Introduction
Cell adhesion molecule 1/tumor suppressor in lung
cancer-1 (CADM1/TSLC1) is a tumor suppressor gene that was first
identified by Murakami et al in lung carcinoma (1). Murakami et al observed that
loss of heterozygosity (LOH) on chromosome 11q23 occurred in
patients with non-small cell lung carcinoma and found it had growth
suppression effects on the tumor cells. Therefore, the gene was
named tumor suppressor in lung carcinoma-1 (TSLC1).
Loss or reduction of CADM1/TSLC1 expression was
frequently observed and demonstrated to be involved in the
progression and metastasis of a growing number of different tumor
types, including lung cancer (2),
gastric cancer (3), T-cell
leukemia (4), ovarian carcinoma
(5), pancreatic cancer (6) and breast cancer (7). In our previous study (8), it was demonstrated that silencing of
CADM1/TSLC1 in melanoma is consistent with promoter methylation,
and that the incidence of the loss of expression and methylation of
CADM1/TSLC1 significantly increased as the tumor stage advanced.
The present study focuses on the functional role of CADM1/TSLC1 in
the tumorigenesis of melanoma. To examine the possible
growth-suppressive activity of CADM1/TSLC in melanoma, a stable
CADM1/TSLC1-expressing cell line A375-CADM1/TSLC1 (A1) and empty
vector-transfected control cell line A375-pcDNA3.1 (A0) were
established by transfection with pcDNA-CADM1/TSLC1 or empty
pcDNA3.1 vectors, respectively. An
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
assay, flow cytometry and transwell chambers were utilized to
detect proliferation, invasion and cell apoptosis,
respectively.
Metastasis is a complex process during which tumor
cells become invasive, migrating away from the primary tumor and
passing through natural extracellular matrix (ECM)-based barriers
impeding access to vascular or lymphatic vessels.
Matrix metalloproteinase-2 (MMP-2) and matrix
metalloproteinase-9 (MMP-9) are two important members in the
metalloproteinase superfamily which are structurally related
proteolytic enzymes that facilitate the degradation of ECM and the
basement membrane. It has been demonstrated that MMPs are closely
correlated with tumor invasion, since their upregulation markedly
facilitated cancer cell migration through the ECM (9).
To further examine the possible anti-invasive
mechanism of CADM1/TSLC1, the expression of MMP-2 and MMP-9 in A375
cells with different treatment were analyzed by western
blotting.
Materials and methods
Cell line and culture
The human melanoma cell line A375 was provided by
Tumor Institute of Harbin Medical University (Harbin, Heilongjiang,
China). The cells were maintained in the RPMI-1640 (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China),
supplemented with 10% fetal bovine serum (Gibco, Eggenstein,
Germany), 100 U/ml of penicillin and 100 ug/ml of streptomycin at
37°C in a 5% CO2 atmosphere.
Construction of plasmid expressing human
CAMD1/TSLC1
Full length fragment of TSLC1 was amplified from RNA
of hepatic cell L-02 by RT-PCR. The sequences of human CAMD1/TSLC1
primers were as follows: the forward primer, GACATGGCGAGTGTAGTGCT;
the reverse primer, TGGGTCTGCAGGTTTCCAGT. PCR was performed using
LA Taq System (TaKaRa Biotechnology (Dalian) Co., Ltd, Dalian,
China) in 25 cycles of 94°C for 50 sec, 58°C for 30 sec and 72°C
for 90 sec. PCR products were ligated into the pcDNA3.1 vector
(Invitrogen Life Technologies, Carlsbad, CA, USA) to obtain the
pcDNA-CADM1/TSLC1 vector.
Establishment of stable cell lines
The A375 cells were maintained in high glucose
RPMI-1640 supplemented with 10% PBS, 100 U/ml penicillin and 100
μg/ml streptomycin. The A375 cells were transfected with
pcDNA-CAMD1/TSLC1 and empty pcDNA3.1 vector, respectively, using
Lipofectamine 2000 (Invitrogen Life Technologies) according to the
manufacturer’s instructions. The transfected cells were selected
and maintained in full medium containing 600 μg/ml G418 (Invitrogen
Life Technologies). The cells stably expressing CAMD1/TSLC1 were
then isolated and the expression of CAMD1/TSLC1 was confirmed by
PCR and western blotting.
Western blotting analysis
Western blotting was conducted according to standard
methods as described previously (8). Briefly, the cells were washed with
PBS, then treated with a lysis buffer and protease inhibitor
mixture on ice for 25 min and centrifuged. The protein samples were
separated with 12% SDS-PAGE and subsequently transferred onto PVDF
membranes (Millipore, Billerica, MA, USA). The membranes were
blocked with 5% skimmed milk solution in PBS for 60 min at room
temperature, and incubated with rabbit anti-CADM1/TSLC1, MMP-2 and
MMP-9 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA),
followed by biotinylated goat anti-rabbit IgG (Sigma, St. Louis,
MO, USA) each for 2 h at 37°C. The membranes were stained with an
enhanced chemiluminescence solution (PerkinElmer, Boston, MA, USA).
The images were acquired using an Image Quant350 digital image
system (GE Healthcare, Uppsala, Sweden).
Cell proliferation analysis
To examine the cell proliferation, the cell growth
of A375, A0 and A1 cells were analyzed by an MTT assay. Briefly,
1×105 cells were seeded 96-well plates and the cell
growth was measured per 24 h until day 4. A volume of 30 μl of MTT
(Sigma Chemical Co., St. Louis, MO, USA) solution (5 mg/ml) was
then added and the cells were further incubated at 37°C for 4 h.
After 100 μl of DMSO was added, the photodensity value at 540 nm
was determined by an Opsys MR microplate reader (Thermo Labsystems,
Beverly, MA, USA). Measurements of cell growth by the MTT assay
were expressed as a percentage of the inhibition according to the
following formula: Inhibition rate (%) = [(A value of vector-alone
transfectant - A value of CADM1 transfectant)/A value of
vector-alone transfectant] × 100%.
Cell migration assay
The effect of CADM1/TSLC1 on tumor cell invasion was
investigated in vitro by an cell migration assay that was
performed using 96-well plate transwell chambers (BD Biosciences,
Franklin Lakes, NJ, USA) according to the manufacturer’s
instructions. In brief, 2×105 of A375, A0 and A1 cells
in 500 μl serum-free medium were seeded into the upper part of each
chamber of 96-well Matrigel chambers, respectively. A total of 24 h
later, the cells were fixed and stained, and the number of cells on
the lower surface of the filters was counted under the microscope
(Olympus BX51; Olympus Corporation, Tokyo, Japan).
Cell apoptosis assay
For the apoptosis assay, 1×105 A375, A0
and A1 cells were trypsinized at 48 h, washed with cold PBS, and
resuspended in PBS. Then, 10 μl of Annexin V-FITC (BD Biosciences)
and 5 ul of propidium iodide (PI) were added. After the cells were
vortexed and incubated for 15 min at room temperature, 400 μl of
binding buffer was added to the mixture. Flow cytometry was
conducted on a FAC Scan instrument (BD Biosciences).
Statistical analyses
Statistical analysis was performed using SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA). Summary results were
described as the mean ± standard error of the mean, and analysis of
variance was used for the comparison of multiple means among the
three groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
CAMD1/TSLC1 expression in A375 cells with
different treatment
To evaluate the biological role of CAMD1/TSLC1 in
melanoma, we established stable CAMD1/TSLC1-expressing cell line
A375-CAMD1/TSLC1 (A1) and empty vector-transfected control cell
line A375-pcDNA3.1 (A0) by transfection with pcDNA-CAMD1/TSLC1 or
empty pcDNA3.1 vectors, respectively. The results of RT-PCR and
western blotting demonstrated that the level of CAMD1/TSLC1 mRNA
(Fig. 1) and protein expression
(Fig. 2) were significantly
increased in A1 cells, compared with the A375 and A0 cells.
However, there were no significant differences in the levels of
CAMD1/TSLC1 mRNA and protein expression between the A375 cells and
A0 cells (P>0.05).
 | Figure 2CADM1/TSLC1 and MMP-2, MMP-9 protein
expression as determined by western blot analysis with an
anti-CADM1/TSLC1 and MMP-2, MMP-9 antibody. The protein expression
levels were semi-quantified by measuring the gray scale normalized
to that of the housekeeping protein, β-actin. The relative protein
expression levels were calculated by CADMI/TSLC1/β-actin ratios.
The values are expressed as the mean ± standard error of the mean.
(A) Compared with A375 and A0, A1 demonstrated a higher level of
CADM1/TSLC1 protein expression (P<0.05); (B) compared with A375
and A0, A1 demonstrated a lower level of MMP-2 protein expression
(P<0.05); (C) compared with A375 and A0, A1 demonstrated a lower
level of MMP-9 protein expression (P<0.05). A0, A375-pcDNA3.1;
A1, A375-CADMI/TSLC1; CADM1/TSLC1, cell adhesion molecule 1/tumor
suppressor in lung cancer-1; MMP-2/9, matrix
metalloproteinase-2/9. |
Expression of CAMD1/TSLC1 inhibits cell
proliferation
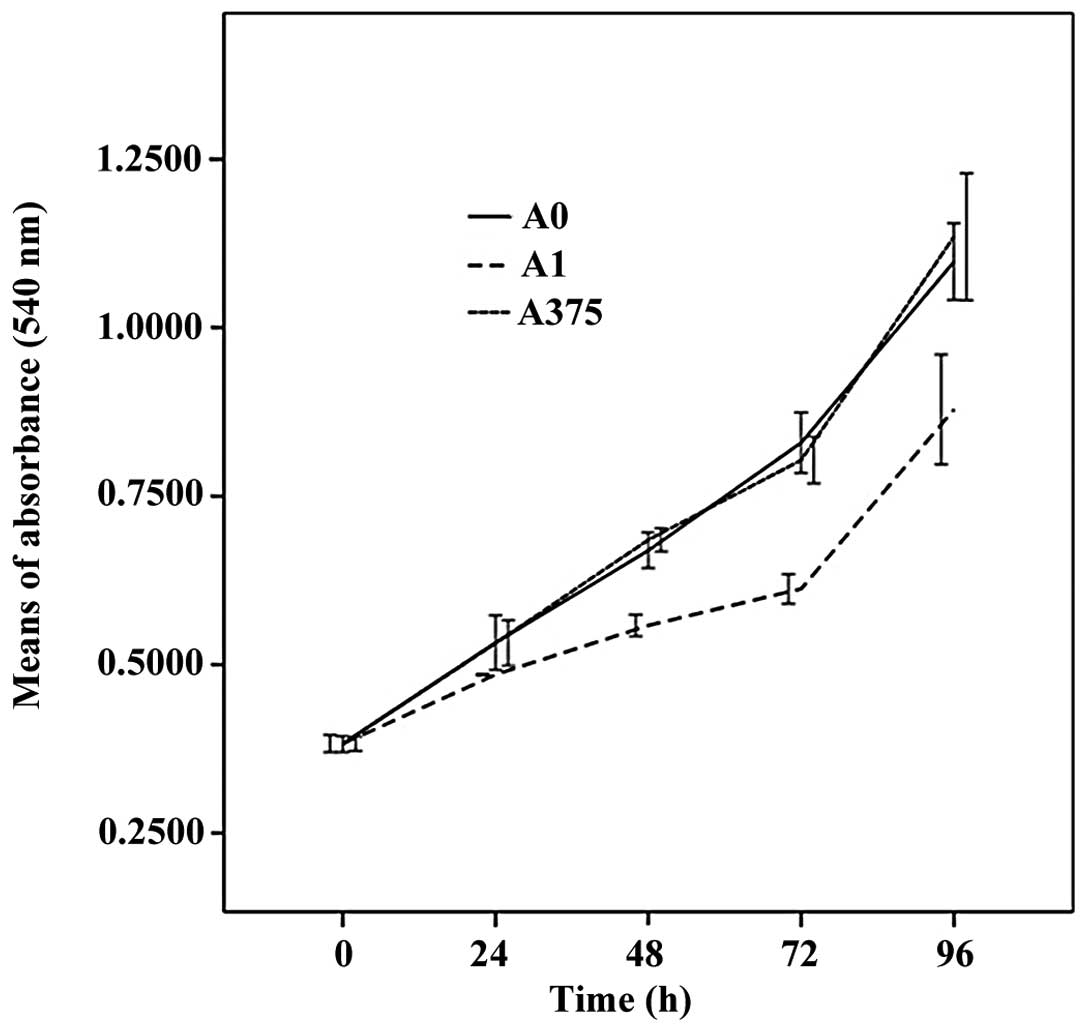
To study the effects of CAMD1/TSLC1 on cell growth,
the cell viability of the transfected and non-transfected A375
cells was measured by an MTT assay. As demonstrated in Fig. 3, the growth of A1 clone was slower
than that of A0 and A375 cells during the 96 h incubation period,
but no significant differences between the A0 and A375 cells were
observed (P>0.05).
Expression of CADM1/TSLC1 induces
apoptosis
To determine the apoptotic cell death in A375 cells
induced by CAMD1/TSLC1, A375, A0 and A1 cells were stained with
Annexin V/PI. As demonstrated in Fig.
3 and Table I, the results of
FACS analyses revealed that the proportion of positive cells were
evidently increased in A1 cells (37.60±0.1%; P<0.01), compared
with the A375 cells and A0 cells (3.98±0.4 and 5.62±0.9%,
respectively). Furthermore, there was a significant increase in the
number of apoptotic cells both in the early phase and late phase in
A375-TSLC1 cells as well. However, there was no difference in
apoptosis rate between the A375 and A0 cells (P>0.05),
indicating that the overexpression of CAMD1/TSLC1 has an inductive
effect on apoptosis of A375 cells.
 | Table IAnalysis of cell apoptosis by Annexin
V/PI staining. |
Table I
Analysis of cell apoptosis by Annexin
V/PI staining.
| Cell line | Apoptotic cell
(%) | Late phase apoptosis
(%) | Living cell (%) | Early phase apoptosis
(%) | Number of apoptotic
cells (both phases) |
|---|
| A1 | 0.59±0.01 | 18.11±0.3a | 68.32±0.6 | 16.59±0.1a | 37.6±0.1a |
| A0 | 0.23±0.05 | 2.27±0.1 | 96.00±0.3 | 1.53±0.3 | 3.98±0.4 |
| A375 | 0.08±0.03 | 3.36±0.2 | 93.58±0.1 | 2.58±0.5 | 5.62±0.9 |
CADM1/TSLC1 suppresses cell
migration
To investigate the effect of CAMD1/TSLC1 on cell
metastasis, the cell migration ability of A375, A0 and A01 cells
was examined by transwell assay. As demonstrated in Fig. 4, the cell migration assay revealed
that the proportion of cells that transferring through the matrigel
was significantly suppressed by 86.7% in A1 cells compared with
that in the A0 (43.4%) and A375 (25.8%) cells. However, no
significant differences between the A0 and A375 cells were observed
in the transwell assay.
CADM1/TSLC1 downregulates MMP-2 and MMP-9
expression
To further investigate the molecular mechanisms
involved in the effect of CADM1/TSLC1 overexpression on tumor cell
invasion, the expression of MMP-2, -9, were determined by western
blotting in A375, A0 and A1 cells. As demonstrated in Fig. 2B and C, the expression of MMP-2 and
MMP-9 were significantly downregulated in the A1 cells, compared
with that in the A0 and A375 cells.
Discussion
Melanoma is a malignant tumor of melanocytes that
causes the majority of skin cancer-associated mortalities (10). The 5-year survival rate decreases
from 95% for patients with a maximum tumor thickness of 1 mm
lacking metastases, to <10% for patients with visceral
metastasis. There is a conspicuous difference in survival between
localized and metastatic disease (5-year survival of 98 and 15–62%,
respectively) (11). The
occurrence of metastasis is associated with high mortality rates
due to the aggressiveness characteristics of the tumor and the lack
of effective therapies to combat its spread. Therefore, identifying
the molecular mechanism underlying tumor progression and metastasis
in cancer is urgently required.
CADM1, also known as TSLC1, as a novel tumor
suppressor, has been extensively investigated in various tumors
(12–16). Loss or reduction of TSLC1
expression has been frequently found and demonstrated to be
involved in the occurrence and progression of multiple different
human tumors (17–20). Our previous results demonstrated
that the silencing of TSLC1 through methylation is an important
event in the pathogenesis of melanoma. Loss of the expression of
TSLC1 in the cytoplasm of melanoma is associated with later tumor
stage and decreased patient survival. TSLC1 thus constitutes a
clinically important prognostic marker and a potential target for
the development of novel therapies.
Accumulative evidence has revealed that CAMD1/TSLC1
is a crucial regulator of cell proliferation, invasion and
apoptosis (21–23). In the present study, to verify the
molecular mechanism of the tumor-suppressing effect of CAMD1/TSLC1
in melanoma, a A375 cell line stably expressing CAMD1/TSLC1, A1,
was successfully established and was used to investigate the role
of CAMD1/TSLC1. In vitro, cell growth suppression by
CAMD1/TSLC1 expression was demonstrated in that the cell
proliferation was evidently inhibited in the A1 cells compared with
the A375 and A0 cells (P<0.05), whereas there was no significant
difference in the proliferation between the A375 and A0 cells
(P>0.05). It was concluded that the inhibition of CAMD1/TSLC1 on
cell invasion was, at least in part, due to the inhibition of
proliferation.
It is well established that apoptosis is an
important physiological process responsible for maintaining the
balance of homeostasis and that it has a central role in the
progression and development of tumors. In the present study,
Annexin V/PI staining demonstrated a significant increase in the
number of total apoptotic cells of A1 other than in A375 and A0
cells. This indicated that the overexpression of CAMD1/TSLC1 has an
inductive effect on the apoptosis of A375 cells.
Malignant tumor invasion is a dynamic, continuous
process. Tumor cells migrate away from the primary site, first
invading the extracellular matrix (ECM) and the basement membrane
and the interstitial cells in some molecular adhesion, and then
activating cell synthesis and the secretion of various degradation
enzymes, that assist in the migration of tumor cells through the
ECM into the blood vessels. Then under the regulation of certain
factors, including running through the vessel wall leakage to the
secondary site, they continue to proliferate and subsequently
result in the formation of metastases with peeling, adhesion,
degradation, mobility, proliferation and transfer throughout the
course of malignant tumor invasion.
In the present study, to determine whether the
invasion ability of the A375 cells, matrigel, which simulates the
metastatic process of tumor cells to travel through the ECM and
basement membrane components, was inhibited, the cell migration
ability of A375, A0 and A01 cells was examined by a transwell
assay. As demonstrated in Fig. 4,
the transwell assay revealed that the invasive capacity was
significantly suppressed in A1 cells compared with that in the A0
and A375 cells, but no significant differences between the A0 and
A375 cells were observed, which indicated that the overexpression
of CAMD1/TSLC1 had an inductive effect on the migration ability of
melanoma.
MMPs are a family of structurally related
proteolytic enzymes that facilitate the degradation of ECM and the
basement membrane. MMP-2 and MMP-9 are two important members in the
metalloproteinase superfamily, which are involved in a wide range
of proteolytic events, including tumor growth, migration,
metastasis and angiogenesis (24).
In the present study, to further examine the probable anti-invasive
mechanism of CAMD1/TSLC1, the expression of MMP-9 and MMP-2 in
A375, A0 and A1 cells was examined. As is demonstrated in Fig. 2., the A1 cells revealed a marked
expression of MMP-9 and MMP-2. Therefore, it was hypothesized that
the invasion inhibitory effect of CAMD1/TSLC1 may be due to the
suppression of MMP-9 and MMP-2 expression, which subsequently
prompts tumor metastasis and invasion. The details of the mechanism
involved required further validation.
In conclusion, the present study demonstrated that
CADM1/TSLC1 had anti-invasive effects on A375 cells. This
inhibition was correlated with the expressional downregulation of
MMP-2and MMP-9, which are associated with tumor metastasis and
progression.
Acknowledgements
This study was supported by the Foundation of
Technique of Heilongjiang Province, China (no. C201317). The
authors would like to thank Xiwen Sun for providing valuable
suggestions and assistance and Yuyan Ma for his excellent technical
assistance.
References
|
1
|
Kuramochi M, Fukuhara H, Nobukuni T, et
al: TSLC1 is a tumor suppressor gene in human non-small cell lung
cancer. Nat Genet. 27:427–430. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uchino K, Ito A, Wakayama T, et al:
Clinical implication and prognostic significance of the tumor
suppressor TSLC1 gene detected in adenocarcinoma of the lung.
Cancer. 98:1002–1007. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tamura G: Promoter methylation status of
tumor suppressor and Tumor related genes in neoplastic and
non-neoplastic gastric epithelia. Histol Histopathol. 19:221–228.
2004.PubMed/NCBI
|
|
4
|
Dewan MZ, Takamatsu N, Hidaka T, et al:
Critical role for TSLC1 expression in the growth and organ
infiltration of adult T-cell leukemia cells in vivo. J
Virol. 82:11958–11963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang G, He W, Cai M, et al:
Loss/Down-regulation of tumor suppressor in lung cancer 1
expression is associated with tumor progression and is a biomarker
of poor prognosis in ovarian carcinoma. Int J Gynecol Cancer.
21:486–493. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jansen M, Fukushima N, Rosty C, et al:
Aberrant methylation of the 5-CpG island of TSLC1 is common in
pancreatic ductal adenocarcinoma and is first manifest in
high-grade PanlNs. Cancer Biol Ther. 1:293–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crawford NP, Qian X, Ziogas A, et al:
Rrp1b, a new candidate susceptibility gene for breast cancer
progression and metastasis. PLoS Genet. 3:e2142007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
You Y, Ma L, You M, et al: TSLC1 gene
silencing in cutaneous melanoma. Melanoma Res. 20:179–183.
2010.PubMed/NCBI
|
|
9
|
Herszényi L, Hritz I, Lakatos G, Varga MZ
and Tulassay Z: The behavior of matrix metalloproteinases and their
inhibitors in colorectal cancer. Int J Mol Sci. 13:13240–13263.
2012.PubMed/NCBI
|
|
10
|
Meyskens FL Jr, Farmer PJ, Yang S and
Anton-Culver H: New perspectives on melanoma pathogenesis and
chemoprevention. Recent Results Cancer Res. 174:191–195. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balch CM, Buzaid AC, Soong SJ, et al:
Final version of the American Joint Committee on Cancer staging
system for cutaneous melanoma. J Clin Oncol. 19:3635–3648.
2001.PubMed/NCBI
|
|
12
|
Takahashi Y, Iwai M, Kawai T, et al:
Aberrant expression of tumor suppressors CADM1 and 4.1B in invasive
lesions of primary breast cancer. Breast Cancer. 19:242–252. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heller G, Fong KM, Girard L, et al:
Expression and methylation pattern of TSLC1 cascade genes in lung
carcinomas. Oncogene. 25:959–968. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Houshmandi SS, Surace EI, Zhang HB, et al:
Tumor suppressor in lung cancer-1 (TSLC1) functions as a glioma
tumor suppressor. Neurology. 67:1863–1866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukami T, Fukuhara H, Kuramochi M, et al:
Promoter methylation of the TSLC1 gene in advanced lung tumors and
various cancer cell lines. Int J Cancer. 107:53–59. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamada D, Yoshida M, Williams YN, et al:
Disruption of spermatogenic cell adhesion and male infertility in
mice lacking TSLC1/IGSF4, an immunoglobulin superfamily cell
adhesion molecule. Mol Cell Biol. 26:3610–3624. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He G, Lei W, Wang S, et al: Overexpression
of tumor suppressor TSLC1 by a survivin-regulated oncolytic
adenovirus significantly inhibits hepatocellular carcinoma growth.
J Cancer Res Clin Oncol. 138:657–670. 2012. View Article : Google Scholar
|
|
18
|
Surace EI, Lusis E, Murakami Y, et al:
Loss of tumor suppressor in lung cancer-1 (TSLC1) expression in
meningioma correlates with increased malignancy grade and reduced
patient survival. J Neuropathol Exp Neurol. 63:1015–1027. 2004.
|
|
19
|
Chen K, Wang G, Peng L, et al: CADM1/TSLC1
inactivation by promoter hypermethylation is a frequent event in
colorectal carcinogenesis and correlates with late stages of the
disease. Int J Cancer. 128:266–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ando K, Ohira M, Ozaki T, et al:
Expression of TSLC1, a candidate tumor suppressor gene mapped to
chromosome 11q23, is downregulated in unfavorable neuroblastoma
without promoter hypermethylation. Int J Cancer. 123:2087–2094.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sussan TE, Pletcher MT, Murakami Y and
Reeves RH: Tumor suppressor in lung cancer 1 (TSLC1) alters
tumorigenic growth properties and gene expression. Mol Cancer.
4:282005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qin L, Zhu W, Xu T, et al: Effect of TSLC1
gene on proliferation, invasion and apoptosis of human
hepatocellular carcinoma cell line HepG2. J Huazhong Univ Sci
Technolog Med Sci. 27:535–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mao X, Seidlitz E, Truant R, Hitt M and
Ghosh HP: Re-expression of TSLC1 in a non-small-cell lung cancer
cell line induces apoptosis and inhibits tumor growth. Oncogene.
23:5632–5642. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giannelli G and Antonaci S: MMP and TIMP
assay in cancer: Biological and clinical significance. Int J
Cancer. 116:1002–1003. 2005. View Article : Google Scholar
|