Introduction
Cervical cancer is the third most commonly diagnosed
cancer and the fourth leading cause of cancer-associated mortality
in females worldwide, accounting for 9% of the total cancer
diagnoses and 8% of the total cancer-related mortalities among
females in 2008; >85% of these cases occur in developing
countries (1). The frequency of
lymph node metastasis increases with clinical stage; and invasive
cervical cancer is a serious problem and a leading cause of
cancer-related mortality in women (2). The majority of patients with cervical
cancer receive standard radiotherapy and chemotherapy, but clinical
outcomes vary significantly and are difficult to predict.
It has been established that invasion and adhesion
are fundamental properties of malignant cancer cells (3). The formation of metastatic nodules
involves multiple mechanisms, including the loss of cellular
adhesion; increased motility and invasiveness; entry to and
survival in the circulatory system, exit to new tissue; and the
eventual colonization of a distant metasasis site. A wide variety
of factors contribute to the metastasis of tumor cells, including
cytokines, hormones, growth factors, cell adhesion molecules and
matrix metalloproteinases (MMPs). Therefore, natural agents that
are able to suppress the expression of these factors may be
considered for development as treatments to prevent cervical cancer
invasion and metastasis (4).
Isothiocyanates are natural compounds located in
consumable cruciferous vegetables such as broccoli, watercress,
brussel sprouts, cabbage, Japanese radishes and cauliflower. They
have been indicated to inhibit the chemical carcinogenesis caused
by a wide variety of chemical carcinogens in animal models
(5). Previous studies have also
demonstrated that isothiocyanates exhibit antitumor activity,
inhibiting growth and metastasis in various human cancer cell
models. Other studies have revealed that phenethyl isothiocyanate
(PEITC) suppresses the growth of cancer cells in vitro and
in vivo (6). Several
cellular responses to this potential chemopreventative agent have
been previously described, including apoptosis mediated by c-Jun
amino terminal kinase and extracellular signal-regulated kinase
(7,8). However, the mechanisms behind the
anticancer effects of PEITC are not clearly understood.
Although numerous studies have indicated that PEITC
can be used as an inducer of apoptosis, there are thus far no
reports implicating PEITC in the inhibition of the metastasis
potential of cervical cancer cells. Therefore, the present study
analyzed the inhibitory effects of PEITC on the metastatic
potential of cervical cancer cells. HeLa cervical carcinoma cells
were used to examine the effect of PEITC on cell proliferation,
invasion, migration and the expression of metastasis-related
genes.
Materials and methods
Cell culture
The human HeLa cervical carcinoma cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA). The cells were grown and maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS) and 2 mM glutamine
(Gibco Life Technologies, Carlsbad, CA, USA) at 37°C, 5%
CO2. Penicillin and streptomycin were not added to the
culture medium to avoid interaction with isothiocyanates. A stock
solution of 1 mM PEITC dissolved in DMSO (Sigma-Aldrich, St. Louis,
MO, USA) was made and added to cell medium at various
concentrations for the treatment of cells.
Cell proliferation assay
Cells were seeded at a density of 2×103
into a 96-well plate overnight and incubated with 0, 0.1, 0.2, 0.5,
1, 2, 5, 10, 20, 50 and 100 μM PEITC for 24 h at 37°C. The cell
viability was determined with a Vi-CELL cell viability analyzer
(Beckman Coulter, Brea, CA, USA) according to the manufacturer’s
instructions, and the IC50 values were calculated.
Adhesion assay
Matrigel was purchased from BD Biosciences (Franklin
Lakes, NJ, USA) and a concentration was used according to the
manufacturer’s instructions. The confluent cultures of
2×105 cells were plated onto the Matrigel-coated insert
of the 96-well culture plates and incubated with 5 or 10 μM PEITC
for 6 h in standard culture medium. Next, the liquid was discarded
and the cells were gently washed once with PBS. The cells were then
analyzed by MTS assay (Promega Corporation, Madison, WI, USA).
Briefly, 20 μl MTS was added to the fresh culture medium and
incubated for 4 h, then the absorbance was determined using a
Synergy 2 Multi-Mode Microplate Reader (BioTek Instruments, Inc.,
Shoreline, WA, USA) at 490 nm (A490) to calculate the percentage
cell viability. The background absorbance was measured at 630 nm
(A630).
Cell invasion assay
A cell invasion assay was performed using Transwell
cell culture inserts (Invitrogen Life Technologies, Carlsbad, CA,
USA). Briefly, 3×104 cells were harvested, resuspended
in serum-free medium, and then transferred to the hydrated Matrigel
(BD Biosciences) upper chambers. The chambers were then incubated
with 5 and 10 μM PEITC for 24 h in culture medium with 10% FBS,
then the invaded cells on the lower surface were fixed and stained
with 0.05% crystal violet (Sigma-Aldrich) for 2 h. Finally, the
invaded cells were counted under a microscope at a magnification of
×40 and the relative number of invaded cells to total cells was
calculated.
Cell cycle analysis
Cells were cultured in 6-well plates at a density of
3×105, in medium without FBS for 24 h for
synchronization, then incubated with 5 μM and 10 μM PEITC for a
further 24 h. The cells were harvested using trypsin
(Sigma-Aldrich) and fixed in 70% cold ethanol overnight at 4°C. The
cells were then resuspended in 1 ml PBS containing 1 mg/ml RNase A
Solution and 0.5 mg/ml Propidium Iodide (Sigma-Aldrich) for 30 min.
Next, 1×104 events were collected and analyzed by flow
cytometry (BD Biosciences). Modfit LT software, version 3.0 (Verity
Software House, Inc., Topsham, ME, USA) was used to analyze the
results.
ELISA analysis
The ELISA kits (R&D Systems, Inc., Minneapolis,
MN, USA) were used to detect the concentration of TGF-β, IL-6 and
IL-8 in the cell culture supernatant, according to the
manufacturer’s instructions. Briefly, 3×105 cells were
cultured in 6-well plates overnight and incubated with 5 and 10 μM
PEITC for 24 h, then the culture was replaced with DMEM without FBS
for 24 h, and the cell culture supernatant was collected. A total
of 100 μl of the supernatant was added to microplate wells, and 200
μl anti-TGF-β, anti-IL-6 and anti-IL-8 were added 10 sec later.
Subsequent to incubation at 37°C for 30 min, the sample was washed
5 times with wash buffer, followed by the addition of 200 μl
horseradish peroxidase (Santa Cruz Biotechnology, Dallas, TX, USA)
to each well following incubation at 37°C for 30 min. Next, the
wells were washed 5 times with Tris-buffered saline with Tween 20
(20 nm Tris, pH 7.5, 150 nM NaCl, 0.1% Tween 20), followed by the
addition of 100 μl tetramethylbenzidine (Sigma-Aldrich) and
incubated at room temperature in the dark. After a 20 min
incubation, 100 μl stop solution was added, and the absorbance was
read on a microplate reader at 450 nm. All experiments were
performed in duplicate. The values were calculated from standard
curves.
Quantitative polymerase chain reaction
(qPCR)
A total of 3×105 cells were cultured in a
6-well plate overnight and incubated with 5 or 10 μM PEITC for 24
h, then total cellular RNA was isolated with TRIzol®
reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. cDNA for each cell line was
synthesized from 1 μg total RNA and SuperScript Reverse
Transcriptase as described in the manufacturer’s protocol (Takara
Bio, Inc., Otsu, Japan). qPCR assays were performed by SYBR-Green
(Takara Bio, Inc.) incorporation. The relative gene expression was
determined by cycle threshold calculation, utilizing actin for
normalization and GAPDH as a reference gene. The following primers
were used to amplify the cDNA fragments: CD44 F 5′-CAGATGGCC
CATACCTTCAAAT-3′ and R 5′-CGGAAACGAAATCCT CTCTGTT-3′; ICAM-1, F
5′-CTCCAATGTGCCAGG CTTG-3′ and R 5′-CAGTGGGAAAGTGCCATCCT-3′; MMP-2
F 5′-CTTCCAAGTCTGGAGCGATGT-3′ and R 5′-TACCGTCAAAGGGGTATCCAT-3′;
MMP-9 F 5′-GGGACGCAGACATCGTCATC-3′ and R 5′-TCG
TCATCGTCGAAATGGGC-3′; CDK1 F 5′-TTCAGG ATGTGCTTATGC-3′ and R
5′-AGAGCAATTCCA AGCCAT-3′; GAPDH F 5′-TGTTGCCATCAATGACCC CTT-3′ and
R 5′-CTCCACGACGTACTCAGCG-3. The PCR cycling conditions were as
follows: Denaturation at 95°C for 60 sec, 40 cycles of 95°C for 5
sec and hybridization at 72°C for 60 sec. GAPDH was used as an
internal control for loading.
Western blotting
A total of 3×105 cells were cultured in
6-well plates overnight and incubated with 5 or 10 μM PEITC for 24
h, then cells were lysed with 1X modified radioimmunoprecipitation
assay buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, and 0.5%
deoxycholate) containing 25 μg/ml leupeptin (Sigma-Aldrich), 10
μg/ml aprotinin (Sigma-Aldrich), 1 mM sodium orthovanadate and 2 mM
EDTA. The protein concentrations of the samples were determined
using a Bicinchoninic Acid Protein Assay Reagent kit (Qiagen,
Valencia, CA, USA), and the cell lysates were analyzed by 8%
SDS-PAGE. The samples were then transferred to polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). The blots
were blocked for 1 h at room temperature with 5% milk and incubated
with the following monoclonal antibodies: CD44, (1:800); ICAM-1
(1:800); MMP-2 (1:800); MMP-9 (1:500); CDK1 (1:500); and p-Smad2
(1:500) (Santa Cruz Biotechnology) and mouse β-actin (1:2,000;
Sigma-Aldrich) which was used for protein loading analyses. The
blots were developed using an enhanced chemiluminescence (ECL) Plus
Western Blotting Detection Reagents kit (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA). The intensity of the protein
bands was determined by densitometry using a Gene Genius Super
system (Gene Tech Co., Ltd., Hong Kong, China).
Statistical analysis
The data are presented as the means ± standard
deviation. All the data from the current study were evaluated using
SPSS software, version 11.5 (SPSS, Inc., Chicago, IL, USA).
Assessment of the differences between groups was performed by
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
Effect of PEITC on the proliferative
potential of HeLa cells
To study the effect of PEITC on the growth of
cervical carcinoma cells, HeLa cells were incubated with various
concentrations of PEITC. The results indicated a dose-dependent
inhibition of cell growth; the IC50 value was 31.3 μM
and the proliferation inhibition rates of 24-h treatment with 5 and
10 μM PEITC were 8.4 and 12.6%. These concentrations were selected
as no-toxity (<10%) and low-toxity (<15%) doses for the
adhesion, invasion, cell cycle, cytokine, western blotting, qPCR
and reporter gene assays, to avoid interference of toxity with the
results (Fig. 1).
PEITC reduces the adhesive potential of
HeLa cells
The effect of PEITC on the adhesion of HeLa cells
was assessed by adhesion assay. When HeLa cells were incubated with
PEITC, cellular adhesion was inhibited, and the adhesion rates were
reduced to 71.2 and 55.7% of the control group following 24-h
treatment with 5 and 10 μM PEITC (P<0.05), respectively
(Fig. 2).
PEITC reduces the invasive potential of
HeLa cells
Invasion is another important step for metastasis,
so the inhibitory effect of PEITC on the ability of HeLa cells to
invade a reconstituted extracellular matrix was investigated. It
was demonstrated that PEITC inhibited cell invasion in a
dose-dependent manner. When HeLa cells were grown on Matrigel, a
significant reduction in the number of invading cells was observed
following treatment with PEITC. The levels of invasion were reduced
to 65.2 and 21.7% of control levels by 5 and 10 μM PEITC treatment
(P<0.05), respectively (Fig.
3).
PEITC treatment leads to G2/M
phase cell cycle arrest in HeLa cells
Cell cycle phase assay was performed by flow
cytometry, subsequent to staining of the fixed cells with PI. As
displayed in Fig. 4, PEITC
markedly induced G2/M arrest in HeLa cells; the
percentages of G2/M phase cells were 10.34 and 22.8% in
the 5 and 10 μM PEITC groups, respectively, and 3.65% in the
control group (P<0.05). PEITC significantly induced cell cycle
arrest at the G2/M phase.
PEITC reduces the production of TGF-β,
IL-6 and IL-8 in HeLa cells
To observe the effects of PEITC on TGF-β, IL-6 and
IL-8 production, the expression levels of TGF-β, IL-6 and IL-8 were
examined by ELISA. The results indicated that the production of
TGF-β, IL-6 and IL-8 was significantly reduced in HeLa cells
incubated with 5 or 10 μM PEITC, as compared with the control group
(Fig. 5).
PEITC downregulates metastasis-related
genes and proteins in HeLa cells
CDK1, MMP-2/9, CD44 and ICAM-1 all function in
cervical cancer metastasis. The mRNA and protein expression levels
of CDK1, MMP-2/9, CD44 and ICAM-1 were investigated. The results
indicated that cells incubated with 5 or 10 μM PEITC exhibited
reduced mRNA and protein levels of CDK1, MMP-2/9, CD44 and ICAM-1,
compared with the control group (Fig.
6). These results were consistent with the assays for adhesion,
invasion and cell cycle.
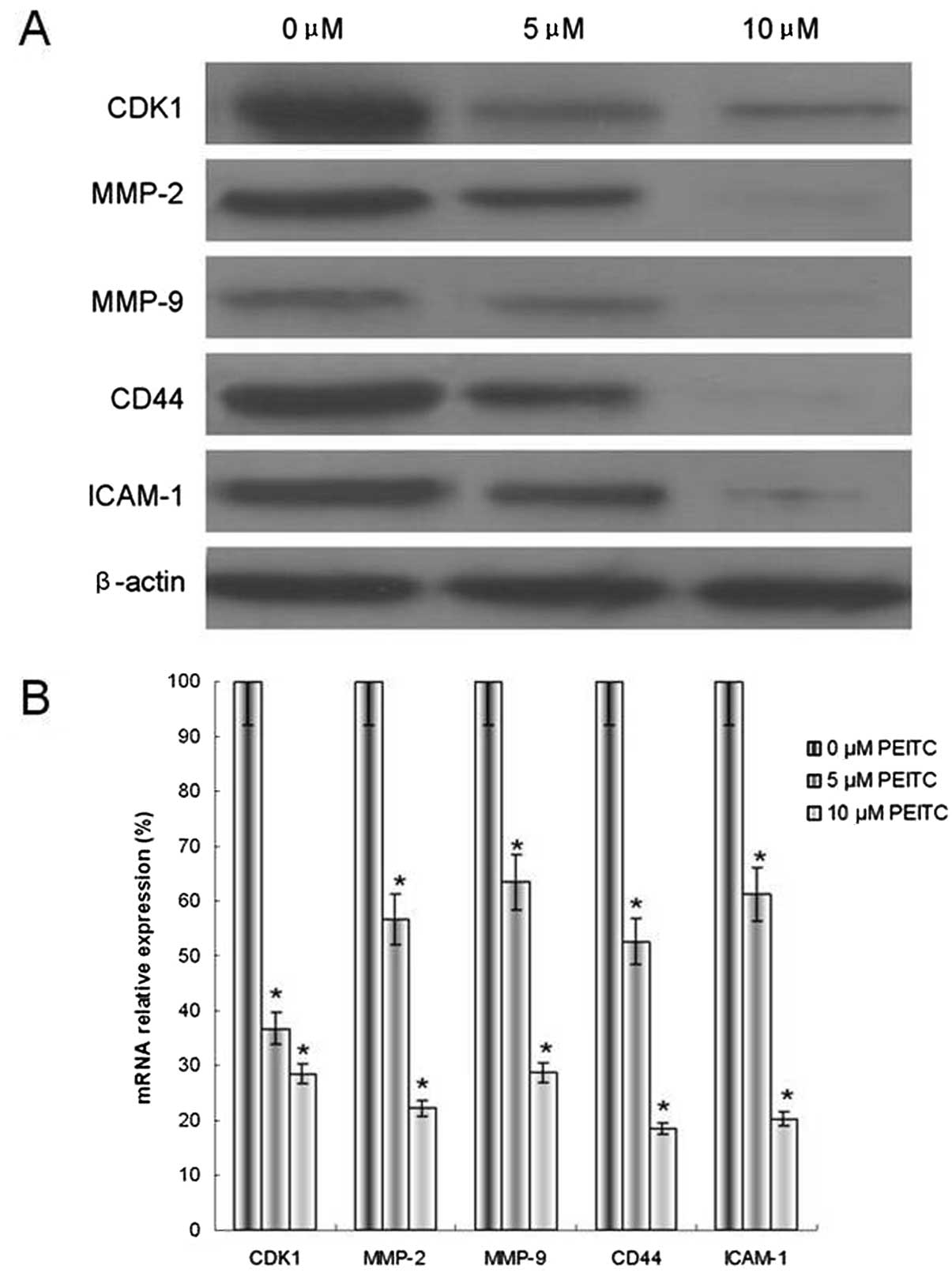 | Figure 6Effect of PEITC on the modulation of
CDK1, MMP-2/9, CD44 and ICAM-1 metastasis-related genes in HeLa
cells. (A) Protein expression, as determined by western blotting
with β-actin as an internal control for loading, and (B) mRNA
expression, determined by quantitative polymerase chain reaction,
with GAPDH as an internal control for loading. The data are
presented as the means ± standard deviation. *P<0.05,
n=5. PEITC, phenethyl isothiocyanate; CDK, cyclin-dependent kinase;
MMP, matrix metalloproteinase; ICAM, intercellular adhesion
molecule. |
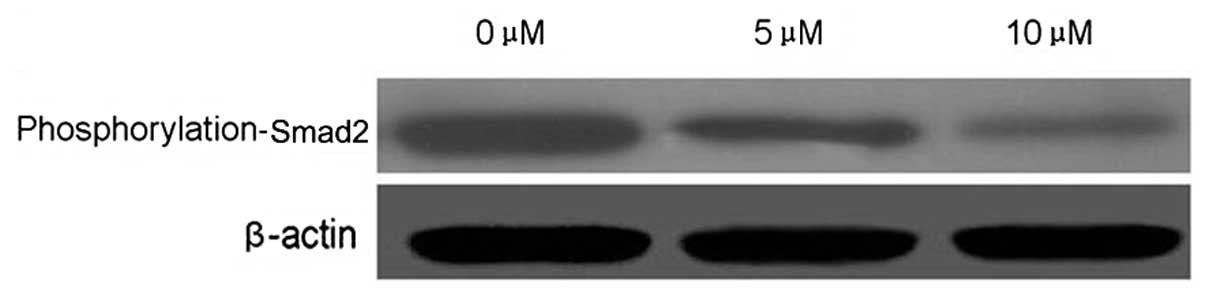
PEITC alters the metastasis-related
signal transduction pathway of HeLa cells
Smad2 is an important cell signaling molecule. To
evaluate whether Smad2 is target of PEITC in the inhibition of
cervical carcinoma cell metastasis, Smad2 phosphorylation was
detected by western blotting. PEITC reduced the phosphorylation of
Smad2 in a dose-dependent manner in HeLa cells incubated with 5 or
10 μM PEITC, as compared with the control group (Fig. 7).
Discussion
PEITC, a constituent of cruciferous plants and a
member of the isothiocyanate family, has been identified to have
antitumor properties. The development of novel anti-metastatic
drugs is currently one of the most important areas of tumor
research. It has been reported recently that PEITC serves an import
function in anti-metastatic processes in various tumor cells,
including human hepatoma, human colon cancer and breast carcinoma
cells (9–11). This effect is associated with the
regulation of the expression of metastasis-related genes, cytokines
and signal transduction molecules. However, little is known
regarding the potential of PEITC in cervical cancer. In the present
study, the effects of PEITC on cervical carcinoma cell metastasis
potential was evaluated by adhesion and invasion assays following
treatment with two concentrations of PEITC. The results suggested
that PEITC effectively inhibited cervical carcinoma cell metastasis
in vitro.
The effect of PEITC on metastasis-related gene
expression was then evaluated. MMPs are members of a superfamily of
zinc endopeptidases, and MMP-2 and MMP-9 are hypothesized to be
crucial in the degradation of extracellular matrix due to their
ability to cleave type IV collagen (12). CD44 and ICAM-1 are important
adhesion molecules that induce heterogeneous cell adhesion and
polarity alteration (13,14). The data indicated that PEITC
reduced MMP-2, MMP-9, CD44 and ICAM-1 mRNA and protein expression
levels. TGF-β, IL-6 and IL-8 have been indicated to be important
cytokines that serve a critical function in cancer invasion and
metastasis (15). In the present
study, reduced production of these cytokines was observed,
indicating that PEITC has the ability to inhibit the metastasis of
cervical carcinoma through the inhibition of TGF-β, IL-6 and IL-8
production.
A possible mechanism by which PEITC may protect
against cancer is through cell cycle arrest. PEITC has been
demonstrated to suppress growth and metastasis by this method in
lung, prostate and ovarian cancer. In the present study, the
results indicated that PEITC induced a dramatic accumulation of
HeLa cells in the G2/M phase. It was previously reported
that CDK1 regulates the cell cycle in mammalian cells, and is
activated primarily in G2/M phase progression (16). Results of the present study also
indicated that PEITC reduced the protein levels of CDK1, which
suggests that PEITC induced cell cycle arrest by suppressing the
expression of CDK1.
It is well-established that the Smad2 activation
pathway is a major cell survival pathway involved in immunity,
stress responses, inflammation and the inhibition of apoptosis and
metastasis (17,18). In the present study, the effect of
PEITC on Smad2 phosphorylation was examined. It was demonstrated
that PEITC inhibited Smad2 phosphorylation in a dose-dependent
manner, suggesting that p-Smad2 is a potential target of PEITC.
The present study provides support to the hypothesis
that PEITC promotes strong inhibition of metastasis by blocking the
TGF-β/Smad2 pathway, which leads to the inhibition of expression of
metastasis-related genes in HeLa cells. Understanding the mechanism
of action of PEITC may provide valuable information for its
possible application in anti-tumor therapy. A number of studies
have evaluated the effects of PEITC in human subjects, and the
present study may potentially facilitate the clinical development
of isothiocyanates for cancer therapy.
Acknowledgements
This study was supported by Tianjin Medical
University Science Fund (2012KYM04).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Wen Y, Pan XF, Zhao ZM, Chen F, et al:
Knowledge of human papillomavirus (HPV) infection, cervical cancer,
and HPV vaccine and its correlates among medical students in
southwest China: a multi-center cross-sectional survey. Asian Pac J
Cancer Prev. 15:5773–5779. 2014. View Article : Google Scholar
|
|
3
|
Ortega-Calderón YN and López-Marure R:
Dehydroepiandrosterone inhibits proliferation and suppresses
migration of human cervical cancer cell lines. Anticancer Res.
34:4039–4044. 2014.PubMed/NCBI
|
|
4
|
Kidd EA, Siegel BA, Dehdashti F, Rader JS,
et al: Lymph node staging by positron emission tomography in
cervical cancer: relationship to prognosis. J Clin Oncol.
28:2108–2113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wolf MA and Claudio PP: Benzyl
isothiocyanate inhibits HNSCC cell migration and invasion, and
sensitizes HNSCC cells to cisplatin. Nutr Cancer. 66:285–294. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Wei S, Wang J, Fang Q and Chai Q:
Phenethyl isothiocyanate inhibits growth of human chronic myeloid
leukemia K562 cells via reactive oxygen species generation and
caspases. Mol Med Rep. 10:543–549. 2014.
|
|
7
|
Zhu Y, Zhuang JX, Wang Q, Zhang HY and
Yang P: Inhibitory effect of benzyl isothiocyanate on proliferation
in vitro of human glioma cells. Asian Pac J Cancer Prev.
14:2607–2610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan H, Zhu Y, Liu B, Wu H, Li Y, Wu X,
Zhou Q and Xu K: Mitogen-activated protein kinase mediates the
apoptosis of highly metastatic human non-small cell lung cancer
cells induced by isothiocyanates. Br J Nutr. 106:1779–1791. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hunakova L, Sedlakova O, Cholujova D,
Gronesova P, Duraj J and Sedlak J: Modulation of markers associated
with aggressive phenotype in MDA-MB-231 breast carcinoma cells by
sulforaphane. Neoplasma. 56:548–556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hwang ES and Lee HJ: Benzyl isothiocyanate
inhibits metalloproteinase-2/-9 expression by suppressing the
mitogen-activated protein kinase in SK-Hep1 human hepatoma cells.
Food Chem Toxicol. 46:2358–2364. 2008. View Article : Google Scholar
|
|
11
|
Lai KC, Huang AC, Hsu SC, et al: Benzyl
isothiocyanate (BITC) inhibits migration and invasion of human
colon cancer HT29 cells by inhibiting matrix metalloproteinase-2/-9
and urokinase plasminogen (uPA) through PKC and MAPK signaling
pathway. J Agric Food Chem. 58:2935–2942. 2010. View Article : Google Scholar
|
|
12
|
Groblewska M, Siewko M, Mroczko B and
Szmitkowski M: The role of matrix metalloproteinases (MMPs) and
their inhibitors (TIMPs) in the development of esophageal cancer.
Folia Histochem Cytobiol. 50:12–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kung CI, Chen CY, Yang CC, et al: Enhanced
membrane-type 1 matrix metalloproteinase expression by hyaluronan
oligosaccharides in breast cancer cells facilitates CD44 cleavage
and tumor cell migration. Oncol Rep. 28:1808–1814. 2012.
|
|
14
|
Kwiatkowska A and Symons M: Signaling
determinants of glioma cell invasion. Adv Exp Med Biol.
986:121–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yigit R, Massuger LF, Zusterzeel PL, et
al: Cytokine profiles in cyst fluids from ovarian tumors reflect
immunosuppressive state of the tumor. Int J Gynecol Cancer.
21:1241–1247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lamberto I, Plano D, Moreno E, et al:
Bisacylimidoselenocarbamates cause G2/M arrest associated with the
modulation of CDK1 and Chk2 in human breast cancer MCF-7 cells.
Curr Med Chem. 20:1609–1619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu D, Han C and Wu T:
15-hydroxyprostaglandin dehydrogenase-derived 15-keto-prostaglandin
E2 inhibits cholangiocarcinoma cell growth through interaction with
peroxisome proliferator-activated receptor-γ, SMAD2/3, and TAP63
proteins. J Biol Chem. 288:19484–19502. 2013.PubMed/NCBI
|
|
18
|
Huang C, Shen S, Ma Q, et al: Blockade of
KCa3.1 ameliorates renal fibrosis through the TGF-β1/Smad pathway
in diabetic mice. Diabetes. 62:2923–2934. 2013.PubMed/NCBI
|