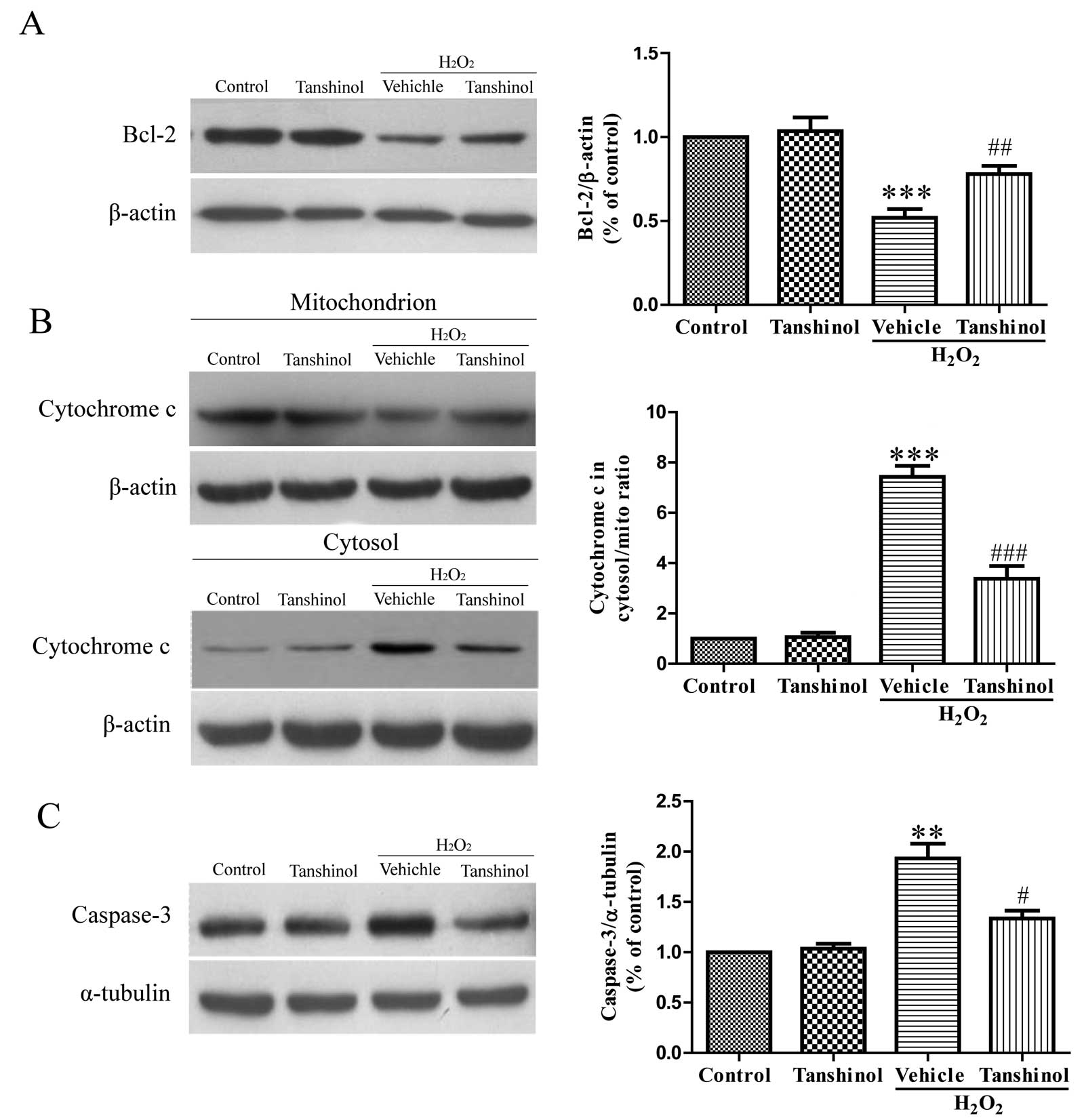
Tanshinol inhibits
H2O2-induced cytochrome c release from the
mitochondria into the cytosol
One mechanism by which oxidative stress may induce
cellular toxicity in HUVECs is through the induction of the
apoptotic pathway, which is caused by the mitochondrial release of
cytochrome c into the cytosol. To demonstrate this
hypothesis, the protective action of tanshinol on
H2O2-induced toxicity was analyzed by
determining the release of cytochrome c from the
mitochondria by western blotting (Fig.
3B). As compared with the control group, the cytochrome
c levels in the H2O2 group were
significantly increased in the cytosol and decreased in the
mitochondria (P<0.01). However, pretreatment with tanshinol
inhibited H2O2-induced release of cytochrome
c, as compared with the vehicle plus
H2O2 group (P<0.001).
Effects of tanshinol on the expression of
Bcl-2 and caspase-3 in HUVECs exposed to
H2O2
The activation of caspase-3 is a key step in
apoptosis. Furthermore, the Bcl-2 protein family has a critical
role in apoptotic cell death, caspase activation and the regulation
of apoptosis. Stimulation of HUVECs with H2O2
at 600 μM for 6 h significantly decreased Bcl-2 protein expression
to 52.3% of the control protein expression levels (P<0.001),
whereas tanshinol at 100 μM attenuated the
H2O2-induced downregulation of Bcl-2 protein
expression (78.6% of control, P<0.01, Fig. 3A). As compared with the control
group, HUVECs exposed to H2O2 alone showed
increased protein expression of caspase-3, and tanshinol reduced
the ability of H2O2 to increase caspase-3
expression (Fig. 3C).
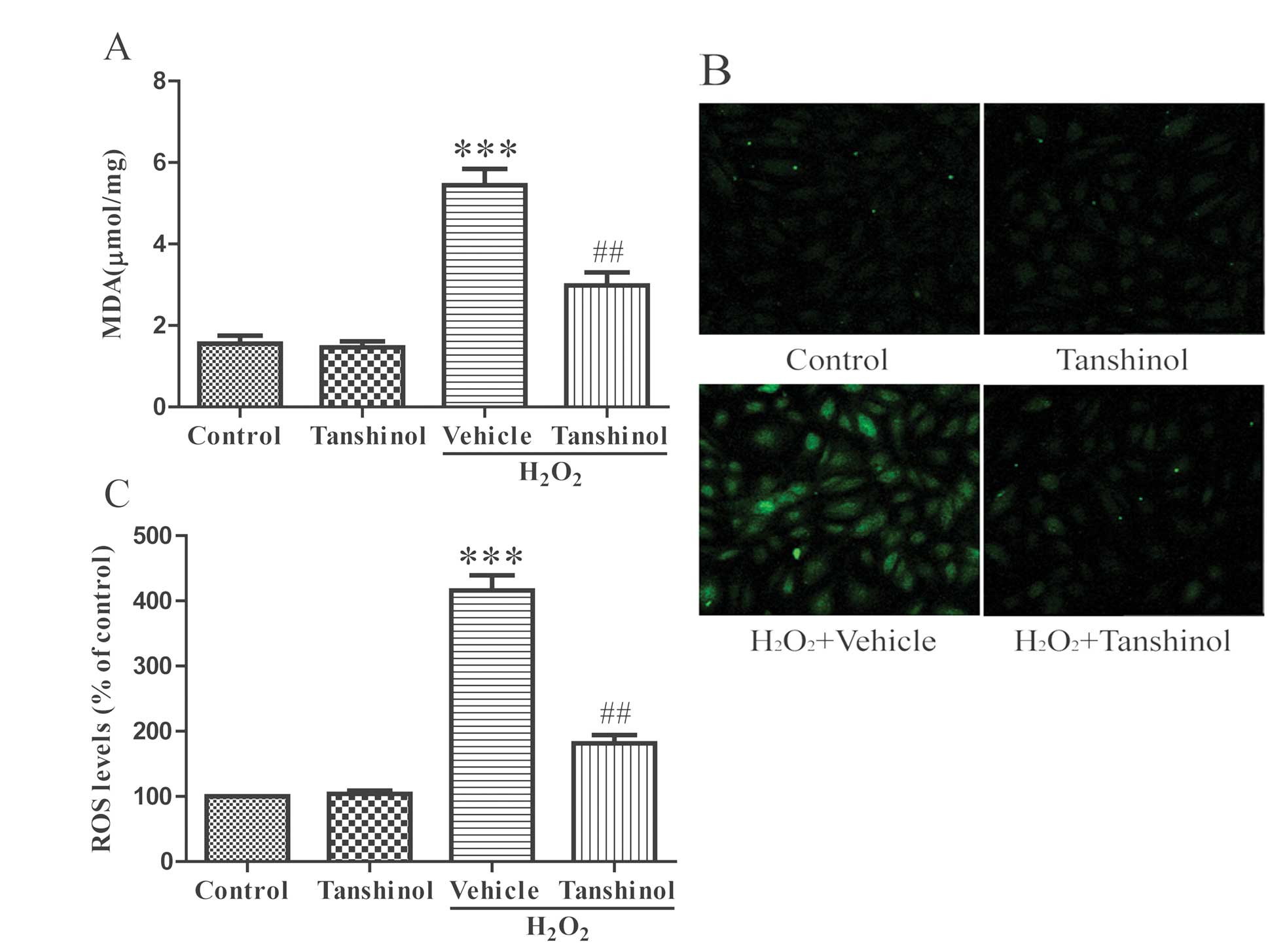
Effects of tanshinol on MDA generation
and ROS production
MDA is a product of lipid peroxidation. The levels
of MDA were low in the control HUVECs and treatment with
H2O2 elevated the cellular MDA levels as
compared with the control levels, thus indicating an increase in
oxidative stress (P<0.001, Fig.
4A). Pretreatment with tanshinol significantly reduced the
generation of MDA induced by H2O2, as
compared with the vehicle plus H2O2 group
(P<0.01). The redox status was observed using the DCFH-DA assay
(Fig. 4B and C). Treatment with
H2O2 induced an increase in the fluorescence
intensity of DCFH-DA, as compared with the control group
(P<0.001). Pre-incubation with tanshinol restrained the
production of ROS induced by H2O2
(P<0.01).
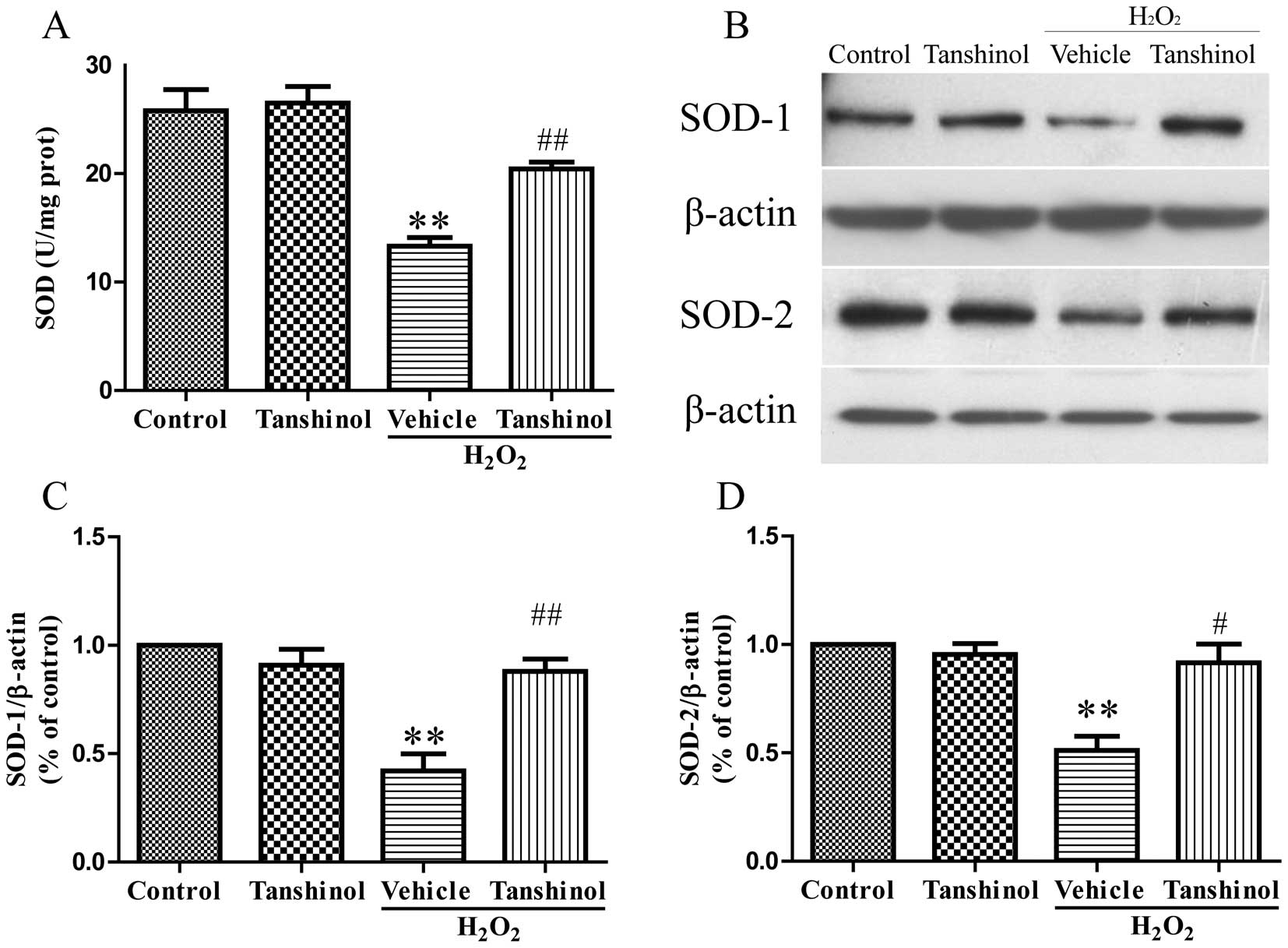
Tanshinol prevents
H2O2-induced decrease of SOD activity
SOD is another indicator of cellular toxicity. To
further examine the protective effects of tanshinol, SOD activity
was measured in H2O2-treated cells. As shown
in Fig. 5A, HUVEC treatment with
H2O2 resulted in a significant decrease in
SOD activity, as compared with the control group (P<0.01).
Conversely, pretreatment with tanshinol increased the SOD activity,
as compared with the vehicle plus H2O2 group
(P<0.01). To support these results, the protein expression of
SOD in HUVECs was measured (Fig 5
B–D). The protein expression levels of SOD-1 and SOD-2 were
decreased in the H2O2 group as compared with
the control group (P<0.01). When compared with the
H2O2 group, higher protein expression levels
were observed in the tanshinol group (SOD-1; P<0.01, SOD-2;
P<0.05). These results were in accordance with the identified
SOD activity of the previous experiment.
Discussion
The present study explored the cardiovascular
protective function of tanshinol on
H2O2-induced injury in HUVECs, as estimated
by measures of antioxidative systems and mitochondrial function.
Oxidative stress is considered to be a potent atherogenic factor at
the initiation of atherosclerotic lesion formation. Oxidized
cholesterol particles promote inflammation, the formation of
plaques and the migration of smooth muscle cells, which are all
conducive to the development of atherosclerotic lesions.
Furthermore, mitochondria can also be damaged by these toxic free
radicals at the cellular level (18).
In the present study, the effects of tanshinol on
H2O2-induced HUVEC apoptosis in vitro,
and its possible underlying mechanisms, were estimated. The results
indicated that tanshinol could protect HUVECs from apoptosis
induced by H2O2, and improve cellular
viability. Tanshinol was also shown to significantly inhibit
cellular apoptosis and increase SOD activity. An underlying
mechanism by which tanshinol protects HUVECs from apoptosis may be
by increasing antioxidant defence systems and preserving
mitochondrial function.
It has been previously reported that increased
levels of ROS from vessels under pathological conditions is a major
process resulting in endothelial injury (17). Following exposure to high levels of
H2O2, HUVECs undergo exceptional
cytotoxicity. In the present study, it was observed that 600 μM
H2O2, a precursor of other ROS, can
significantly reduce the cellular viability and increase the
apoptotic rate of HUVECs. The present findings are in accordance
with previous reports demonstrating increased apoptosis in HUVECs
incubated with H2O2 (19–20).
However, when HUVECs were pretreated with tanshinol for 24 h, cell
viability was significantly enhanced and the apoptotic rate was
significantly reduced. These findings suggest that tanshinol may
protect HUVECs against H2O2-induced
apoptosis.
The mechanisms by which tanshinol prevents EC
apoptosis under oxidative stress are currently unknown. Tanshinol
was shown to protect HUVECs against oxidative stress which was
mainly attributed to the upregulation of cellular antioxidant
defenses, including SOD. Thus, it may be assumed that the
underlying mechanism behind the anti-apoptotic effects of tanshinol
may be due to its upregulation of endogenous cellular antioxidant
systems that are capable of scavenging ROS.
It is well known that the antioxidant enzyme SOD has
a vital role in EC defenses (21).
SOD can dismutate superoxide radicals into hydrogen peroxide, which
can then be detoxified into water by other antioxidant enzymes
(16). As shown in Figure 4, pretreatment with tanshinol
markedly increased SOD activity. These findings suggest that
tanshinol may protect ECs by increasing the ability of antioxidant
enzymes in the scavenging of H2O2.
Consistent with previous findings (20,23,24),
the present study found that H2O2 could
markedly decrease Bcl-2 protein expression. In contrast, tanshinol
treatment significantly increased
H2O2-induced Bcl-2 protein expression. It is
well reported that Bcl-2 is an anti-apoptotic protein that can
inhibit apoptosis through a mitochondria-dependent caspase pathway
(25) Bcl-2 has been shown to
prevent apoptosis induced by different stimuli, either by
restraining the mitochondrial release of cytochrome c, which
stimulates caspase activity, or by serving as an antioxidant
(26). According to the present
study, tanshinol significantly inhibits cytochrome c release
from the mitochondria into the cytosol. Therefore, the protective
effect of tanshinol on H2O2-induced EC injury
may be related to the upregulation of Bcl-2 protein expression and
therefore reduced caspase activity. In addition, as the interior
apoptotic pathway relies on the balance between pro- and
anti-apoptotic members of the Bcl-2 family, including Bax, Bcl-XL,
Bak and Bad (27–29), the effects of tanshinol on
pro-apoptotic proteins requires further study.
In conclusion, the present study showed that
tanshinol had a protective effect against
H2O2-induced cytotoxicity and apoptosis in
HUVECs. This anti-apoptotic effect of tanshinol likely contributed
to the preservation of mitochondrial function through the
upregulation of Bcl-2 protein expression, the inhibition of the
cytochrome c caspase-3 pathway, and the stimulation of
cellular antioxidant defenses by increased SOD activity. Since
oxidative stress-induced EC injury has a vital role in the
development of atherosclerosis (30), the findings of the present study
may illuminate the pharmacological foundation for the clinical
application of tanshinol for the treatment of atherosclerosis.
References
|
1
|
Bai X, Wang X and Xu Q: Endothelial damage
and stem cell repair in atherosclerosis. Vasc Pharmacol.
52:224–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Birukov KG: Cyclic stretch, reactive
oxygen species, and vascular remodeling. Antioxid Redox Signal.
11:1651–1667. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Urso C and Caimi G: Oxidative stress and
endothelial dysfunction. Minerva Med. 102:59–77. 2011.(In
Italian).
|
|
4
|
Irani K: Oxidant signaling in vascular
cell growth, death, and survival: a review of the roles of reactive
oxygen species in smooth muscle and endothelial cell mitogenic and
apoptotic signaling. Circ Res. 87:179–183. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boueiz A and Hassoun PM: Regulation of
endothelial barrier function by reactive oxygen and nitrogen
species. Microvasc Res. 77:26–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakamura H, Nakamura K and Yodoi J: Redox
regulation of cellular activation. Annu Rev Immunol. 15:351–369.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang B, Oo TN and Rizzo V: Lipid rafts
mediate H2O2 prosurvival effects in cultured endothelial cells.
FASEB J. 20:1501–1503. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hao CN, Geng YJ, Li F, Yang T, Su DF, Duan
JL and Li Y: Insulin-like growth factor-1 receptor activation
prevents hydrogen peroxide-induced oxidative stress, mitochondrial
dysfunction and apoptosis. Apoptosis. 16:1118–1127. 2011.
View Article : Google Scholar
|
|
9
|
Jia Y, Ji L, Zhang S, Xu L, Yin L, Li L,
et al: Total flavonoids from Rosa Laevigata Michx fruit attenuates
hydrogen peroxide induced injury in human umbilical vein
endothelial cells. Food Chem Toxicol. 50:3133–3141. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang RX, Huang SY, Yan FF, Lu XT, Xing YF,
Liu Y, et al: Danshensu protects vascular endothelia in a rat model
of hyperhomocysteinemia. Acta Pharmacol Sin. 31:1395–1400. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng TO: Cardiovascular effects of
Danshen. Int J Cardiol. 121:9–22. 2007. View Article : Google Scholar
|
|
12
|
Wang F, Liu YY, Liu LY, Zeng QJ, Wang CS,
Sun K, et al: The attenuation effect of 3,4-dihydroxy-phenyl lactic
acid and salvianolic acid B on venular thrombosis induced in rat
mesentery by photochemical reaction. Clin Hemorheol Microcirc.
42:7–18. 2009.
|
|
13
|
Han JY, Horie Y, Fan JY, Sun K, Guo J,
Miura S and Hibi T: Potential of 3,4-dihydroxy-phenyl lactic acid
for ameliorating ischemia-reperfusion-induced microvascular
disturbance in rat mesentery. Am J Physiol Gastrointest Liver
Physiol. 296:G36–G44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan K, Chui SH, Wong DY, Ha WY, Chan CL
and Wong RN: Protective effects of Danshensu from the aqueous
extract of Salvia miltiorrhiza (Danshen) against
homocysteine-induced endothelial dysfunction. Life Sci.
75:3157–3171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang GD, Zhang H, Lin R, Wang WR, Shi XL,
Liu Y and Ji QL: Down-regulation of CD40 gene expression and
inhibition of apoptosis with Danshensu in endothelial cells. Basic
Clin Pharmacol Toxicol. 104:87–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jaffe EA, Nachman RL, Becker CG and Minick
CR: Culture of human endothelial cells derived from umbilical
veins. Identification by morphologic and immunologic criteria. J
Clin Invest. 52:2745–2756. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu L, Gu L, Ma Q, Zhu D and Huang X:
Resveratrol attenuates hydrogen peroxide-induced apoptosis in human
umbilical vein endothelial cells. Eur Rev Med Pharmacol Sci.
17:88–94. 2013.PubMed/NCBI
|
|
18
|
Wen YD, Wang H, Kho SH, Rinkiko S, Sheng
X, Shen HM and Zhu YZ: Hydrogen sulfide protects HUVECs against
hydrogen peroxide induced mitochondrial dysfunction and oxidative
stress. PLoS One. 8:e531472013. View Article : Google Scholar
|
|
19
|
Qian J, Jiang F, Wang B, Yu Y, Zhang X,
Yin Z and Liu C: Ophiopogonin D prevents H2O2-induced injury in
primary human umbilical vein endothelial cells. J Ethnopharmacol.
128:438–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang YK, Hong YJ, Wei M, Wu Y, Huang ZQ,
Chen RZ and Chen HZ: Curculigoside attenuates human umbilical vein
endothelial cell injury induced by H2O2. J Ethnopharmacol.
132:233–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukai T and Ushio-Fukai M: Superoxide
dismutases: role in redox signaling, vascular function, and
diseases. Antioxid Redox Signal. 15:1583–1606. 2011. View Article : Google Scholar
|
|
22
|
Hu L, Sun Y and Hu J: Catalpol inhibits
apoptosis in hydrogen peroxide-induced endothelium by activating
the PI3K/Akt signaling pathway and modulating expression of Bcl-2
and Bax. Eur J Pharmacol. 628:155–163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li ZL, Liu JC, Hu J, Li XQ, Wang SW, Yi DH
and Zhao MG: Protective effects of hyperoside against human
umbilical vein endothelial cell damage induced by hydrogen
peroxide. J Ethnopharmacol. 139:388–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duan W, Yang Y, Yi W, Yan J, Liang Z, Wang
N, et al: New role of JAK2/STAT3 signaling in endothelial cell
oxidative stress injury and protective effect of melatonin. PLoS
One. 8:e579412013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kane DJ, Sarafian TA, Anton R, Hahn H,
Gralla EB, Valentine JS, et al: Bcl-2 inhibition of neural death:
decreased generation of reactive oxygen species. Science.
262:1274–1277. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hockenbery DM, Oltvai ZN, Yin XM, Milliman
CL and Korsmeyer SJ: Bcl-2 functions in an antioxidant pathway to
prevent apoptosis. Cell. 75:241–251. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shroff EH, Snyder C and Chandel NS: Role
of Bcl-2 family members in anoxia induced cell death. Cell Cycle.
6:807–809. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong WW and Puthalakath H: Bcl-2 family
proteins: the sentinels of the mitochondrial apoptosis pathway.
IUBMB Life. 60:390–397. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lalier L, Cartron PF, Juin P, Nedelkina S,
Manon S, Bechinger B and Vallette FM: Bax activation and
mitochondrial insertion during apoptosis. Apoptosis. 12:887–896.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen WL, Qian Y, Meng WF, Pang JY, Lin YC,
Guan YY, Chen SP, Liu J, Pei Z and Wang GL: A novel marine compound
xyloketal B protects against oxidized LDL-induced cell injury in
vitro. Biochem Pharmacol. 78:941–950. 2009. View Article : Google Scholar : PubMed/NCBI
|