Introduction
Oral squamous cell carcinoma (OSCC; ICD9 code
140-149, excluding 142 and 147) is the sixth most common human
malignancy worldwide. Due to the high recurrence rate of primary
tumors or the presence of a second primary tumor, the five-year
survival rate of OSCC following diagnosis remains ~50% despite
advances in surgical and radiotherapy treatment (1). Another important reason for this low
survival rate is mainly due to the lack of understanding of the
basic biological mechanisms involved in OSCC (2). In the last decade, epigenetic
information, including DNA methylation, histone modification and
chromatin structure has been demonstrated to be important in
carcinogenesis (3,4). Similarly, several studies
investigating DNA hypermethylation in head and neck cancer
suggested that this phenomenon may be critical in OSCC development
(5,6).
The Wnt signaling pathway is involved in numerous
developmental processes and in tumorigenesis (7,8).
Usually, the binding of Wnt to frizzled receptors leads to
cytosolic stabilization and accumulation of β-catenin, which
translocates into the nucleus and interacts with TCF/LEF
transcription factors thereby regulating the expression of
downstream target genes, including cyclin D1. The Wnt signaling
pathway can be partially regulated by Wnt antagonists, including
members of the Dickkopf family, Wnt inhibitory factor 1 and
secreted frizzled-related proteins (SFRPs). The SFRPs are a family
composed of five soluble glycoproteins (SFRP1-5), each containing a
cysteine-rich domain. These domains are homologous to the putative
Wnt-binding sites of frizzled proteins. Therefore, SFRPs may
prevent frizzled receptors from binding to Wnt proteins and, in
turn, downregulate Wnt signaling.
A previous study confirmed that the epigenetic
inactivation of SFRP2, a member of the SFRP family, is important in
the progression of several types of human tumor, including head,
colorectal and breast cancer (9–11).
However, few studies have been performed to investigate the
functional significance of SFRP2 in the development of OSCC. Based
on analysis of the SFRP2 promoter methylation status and the
expression levels of SFRP2 in cancer tissues and adjacent
non-cancer tissues from OSCC patients, the present study
investigated the effects of SFRP2 on the Wnt signaling pathway in
the development of OSCC in vivo and in vitro.
Materials and methods
Human OSCC samples
A total of 49 cancer tissues and their adjacent
non-cancer specimens were obtained from patients diagnosed with
OSCC, who had undergone curative surgery at the Department of Oral
and Maxillofacial Surgery, The First Affiliated Hospital of Soochow
University, (Suzhou, China). The collected specimens were
immediately snap-frozen in liquid nitrogen and stored at −196°C for
further analysis. The present study was approved by the Clinical
Research Ethics Committee of Soochow University and all patients
provided written informed consent for obtaining the study
materials.
Cell lines and cell culture
The human tongue squamous cell carcinoma cell line
Tca8113 was obtained from the China Center for Type Culture
Collection (Shanghai, China). Cells were cultured in RPMI-1640
containing 10% fetal bovine serum (Invitrogen Life Technologies,
Carlsbad, CA, USA) at 37°C in 5% CO2 and 95%
humidity.
DNA and RNA extraction
Genomic DNA was extracted from the cancer tissues
and their adjacent non-cancer specimens using a DNA mini kit
(Qiagen, Hilden, Germany) according to the manufacturer’s
instructions. The DNA was subsequently resuspended in 500 μl LoTE
(2.5 mmol/l EDTA and 10 mmol/l Tris-HCl; MayBiotech, Nanjing,
China) and stored at −80°C until use.
Total RNA was extracted from 49 pairs of tissue
specimens and Tca8113 cells using TRIzol reagent (Invitrogen Life
Technologies) according to the manufacturer’s instructions. The RNA
was resuspended in 500 μl LoTE buffer and stored at −80°C until
use.
Methylation-specific polymerase chain
reaction (MSP)
The methylation status of the SFRP2 promoter in the
OSCC tissues and Tca8113 cells was determined by MSP. Briefly, 2 mg
genomic DNA was bisulphite-treated using a Zymo DNA Modification
kit (Zymo Research, Orange, CA, USA) and was then used as a
template for MSP. The MSP conditions were as follows: One cycle at
95°C for 3 min followed by 30 cycles of 95°C for 30 sec, 53.5°C for
30 sec and 72°C for 1 min. The detection of methylation included
use of a methylated (M) reaction and an unmethylated (U) reaction.
The primer sequences used were as follows: M, forward
5′-TTTTTACGGTATTGGGGAGTATATC-3′ and reverse
5′-AAAAACCAATAAAAAATAATCCGA-3′; U, forward
5′-TTATGGTATTGGGGAGTATATTGA-3′ and reverse 5′-AAAACCAATAAAAAATAA
TCCAAA-3′.
Reverse transcription polymerase chain
reaction (RT-PCR)
A reverse transcription reaction was performed using
2 mg total RNA using a first strand cDNA kit (Roche Diagnostics,
Mannheim, Germany). The mRNA expression of SFRP2 was detected by
RT-PCR and GAPDH was used as an internal control of RNA integrity.
The primer sequences used were as follows: SFRP2, forward
5′-TGGAGACCAAGAGCAAGAC-3′ and reverse 5′-GTGGGACAAAGACAGGGTA-3′;
GADPH, forward 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse 5′-GAAGATGGTG
ATGGGATTTC-3′.
Plasmid construction and generation of
stable SFRP2 cell lines
A human SFRP2 expression vector, pcDNA3.1/SFRP2, was
constructed by subcloning the full-length cDNA of SFRP2 into the
HindIII-XhoI site of the pcDNA3.1(+) vector (Invitrogen Life
Technologies). Following confirmation by sequencing, the
pcDNA3.1/SFRP2 and pcDNA3.1 (control) were transfected into Tca8113
cells using FuGENE® HD (Roche Diagnostics) according to
the manufacturer’s instructions. Using 500 μg/ml G418 (Invitrogen
Life Technologies) and confirming with RT-PCR and western blot
analysis, single colonies of transfected cells exhibiting stable
SFRP2 expression were selected for generation.
Cell cycle analysis
The cell cycle was analyzed using flow cytometry
(Cell Lab Quanta SC; Beckman Coulter, Miami, FL, USA) with
4′,6-diamidino-2-phenylindole staining (Sigma-Aldrich, St. Louis,
MO, USA), as described in a previous study (12). Measurements were repeated
independently three times.
In vivo tumor growth
Suspensions of the stable SFRP2-expressing cells and
the control cells (1×107 in 200 μl RPMI-1640) were
injected subcutaneously into 4–5-week old female nude mice (strain
BALB/c nu/nu). Tumor size was measured using calipers every 3 days
for a total of 4 weeks and tumor volume was calculated on the basis
of width (x) and length (y) using the following formula: x2y / 2,
where x<y.
Western blot analysis
The protein extracted from the tissues and cells was
resolved by SDS-PAGE and transferred onto polyvinylidene fluoride
membranes (Hybond-P; cat no. 10600088; GE Healthcare,
Buckinghamshire, UK) followed by western blot analysis. Signals
were detected using an enhanced chemiluminescence detection system
(ECL Plus Western Blotting Detection system; GE Healthcare).
Primary goat anti-SFRP2 antibody was purchased from Sigma-Aldrich
(cat: SAB2500934; Shanghai, China). Goat antibodies against
glycogen synthase kinase 3β (GSK-3β; sc-8257) and β-catenin
(sc-1496), and rabbit antibodies against cyclin D1 (sc-753) and
β-actin (sc-130656) were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). The poly-HRP anti-goat IgG was
purchased from Abcam (ab6741; Cambridge, MA, USA). The goat
anti-rabbit IgG (NA934) were purchased from GE Healthcare.
Immunohistochemistry
The tissue sections were deparaffinized in xylene
and dehydrated in ethanol (Sigma-Aldrich). Following dehydration,
endogenous peroxides were inhibited, the sections were incubated
with the goat polyclonal GSK-3β (sc8257), β-catenin (sc-1496) and
rabbit polyclonal cyclin D1 (sc-753) primary antibodies and
poly-HRP anti-goat (ab6741) or anti-rabbit IgG (ab6721; Abcam)
secondary antibodies, and then incubated with 3,3′-diaminobenzidine
(Sigma-Aldrich). The sections were then counterstained and
dehydrated. Antibodies against GSK-3β, β-catenin and cyclin D1 were
purchased from Santa Cruz Biotechnology, Inc.
Statistical analysis
The SPSS software program for Windows (version 13;
SPSS, Inc., Chicago, IL, USA) was used for statistical analysis.
The association between methylation of the SFRP genes and the
clinical parameters were analyzed using a χ2 test and a
Fisher’s exact test, where necessary. P<0.05 was considered to
indicate a statistically significant difference.
Results
Methylation status of the SFRP2 promoter
in OSCC tissues
Through MSP, the methylation status of the SFRP2
promoter was evaluated in 49 cases of OSCC and corresponding normal
tumor-adjacent tissues. The methylation of the SFRP2 promoter was
detected in 37 tumor samples (75.51%). However, in the
corresponding normal tumor-adjacent tissues, the SFRP2 promoter was
methylated in only three cases (6.12%). The frequency of SFRP2
promoter methylation in OSCC tissues was significantly higher than
that in the adjacent tissues (χ2=46.00; P<0.001).
Expression of SFRP2 mRNA in OSCC
tissues
The present study detected the SFRP2 mRNA expression
in 49 sample pairs of OSCC and their adjacent non-cancerous tissues
by quantitative RT-PCR (Data not shown). SFRP2 mRNA was detectable
in 48 corresponding normal tumor-adjacent tissues (97.96%).
However, of the 49 OSCC tissues, only eight samples exhibited SFRP2
mRNA expression (χ2=63.37; P<0.001). Furthermore, in
35 (94.59%) of the 37 OSCC tissue samples demonstrating methylation
of the SFRP2 promoter, there was no detectable expression of SFRP2
mRNA. In the 12 OSCC tissue samples that did not demonstrate
methylation of the SFRP2 promoter sequence, SFRP2 mRNA was detected
in six samples (χ2=10.13; P<0.01) (Data not
shown).
SFRP2 inhibits OSCC cell
proliferation
The frequent silencing of SFRP2 by methylation in
OSCC, but not in non-cancerous tissues suggests SFRP2 has a
potential tumor-suppressing effect in the development of OSCC. To
assess this hypothesis, the present study examined the effect of
SFRP2 overexpression on the proliferation and apoptosis of Tca8113
cells. Compared with the Tca8113 cells transfected with pcDNA3.1,
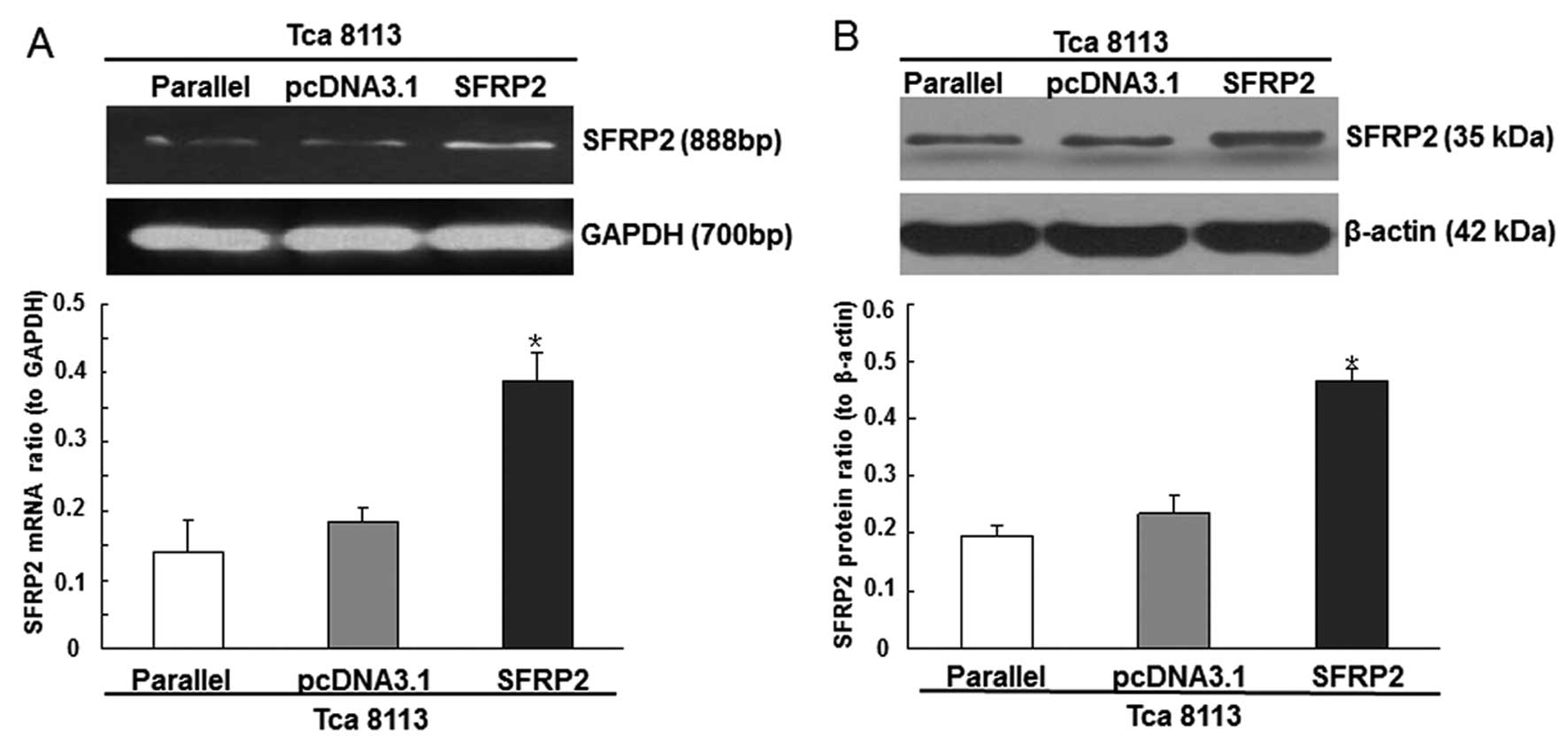
the cells transfected with the pcDNA3.1/SFRP2 plasmids demonstrated
increased expression levels of SFRP2 (Fig. 1A and B). In addition, 48 h after
transfection, overexpression of SFRP2 significantly inhibited the
proliferation of Tca8113 cells and arrested the cell cycle in the
G1 phase (Table I and II).
 | Table IEffects of SFRP2 overexpression on the
proliferation of Tca8113, Tca8113/pcDNA3.1 and Tca8113/SFRP2
cells. |
Table I
Effects of SFRP2 overexpression on the
proliferation of Tca8113, Tca8113/pcDNA3.1 and Tca8113/SFRP2
cells.
| OD570 nm (MTT) |
|---|
|
|
|---|
| Group | Day 1 | Day 2 | Day 3 | Day 4 |
|---|
| Tca8113 | 0.3667±0.0349 | 0.6358±0.0295 | 1.0427±0.1087 | 1.0915±0.1536 |
| Tca8113/pcDNA3.1 | 0.3962±0.0451 | 0.6351±0.1865 | 1.0338±0.1265 | 1.1217±0.2294 |
| Tca8113/SERP2 | 0.3342±0.0667 | 0.6621±0.2333 | 0.6961±0.1661a | 0.8041±0.0277a |
 | Table IIEffects of SFRP2 overexpression on the
cell cycle distribution of Tca8113, Tca8113/pcDNA3.1 and
Tca8113/SFRP2 cells. |
Table II
Effects of SFRP2 overexpression on the
cell cycle distribution of Tca8113, Tca8113/pcDNA3.1 and
Tca8113/SFRP2 cells.
| Cell cycle
distribution (%) |
|---|
|
|
|---|
| Group | G1 | G2 | S |
|---|
| Tca8113 | 46.839±1.516 | 17.431±0.989 | 33.265±1.604 |
| Tca8113/pcDNA3.1 | 48.472±1.879 | 16.748±1.133 | 34.780±1.416 |
| Tca8113/SFRP2 | 56.433±1.348a | 11.758±2.839a | 31.809±1.537 |
Effects of SFRP2 on the Wnt signaling
pathway in OSCC cells
To further understand the effects of SFRP2 on the
Wnt signaling pathway in OSCC, the expression levels of GSK-3β,
β-catenin and cyclin D1 were analyzed in the Tca8113 cells,
Tca8113/pcDNA3.1 cells and Tca8113/SFRP2 cells, respectively.
Compared with the parental and mock-transfected control cells,
increased levels of GSK-3β and β-catenin were observed in the cells
exhibiting SFRP2 overexpression. In addition, the growth promoting
gene cyclin D1, a direct read-out gene of the active Wnt signaling
pathway, was significantly decreased in the Tca8113/SFRP2 cells
(Fig. 2).
SFRP2 inhibits OSCC growth in vivo
Following the observed anti-proliferative effects of
SFRP2 on Tca8113 cells, the present study then assessed whether
SFRP2 inhibits the growth of OSCC in vivo. Following
subcutaneous inoculation with Tca8113/pcDNA3.1 cells and
Tca8113/SFRP2 cells, all the animals developed OSCC and exhibited
the typical histopathological characteristics of human OSCC.
However, as indicated by the tumor growth curve, the mean tumor
volume was significantly smaller in the SFRP2-transfected nude mice
than that in the animals inoculated with the Tca8113/pcDNA3.1
control (P<0.001; Fig. 3).
Effects of SFRP2 on the Wnt signaling
pathway in vivo
In order to investigate whether SFRP2 affects the
growth of OSCC via the Wnt signaling pathway in nude mice, the
expression levels of GSK-3β, β-catenin and cyclin D1 in tumor
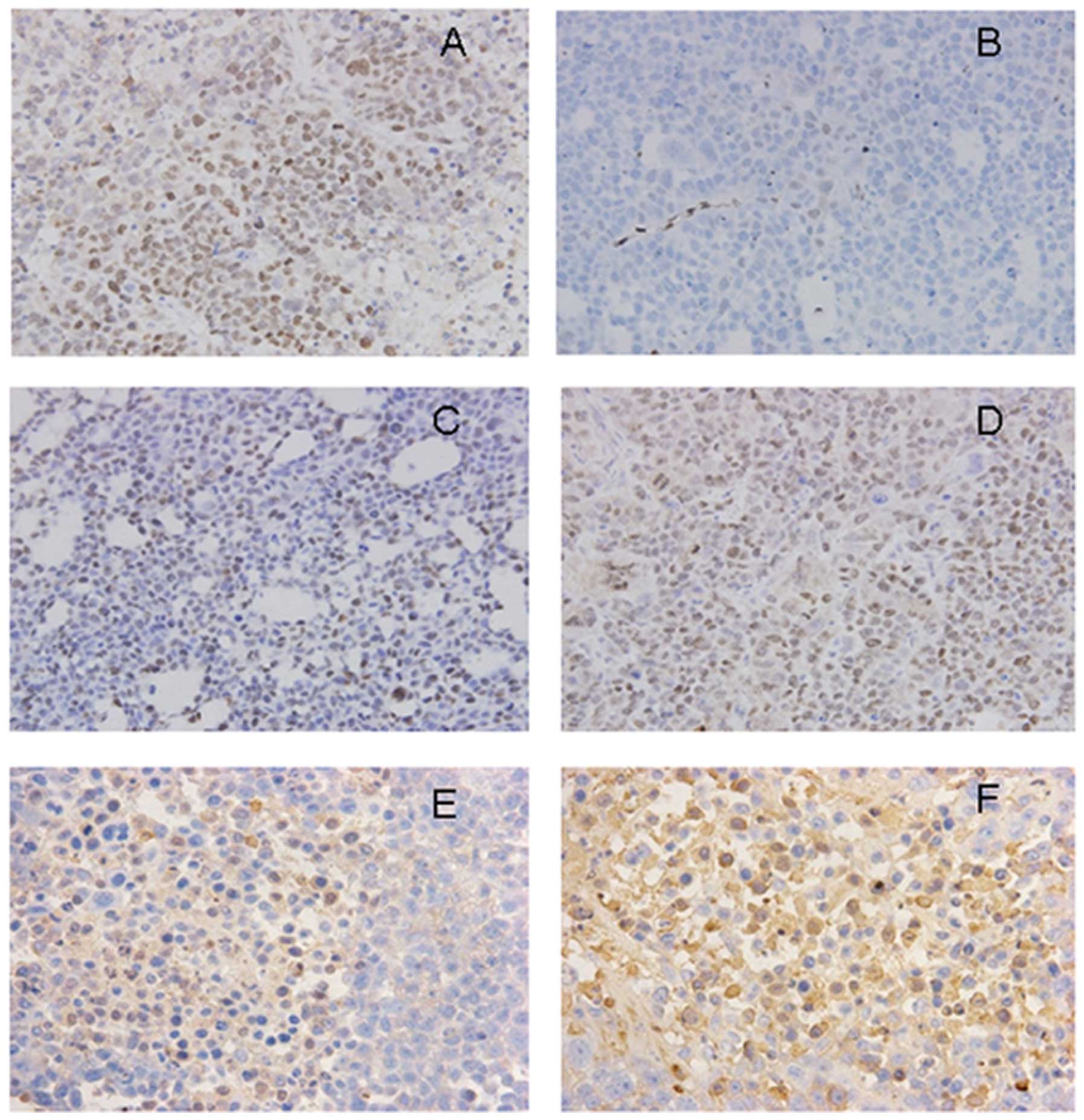
tissues were further analyzed by immunohistochemical analysis.
Compared with the animals in the Tca8113/pcDNA3.1 group, the
expression level of cyclin D1 was markedly decreased (Fig. 4), while the expression levels of
GSK-3β and β-catenin was significantly increased in the cell
cytoplasm and cell membrane in the animals from the Tca8113/SFRP2
group (Fig. 4).
Discussion
Several previous studies indicated that
hypermethylation of a gene promoter is critical in silencing tumor
suppressor genes in various types of human malignancy, including
breast cancer, oral cancer and head and neck cancer (13–15).
The SFRP gene family, which functionally act as Wnt signaling
inhibitors, are a common target of promoter hypermethylation in
several types of tumor (16,17).
Similar to these observations, the results of the present study
demonstrated that methylation of the SFRP2 promoter in OSCC tissues
occurred more frequently than in the normal tumor-adjacent tissues
(75.51 vs. 6.12%). As a result, the SFRP2 mRNA expression was
significantly inhibited in the OSCC tissue samples. In addition, in
the OSCC tissue samples exhibiting SFRP2 promoter methylation, the
mRNA expression of SFRP2 was markedly decreased compared with those
without SFRP2 promoter methylation. Therefore, the results of the
present study indicated that SFRP2 promoter methylation was an
important mechanism in the development of OSCC and suggested that
downregulation in the expression of SFRP2 is likely to be due to
methylation of the SFRP2 promoter.
The Wnt signaling pathway is known to be involved in
tumorigenesis in several types of human cancer (18,19).
The identification of Wnt signaling pathway antagonists, including
SFRPs family members, signified a new era in the study of the Wnt
gene and its effects (20–22). Similar to other members of the SFRP
family, SFRP2 is able to negatively regulate the Wnt signal
transduction pathway (11).
Overexpression of SFRP2 generally causes suppression of the Wnt
signaling pathway and inhibition of cell proliferation (23,24).
In the present study, the overexpression of SFRP2 significantly
inhibited Tca8113 cell proliferation and arrested the cell cycle in
the G1 phase. Additionally, the growth promoting gene cyclin D1, a
direct read-out gene of the active Wnt signaling pathway, was
significantly decreased in the cells overexpressing SFRP2. In
addition, using an OSCC nude mice model, the present study further
confirmed that SFRP2 inhibited the growth of OSCC in vivo.
The expression level of cyclin D1 was also decreased significantly,
while GSK-3β, an inactivated signal protein of the Wnt signaling
pathway, was increased significantly in the animals with SFRP2
overexpression. Therefore, these findings suggested that
methylation of the SFRP2 gene and the consequent regulation of
protein expression may be involved in the development of OSCC and
is likely to be associated with overactivation of the Wnt signaling
pathway.
β-catenin is a multifunctional protein involved in
two independent processes, including cell-cell adhesion and signal
transduction (25). In addition to
its effect on the regulation of cell adhesion, β-catenin is a key
effector in the Wnt signaling pathway (26,27).
When β-catenin accumulates in the cytoplasm and moves into the
nucleus of the cell, it can activate the Wnt signaling pathway and
accelerate the progress of cancer. As expected, accumulation of
β-catenin was detected in the cytoplasm of OSCC cells in the
present study. However, although Tca8113 cell proliferation and
OSCC development were inhibited by the overexpression of SFRP2,
increased expression levels of β-catenin were unexpectedly observed
in the Tca8113/SFRP2 cells and in the animal models. The underlying
mechanisms causing these increased levels of β-catenin and the
biological functions of the increased expression of β-catenin
remain to be elucidated. In previous studies, various patterns of
β-catenin expression have been observed in human cancer tissues
(28). For example, it was
reported that 88% of head and neck squamous cell carcinomas
exhibited reduced β-catenin expression (29,30).
By contrast, increased levels of β-catenin were detected in several
types of tumor, including liver cancer and lung cancer (31,32).
Thus, in order to clarify the biological functions of β-catenin and
its association with SFRP2 in OSCC development, more detailed
studies are required in the future.
Taken together, the data from the present study
demonstrated that hypermethylation of the SFRP2 promoter is
important in the development of OSCC in vivo and in
vitro. In addition, by increasing the expression of GSK-3β and
decreasing the expression of cyclin D1, SFRP2 had the ability to
inactivate the Wnt signaling pathway in OSCC development. Further
understanding of the precise mechanisms of how SFRP2 inhibits the
Wnt signaling pathway and its association with β-catenin is
important for improving the design of anticancer strategies against
OSCC.
Acknowledgements
The authors would like to thank Dr Yang Jiao and Dr
Chen Dong at the Department of Epidemiology and Statistics, School
of Public Health, Soochow University, China for their assistance in
preparation of the manuscript.
References
|
1
|
Huang SH and O’Sullivan B: Oral cancer:
Current role of radiotherapy and chemotherapy. Med Oral Patol Oral
Cir Bucal. 18:e233–e240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma M, Sah P, Sharma SS and
Radhakrishnan R: Molecular changes in invasive front of oral
cancer. J Oral Maxillofac Pathol. 17:240–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silva TD, Vidigal VM, Felipe AV, DE Lima
JM, Neto RA, Saad SS and Forones NM: DNA methylation as an
epigenetic biomarker in colorectal cancer. Oncol Lett. 6:1687–1692.
2013.PubMed/NCBI
|
|
4
|
Tam WL and Weinberg RA: The epigenetics of
epithelial-mesenchymal plasticity in cancer. Nat Med. 19:1438–1449.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marcinkiewicz KM and Gudas LJ: Altered
epigenetic regulation of homeobox genes in human oral squamous cell
carcinoma cells. Exp Cell Res. 320:128–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
González-Ramírez I, García-Cuellar C,
Sánchez-Pérez Y and Granados-García M: DNA methylation in oral
squamous cell carcinoma: molecular mechanisms and clinical
implications. Oral Dis. 17:771–778. 2011.PubMed/NCBI
|
|
7
|
Tsao CM, Yan MD, Shih YL, Yu PN, Kuo CC,
Lin WC, et al: SOX1 functions as a tumor suppressor by antagonizing
the WNT/β-catenin signaling pathway in hepatocellular carcinoma.
Hepatology. 56:2277–2287. 2012.PubMed/NCBI
|
|
8
|
Schütz AK, Hennes T, Jumpertz S, Fuchs S
and Bernhagen J: Role of CSN5/JAB1 in Wnt/β-catenin activation in
colorectal cancer cells. FEBS Lett. 586:1645–1651. 2012.
|
|
9
|
O’Hurley G, Perry AS, O’Grady A, Loftus B,
Smyth P, O’Leary JJ, et al: The role of secreted frizzled-related
protein 2 expression in prostate cancer. Histopathology.
59:1240–1248. 2011.PubMed/NCBI
|
|
10
|
Kinoshita T, Nomoto S, Kodera Y, Koike M,
Fujiwara M and Nakao A: Decreased expression and aberrant
hypermethylation of the SFRP genes in human gastric cancer.
Hepatogastroenterology. 58:1051–1056. 2011.PubMed/NCBI
|
|
11
|
von Marschall Z and Fisher LW: Secreted
Frizzled-related protein-2 (sFRP2) augments canonical Wnt3a-induced
signaling. Biochem Biophys Res Commun. 400:299–304. 2010.PubMed/NCBI
|
|
12
|
Li YY, Qin YZ, Wang RQ, Li WB and Qu XJ:
SL-01, an oral derivative of gemcitabine, inhibited human breast
cancer growth through induction of apoptosis. Biochem Biophys Res
Commun. 438:402–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Y, Ho GH, Koong HN, Sivaramakrishnan
G, Ang WT, Koh QM and Lin VC: Down-regulation of tripartite-motif
containing 22 expression in breast cancer is associated with a lack
of p53-mediated induction. Biochem Biophys Res Commun. 44:600–606.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Freitas Cordeiro-Silva M, Stur E,
Agostini LP, de Podestá JR, de Oliveira JC, Soares MS, et al:
Promoter hypermethylation in primary squamous cell carcinoma of the
oral cavity and oropharynx: a study of a Brazilian cohort. Mol Biol
Rep. 39:10111–10119. 2012.PubMed/NCBI
|
|
15
|
Ovchinnikov DA, Cooper MA, Pandit P, Coman
WB, Cooper-White JJ, Keith P, et al: Tumor-suppressor gene promoter
hypermethylation in saliva of head and neck cancer patients. Transl
Oncol. 5:321–326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jost E, Gezer D, Wilop S, Suzuki H, Herman
JG, Osieka R and Galm O: Epigenetic dysregulation of secreted
Frizzled-related proteins in multiple myeloma. Cancer Lett.
281:24–31. 2009. View Article : Google Scholar
|
|
17
|
Cheng YY, Yu J, Wong YP, Man EP, To KF,
Jin VX, et al: Frequent epigenetic inactivation of secreted
frizzled-related protein 2 (SFRP2) by promoter methylation in human
gastric cancer. Br J Cancer. 97:895–901. 2007.PubMed/NCBI
|
|
18
|
Lachenmayer A, Alsinet C, Savic R,
Cabellos L, Toffanin S, Hoshida Y, et al: Wnt-pathway activation in
two molecular classes of hepatocellular carcinoma and experimental
modulation by sorafenib. Clin Cancer Res. 18:4997–5007. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jia Y, Yang Y, Brock MV, Zhan Q, Herman JG
and Guo M: Epigenetic regulation of DACT2, a key component of the
Wnt signalling pathway in human lung cancer. J Pathol. 230:194–204.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lavergne E, Hendaoui I, Coulouarn C,
Ribault C, Leseur J, Eliat PA, et al: Blocking Wnt signaling by
SFRP-like molecules inhibits in vivo cell proliferation and tumor
growth in cells carrying active β-catenin. Oncogene. 30:423–433.
2011.PubMed/NCBI
|
|
21
|
Guo Y, Guo W, Chen Z, Kuang G, Yang Z and
Dong Z: Hypermethylation and aberrant expression of Wnt-antagonist
family genes in gastric cardia adenocarcinoma. Neoplasma.
58:110–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kongkham PN, Northcott PA, Croul SE, Smith
CA, Taylor MD and Rutka JT: The SFRP family of WNT inhibitors
function as novel tumor suppressor genes epigenetically silenced in
medulloblastoma. Oncogene. 29:3017–3024. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y and Chen H: Genistein attenuates
WNT signaling by up-regulating sFRP2 in a human colon cancer cell
line. Exp Biol Med (Maywood). 236:714–722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chung MT, Lai HC, Sytwu HK, Yan MD, Shih
YL, Chang CC, et al: SFRP1 and SFRP2 suppress the transformation
and invasion abilities of cervical cancer cells through Wnt signal
pathway. Gynecol Oncol. 112:646–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu Y, Zheng S, An N, Athanasopoulos T,
Popplewell L, Liang A, et al: β-catenin as a potential key target
for tumor suppression. Int J Cancer. 129:1541–1551. 2011.
|
|
26
|
Webster MR and Weeraratna AT: A Wnt-er
migration: the confusing role of β-catenin in melanoma metastasis.
Sci Signal. 6:pe112013.PubMed/NCBI
|
|
27
|
Kim W, Kim M and Jho EH: Wnt/β-catenin
signalling: from plasma membrane to nucleus. Biochem J. 450:9–21.
2013.
|
|
28
|
Valenta T, Hausmann G and Basler K: The
many faces and functions of β-catenin. EMBO J. 31:2714–2736.
2012.
|
|
29
|
Umbreit C, Aderhold C, Faber A, Sommer JU,
Sauter A, Hofheinz RD, et al: Unexpected alteration of β-catenin
and c-KIT expression by 5-FU and docetaxel in p16-positive squamous
cell carcinoma compared to HPV-negative HNSCC cells in vitro.
Anticancer Res. 33:2457–2465. 2013.
|
|
30
|
Ebrahimi M, Boldrup L, Wahlin YB, Coates
PJ and Nylander K: Decreased expression of the p63 related proteins
beta-catenin, E-cadherin and EGFR in oral lichen planus. Oral
Oncol. 44:634–638. 2008. View Article : Google Scholar
|
|
31
|
Li GH, Cui YS, Wu QY, Zhang XJ and Gao YF:
Clinicopathologic significance of β-catenin and matrix
metalloproteinase-2 expression in non-small cell lung cancer. Med
Oncol. 30:4372013.
|
|
32
|
Monga SP: Role of Wnt/β-catenin signaling
in liver metabolism and cancer. Int J Biochem Cell Biol.
43:1021–1029. 2011.
|