Introduction
Cardiovascular infections include a group of
entities involving the heart wall, such as myocarditis, dilated
cardiomyopathy and pericarditis. These processes are associated
with high morbidity and mortality. Although early diagnosis is
essential for adequate patient management and leads to improved
prognosis, the clinical manifestations are often non specific
(1).
Myocarditis is clinically and pathologically defined
as an inflammation of the heart muscle. The term myocarditis was
first used in the early 19th century to describe myocardial
diseases not associated with valvular abnormalities (2), but only in the second half of the
20th century was interest in inflammatory myocardial diseases
renewed (3). A number of patients
with acute viral myocarditis may develop dilated cardiomyopathy as
a complication (4–19). Patients who have suffered from a
heart attack may develop pericarditis over the subsequent days or
weeks. Pericarditis is a swelling and irritation of the
pericardium, the thin sac-like membrane that surrounds the heart.
It is most commonly sudden and acute. When the symptoms develop
more gradually or persist, pericarditis is considered chronic
(1,20,21).
Acute pericarditis and myocarditis often occur
together although they are rarely of the same intensity (22,23).
When both are present, they generally trigger clinical syndromes
that are mainly pericarditic or myocarditic (24). The term myopericarditis indicates a
primarily pericarditic syndrome with minor myocardial involvement,
which describes the majority of combined pericarditis and
myocarditis cases encountered in clinical practice. By contrast,
the term perimyocarditis indicates a primarily myocarditic
syndrome. However, these two terms are often used interchangeably
without regard to the predominant type of cardiac involvement
(22,25).
Myocarditis, with or without pericarditis, is
becoming an increasingly common diagnosis. Numerous agents are
known to cause these heart infections and viruses are considered to
be the most important causative agent. Coxsackieviruses B (CV-B)
have been involved in 25–40% cases of acute myocarditis and dilated
cardiomyopathy in infants and young adolescents (26–28).
CV-B belong to the enterovirus group of the Picornaviridae family
and are the causative agents of a broad spectrum of clinically
relevant diseases, including acute and chronic myocarditis,
meningitis and possibly autoimmune diabetes (29). The 7.4 kb positive stranded RNA
genome of CV-B consists of a 5′-untranslated region (UTR) followed
by a single polyprotein coding region and a 3′-UTR, flanked by a
poly A-tail. The first part of the polyprotein (P1) encodes the
four capsid proteins while the second and third part (P2 and P3,
respectively) encode non-structural proteins involved in genome
processing and RNA synthesis. The four capsid proteins, VP1-VP4,
are grouped into a pseudo-icosahedral capsid. The VP1–VP3
constitute the outer surface of the viral particle, whilst VP4 is
embedded within the inner surface of the capsid (30). Outbreaks of myocarditis most
commonly occur in young children, however sporadic cases are
observed in older children and adults (31–34).
Studies on enterovirus infections in heart muscle
disease have been promoted, by methods using the reverse
transcriptase-polymerase chain reaction. As a result of this
technique, the enteroviral genomic RNA was detected in samples of
patients with infectious heart diseases (9,14).
However, when a low copy number of viruses is present in the
samples, the RT-PCR may fail to produce enough amplified products
(amplicons) to be either detected by the ethidium bromide staining
or used for further molecular manipulation (35).
In the present study, the involvement of CV-B as an
etiological agent in infectious heart diseases was investigated by
the detection of the genomic RNA in blood and pericardial fluid
samples from patients suffering from myocardial and/or pericardial
diseases. Immunohistochemical investigations for the detection of
the enteroviral capsid protein, VP1, from heart biopsies were also
performed. Cell cultures for possible virus isolation followed by
indirect immunofluorescence assay (IFA) for the detection of VP1
capsid protein were also conducted. In addition, the
epidemiological data for CV-B heart infections were described. This
information was collected from Tunisian hospitalized patients over
five years (2006–2011) upon admission to the Cardiology Departments
at Fattouma Bourguiba (Monastir, Tunisia) and Sahloul (Sousse,
Tunisia) hospitals.
Materials and methods
Patients
The present study reports 102 patients (89 males and
13 females) clinically selected for presentation with inflammatory
heart diseases, and aged between 17 and 46 years (mean age 30.8
years). Myocardium and/or pericardium inflammation was presumed to
be due to a typical history of viral infection at the time of
cardiac disease onset. A number of clinical signs of viral
impregnation, including rhinorrhea, coughing, myalgia, muscle
soreness, high fever as well as cardiac symptoms, such as chest
pain, breathlessness, cardiac arrhythmia, easy fatigability,
electrocardiographic changes and reduced exercise, were observed.
In terms of susceptibility and history, the patients are most
commonly adolescents and young adults. Further biological tests
were performed. In fact, a complete blood count marked by a
hyperleukosytosis with a lymphocyte predominance, marginally raised
C-reactive protein and procalcitonin, supported the suspicion of a
viral heart infection. The samples were obtained from patients
within 48 h following admission to the Cardiology Departments at
Fattouma Bourguiba and Sahloul hospitals. The sampling was
performed at three university hospitals located on the coast of
Tunisia: Fattouma Bourguiba (Monastir), Farhat Hached (Sousse) and
Sahloul (Sousse) between November 2006 and November 2011. A total
of 100 adult subjects presenting with other cardiac pathologies
than infectious ones were used as the controls. The study was
approved by the ethics committees of the University Hospitals
Fattouma Bourguiba (Monastir, Tunisia), Farhat Hached and Sahloul
(Sousse, Tunisia). Each Ethic Committee is designed by the hospital
where the sampling was performed. Written informed consent forms
were signed by patients and the controls participating in this
study.
Reverse transcriptase-polymerase chain
reaction (RT-PCR)
The viral RNA was extracted from blood, pericardial
fluid samples using the TRIzol® Plus RNA Purification
kit (Invitrogen Life Technologies) according to the manufacturer’s
instructions. DNase treatment during RNA purification was adopted
using the PureLink™ DNase (Invitrogen Life Technologies) in order
to obtain DNA-free total RNA. One-step RT-PCR for the detection of
enterovirus RNA was performed with primers directed to the
conserved sequences in the 5′-UTR of the enterovirus genome. A
fragment of 155 bp of the extracted RNA was amplified by one-step
RT-PCR (Invitrogen SuperScript™ One-Step RT-PCR with
Platinum® Taq) using 006 and 007 primers (36). The RT-PCR was performed on a
mixture containing 25 μl 2X reaction mix (a buffer containing 0.4
mm of each dNTP; 2.4 mm MgSO4); 0.2 μM each of sense and
anti-sense primers, 1 μl of enzyme mix (RT/Platinum®
Taq; Invitrogen Life Technologies) and RNase free water to 50 μl.
The reaction was conducted with an initial reverse transcription
step at 42°C for 30 min, followed by PCR activation at 94°C for 5
min, 30 amplification cycles (94°C, 30 sec; 42°C, 1 min; 72°C, 2
min) and a final 10-min extension at 72°C in an Eppendorf
Mastercycler Thermal Cycler. The PCR products were run on a 2%
agarose gel stained with ethidium bromide and visualized under UV
light.
Sequencing and analysis of PCR
enterovirus amplification products
First, the PCR amplicons were purified using the
ExoSAP-IT-PCR Clean-Up Reagent (USB® Products from
Affymetrix, Inc) which constitutes a one-step enzymatic cleanup of
PCR products. Next, they were sequenced in forward and reverse
directions with the respective PCR primers. The chromatogram
sequencing files were inspected with Finch TV (version 1.4.0). The
obtained enterovirus sequences were compared with the corresponding
ones available in the GenBank using Basic Local Alignment Search
Tool (BLAST) in order to identify the enterovirus type (37,38).
Histopathology: hematoxylin-eosin
staining
Heart biopsies obtained from the patients and
controls were fixed in formalin (neutral-buffered formaldehyde, 30%
diluted to 1/10) for 24 h and embedded in paraffin. The sections (5
μm) were cut from the paraffin-embedded tissues with a microtome.
All of the sections were stained with hematoxylin-eosin staining
(Invitrogen Life Technologies) and the slides were investigated for
the presence of infectious markers (39–42).
Immunohistochemical analysis
In all the heart biopsies, immunohistochemical
investigations were performed on the enteroviral capsid protein VP1
Mab 5-D8/1 (DAKO, Carpenteria, VT, USA). Tris-buffered NaCl
solution with Tween-20, the target retrieval solution, serum-free
protein block, antibody diluent, mayer’s hematoxylin,
EnVision+ system-HRP (AEC) and Glycergel®
Mounting Medium (aqueous) were all purchased from DAKO. The
immunohistochemical procedures included antigen exposure, blocking,
incubation with primary antibody, incubation with the secondary
antibody in the En-Vision detection system and appropriate wash
between steps using Tris-buffered saline with Tween-20. All the
incubations were performed at room temperature (5,43–45).
Briefly, paraffin-embedded tissue sections (5 μm) were dewaxed with
xylene and rehydrated with graded ethanol. Antigen exposure was
achieved by heat in a water bath (95–99°C) mediated by target
retrieval. The endogenous peroxidase activity was blocked with a
peroxidase-blocking reagent for 15 min. The tissue sections were
blocked with the protein block for 10 min and then incubated with
primary antibody (appropriately diluted to 1:100–1:500 in antibody
diluent) for 30 min and washed. The secondary antibody in the
En-Vision detection system was the goat anti-mouse Ig conjugated
with dextran polymer, on which numerous peroxidase molecules were
labeled. The sections were incubated with this reagent for 30 min,
washed and then reacted with substrate chromogen for 5–10 min. The
slides were immersed in aqueous hematoxylin for counterstaining.
The color reaction was stopped by a wash in distilled water.
Finally, the mounted sections were examined and confirmed under a
Nikon Eclipse 50i microscope (Nikon, Tokyo, Japan).
Virus isolation by in vitro cell
culture
Virus isolation was performed on KB cells (human
squamous carcinoma cell line). The cells were grown in 24-well
microplates in RPMI-1640 medium (Invitrogen Life Technologies),
washed twice with phosphate-buffered saline (PBS) and then
inoculated with 50 μl of the patients’ samples (blood and
pericardial fluid). The samples were allowed to adsorb for 1 h at
37°C with gentle swirling every 15 min. Eagle’s minimal essential
medium with 5% fetal calf serum, 100 U/ml penicillin, 200 μg/ml
streptomycin and 1 mg/ml l-glutamine (Gibco-BRL, Invitrogen Life
Technologies) were then added to the infected cells. The cells were
monitored daily for characteristic cytopathic effect (CPE)
(6).
Indirect immunofluorescence assay
(IFA)
To detect viral internalization, the cells were
fixed in cold acetone (15 min) and subjected to an indirect
immunofluorescence assay intended to detect the VP1 capsid protein.
Briefly, the slides were incubated with enterovirus group specific
monoclonal antibody (mAb) 5-D8/1 (diluted at 1/40 in PBS; Dako,
Trappes, France) for 30 min at room temperature. Following three
washes in PBS, fluorescein isothiocyanate (FITC)-conjugated goat
anti-mouse antibody (Jackson ImmnuoResearch Laboratories, Inc.,
West Grove, PA, USA) was applied at a dilution of 1:25 in blue
Evans for a further 30 min at room temperature. Subsequently, the
slides were washed again with PBS, dried, mounted with glycerol
medium and examined using a fluorescence microscope (Olympus,
Rungis, France) (5).
Statistical analysis
To determine the risk groups, a statistical analysis
(chi-squared test) with a 95% significance level was performed
using Epi Info version 6.04 (CDC, Atlanta, GA, USA).
Results
Enterovirus RNA detection by RT-PCR and
epidemiological characteristics
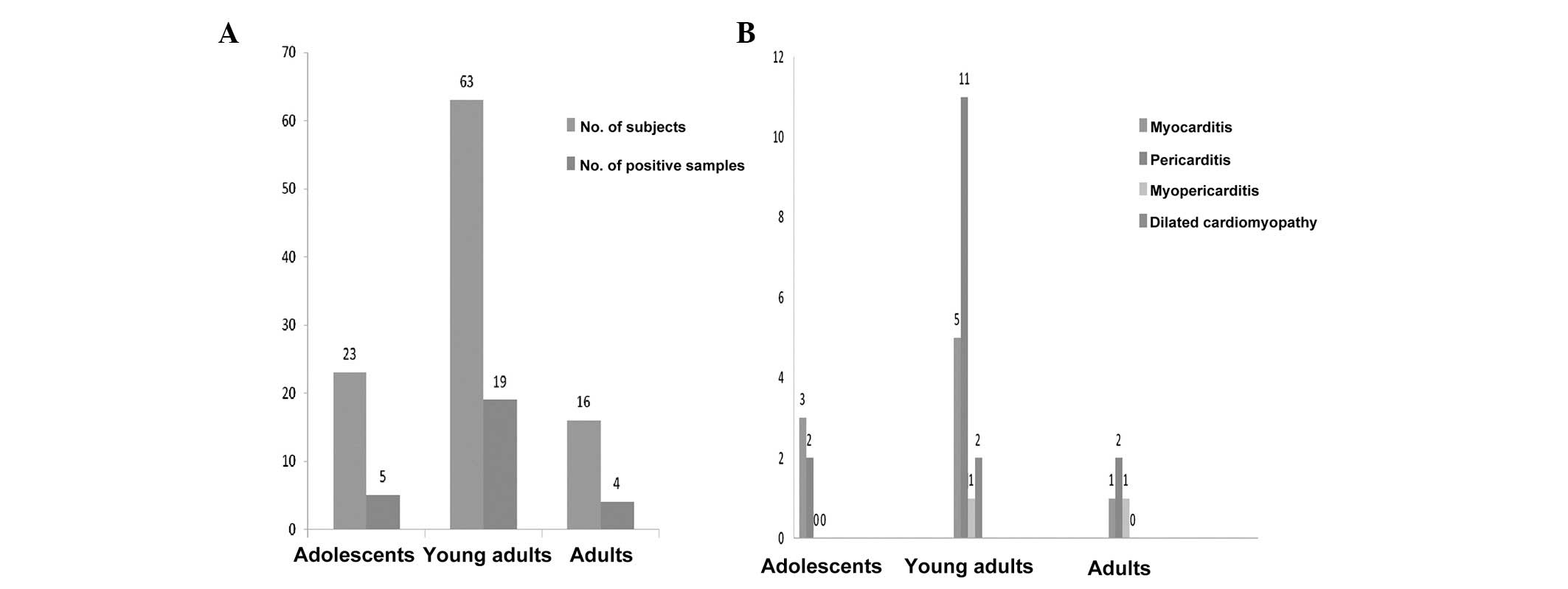
Of the 102 evaluated affected individuals, 51
patients were clinically diagnosed with myocarditis, 31 with
pericarditis, 12 with myopericarditis and 8 with dilated
cardiomyopathy. The patients were divided into groups according to
age: adolescents (13–18 years old; n=23), young adults (19–29 years
old; n=63) and adults (aged >29 years; n=16). The samples were
tested for the presence of genomic RNA by RT-PCR using primers
located in the 5′-UTR of the enterovirus genome. The RT-PCR
amplified the enteroviral genome in 9 of 51 samples obtained from
myocarditis patients, in 15 of 31 samples obtained from
pericarditis patients, in 2 of 12 samples obtained from
myopericarditis patients and in 2 of 8 samples obtained from
dilated cardiomyopathy patients. Pericarditis infection was then
more frequent than myocarditis (P<0.05) or myopericarditis
(P=0.05). Of the 28 positive RT-PCR samples, 17 were simultaneously
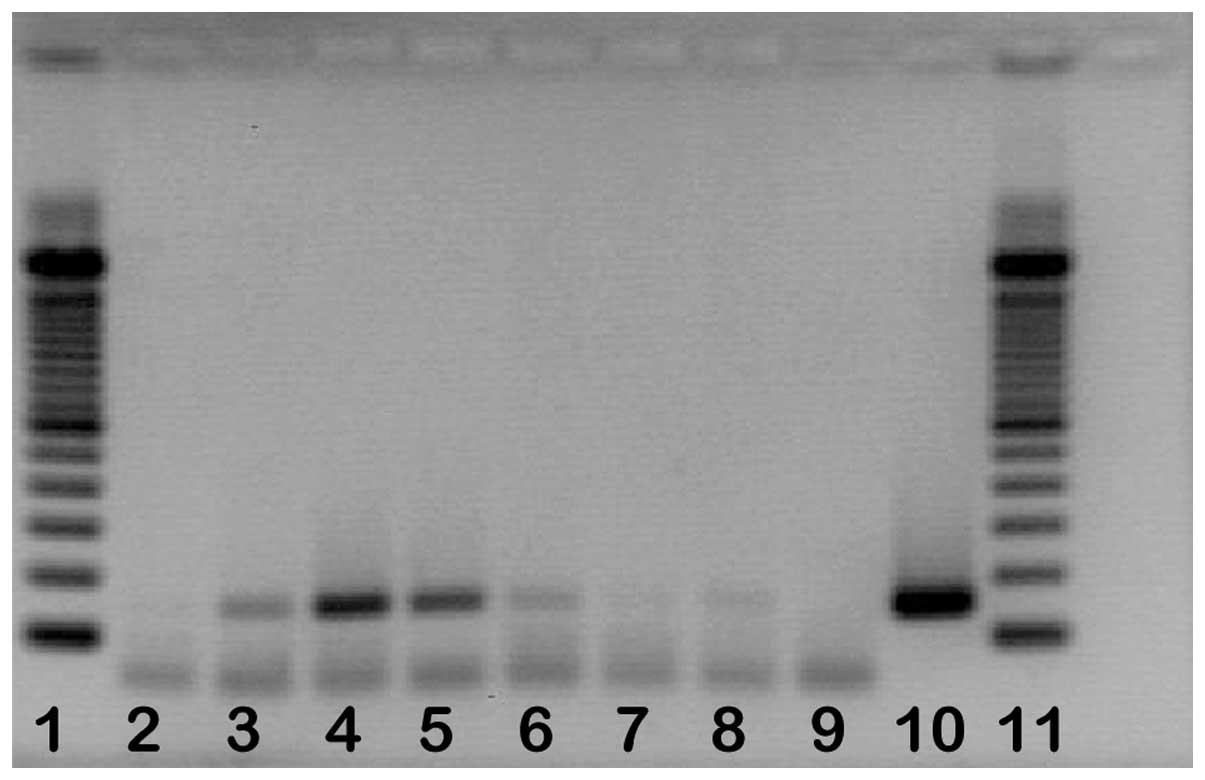
positive in blood and pericardial fluid samples. Fig. 1 shows an example of detection by
agarose gel electrophoresis after RT-PCR. However, of the 100
control samples, none were RT-PCR positive.
When analyzed according to age group, the
differences in outcome were notable between groups (Fig. 2). Five out of 23 adolescents, 19
out of 63 young adults and 4 out of 16 adults tested positive for
enteroviral RNA by RT-PCR. Infection was higher in young adults
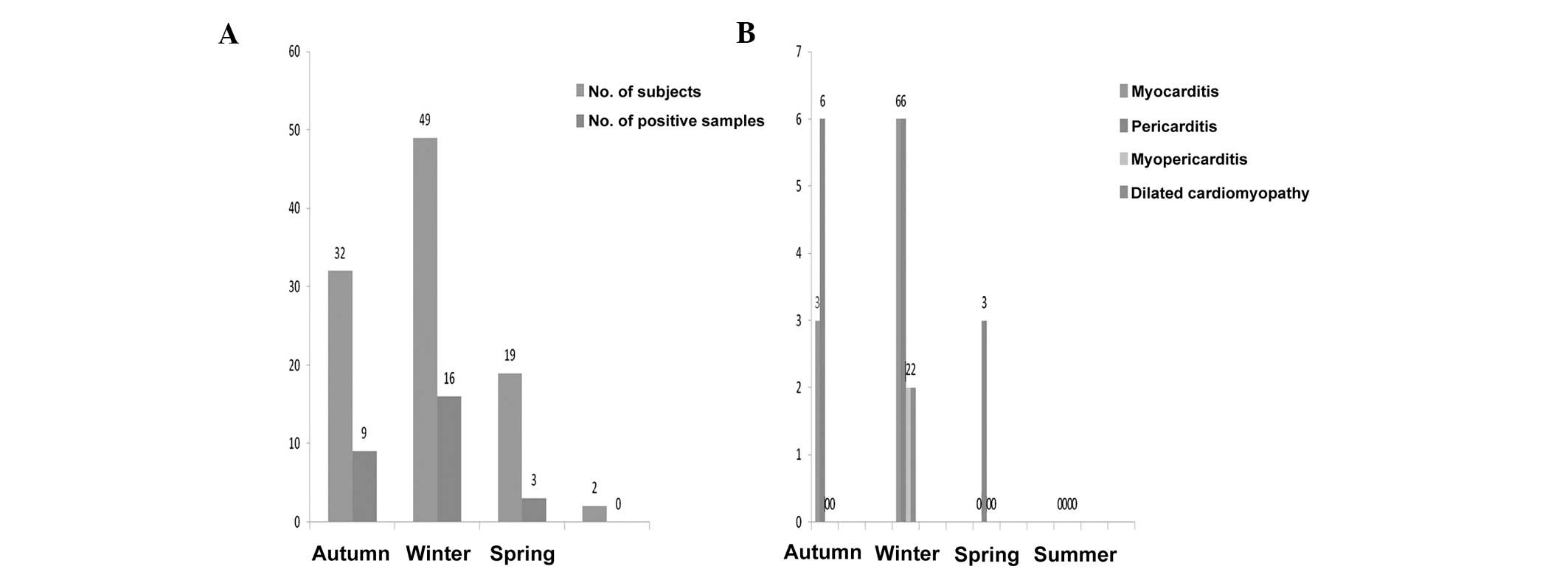
than in adults (P<0.5) or adolescents (P<0.5). When analyzed
by seasonal variation, the differences in outcome were also
significant. Fig. 3 demonstrates
the following distribution: enteroviral RT-PCR was positive in 9 of
32 in autumn, 16 of 49 in winter and 3 of 19 in spring. The
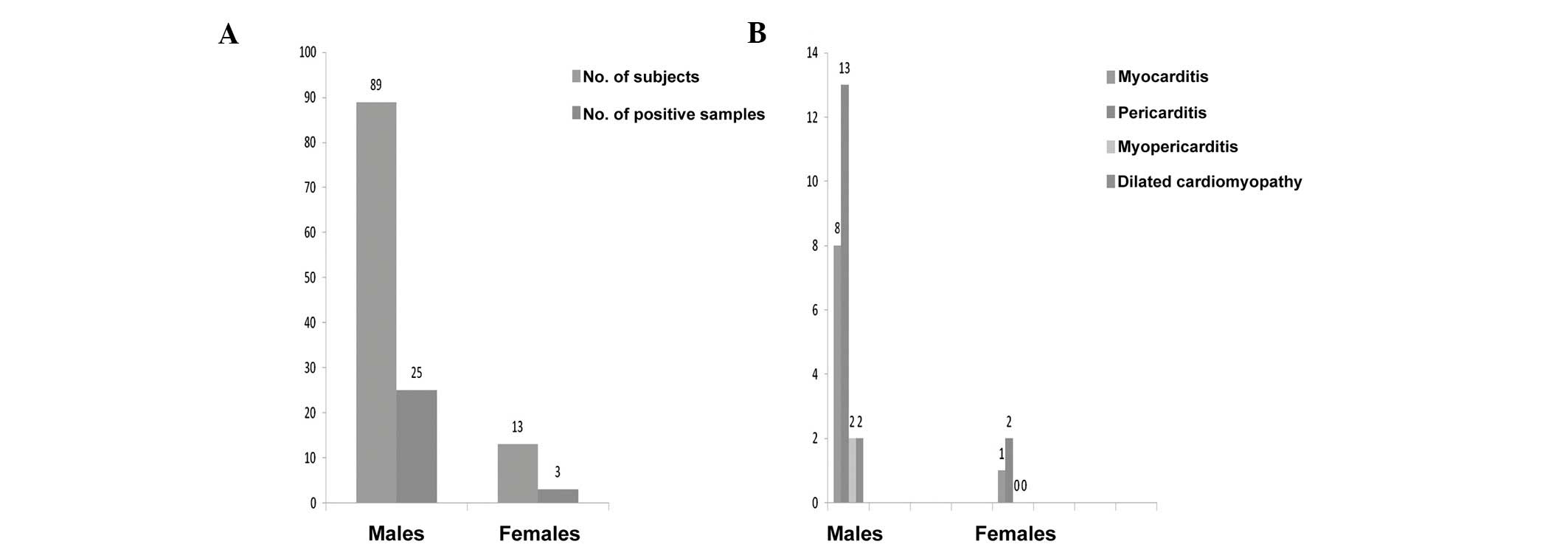
analysis of the enteroviral RT-PCR positivity according to gender
shows the following distribution: 25 of 89 males and 3 of 13
females were positive. Thus, males seem more susceptible (P<0.5;
Fig. 4).
Sequence analysis
The sequence analysis of enterovirus amplimers
showed that 27 strains were identical to the CV-B3 sequence in 9
myocarditis cases, 15 pericarditis cases, 1 myopericarditis case
and 2 dilated cardiomyopathy cases. Only one strain was identical
to the CV-B1 sequence in 1 myopericarditis case.
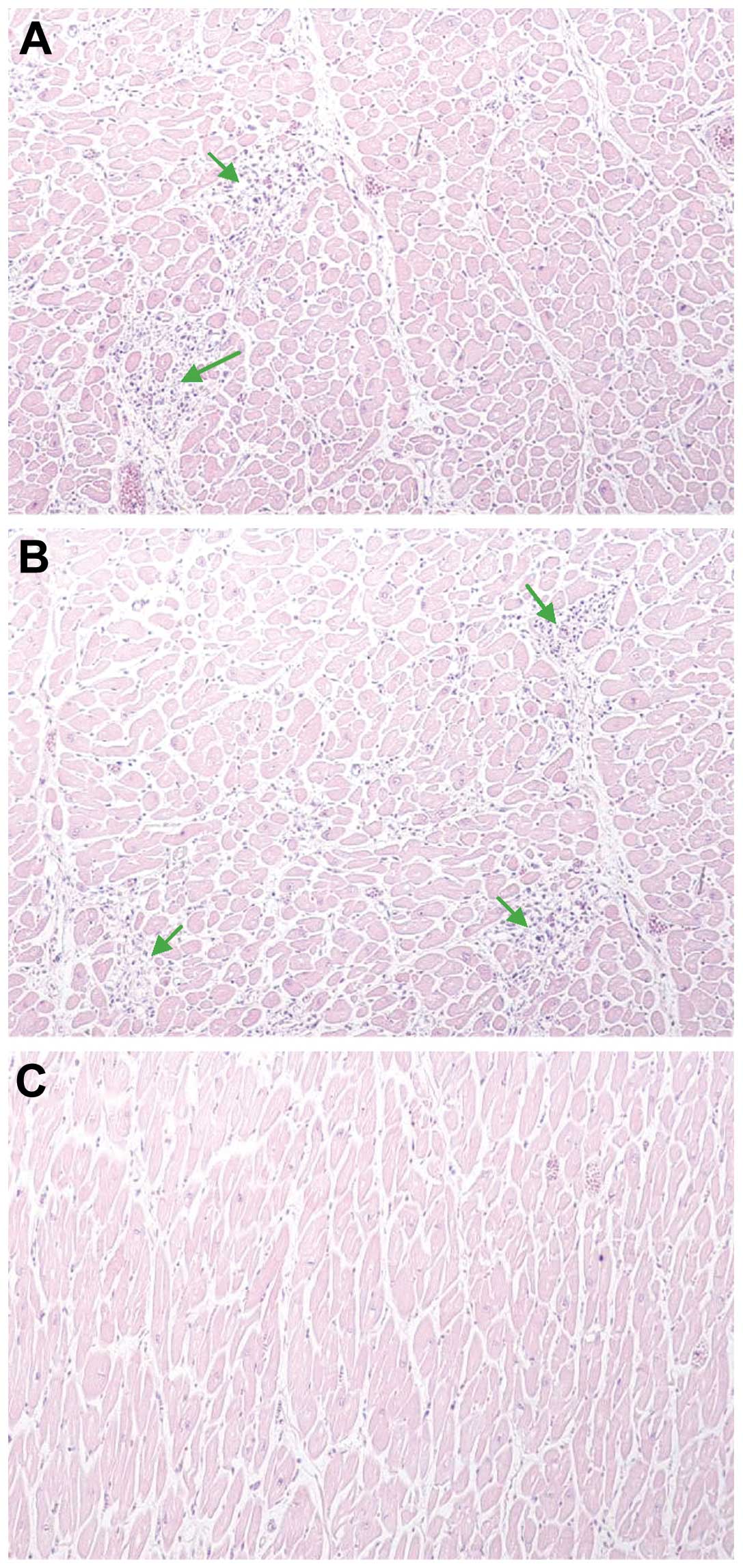
Histopathological investigation
Following investigation of the paraffin tissue
blocks from patients, diffuse inflammation was marked (Fig. 5A and B). The slides from the
paraffin tissue blocks from the controls demonstrated no
significant pathological findings (Fig. 5C).
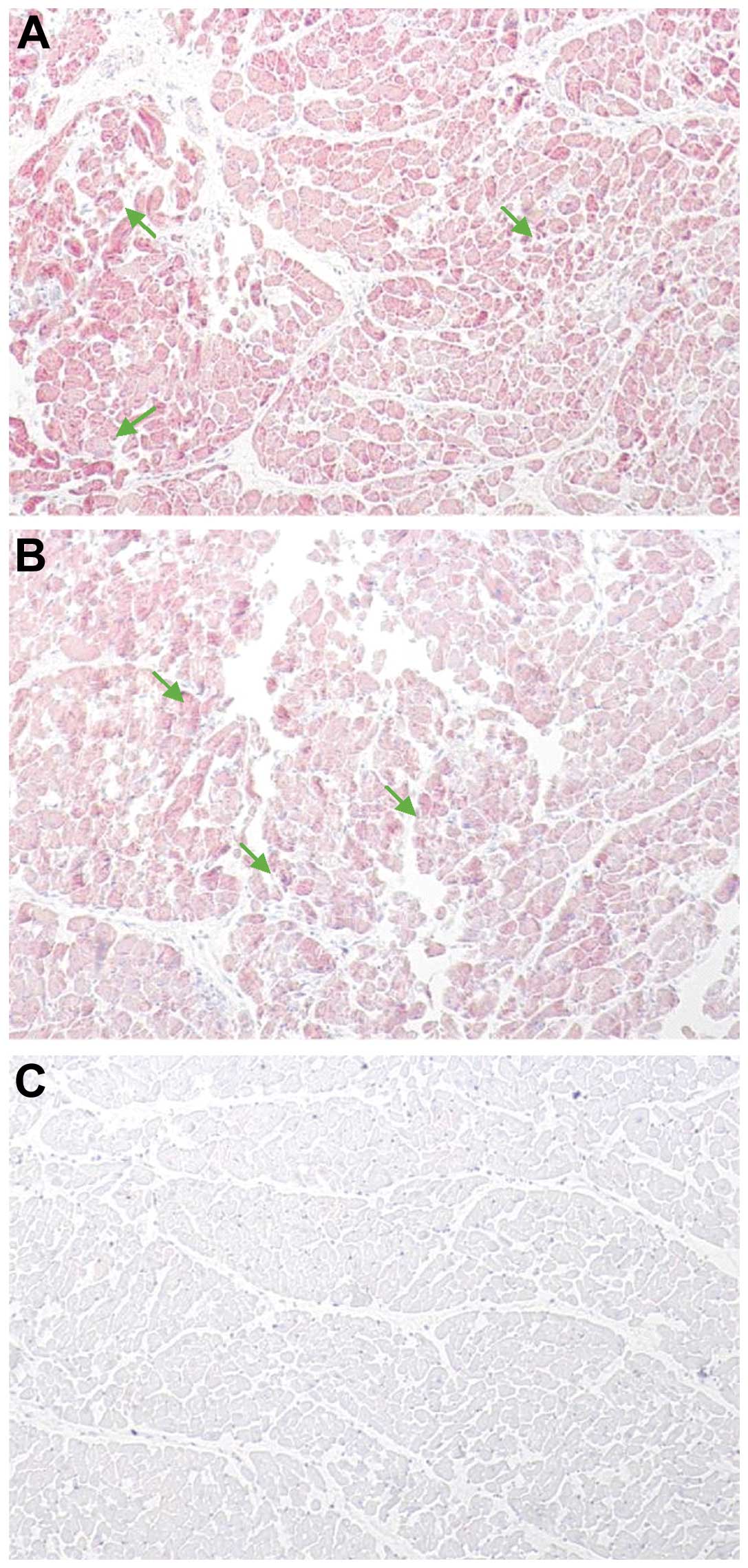
Immunohistochemical detection of
enteroviral capsid protein VP1
To determine whether the enterovirus cardiac
infection was associated with a viral protein synthesis activity,
all of the heart biopsies obtained from hospitalized patients and
controls were examined by immunohistochemical assays specific for
the enteroviral capsid protein VP1. The VP1 capsid protein in the
heart biopsies was detected in enterovirus-positive cases revealed
by means of RT-PCR (Fig. 6A and
B). The slides from the control subjects demonstrated a
negative result with no significant pathological findings (Fig. 6C).
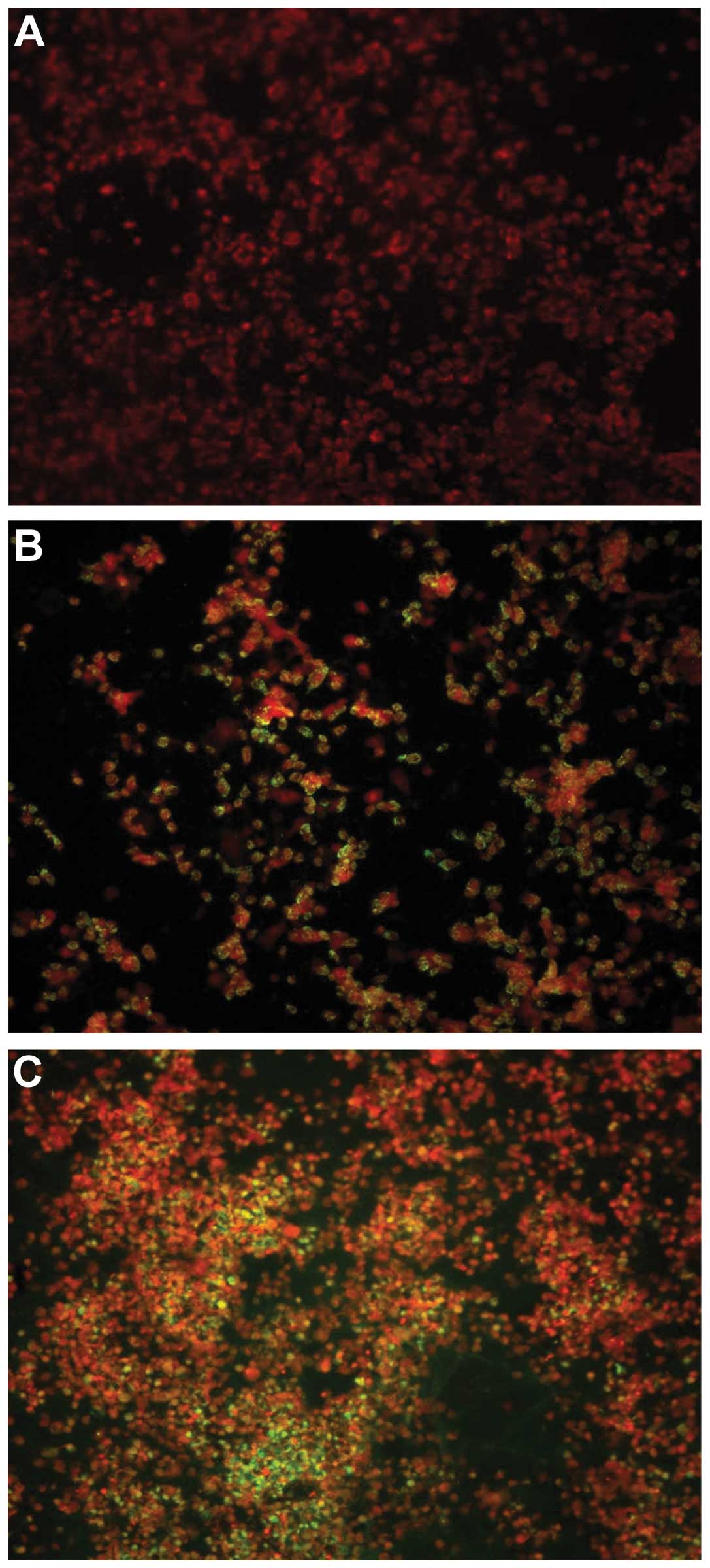
Culture and indirect immunofluorescence
assay detection of VP1 enterovirus
Enterovirus was cultured from 1 of 43 pericardial
fluid samples whereas no positive viral cultures were found in all
of the blood samples (n=102). Within the control group, enteroviral
cell culture was negative. The sample demonstrating a positive
cytopathic effect in cell culture was also positive for VP1 antigen
detection by immunostaining assay (Fig. 7).
Discussion
The present study, conducted in the coastal region
of Tunisia, aimed to investigate the presence of enterovirus
markers of infection (genomic RNA or VP1 antigen) in blood,
pericardial fluid samples and heart biopsies in hospitalized
patients suffering from myocardial and/or pericardial heart
diseases. Enteroviral genomic RNA was identified in 28 cases
(27.45%) using the RT-PCR assays. Having demonstrated that
enteroviral RNA is often involved in different forms of infectious
heart diseases, sequencing was adopted to confirm the involvement
of these viruses.
As an enterovirus group-specific monoclonal antibody
(Mab) is now available, the demonstration of enteroviral antigens
in heart tissue is possible (17).
In the present study, this Mab was used to detect enteroviral
antigens (VP1) in formalin-fixed heart biopsies, paraffin-embedded
sections. Using immunohistochemistry, the enteroviral capsid
protein VP1 in the heart biopsies was detected in all
enterovirus-positive cases revealed by means of RT-PCR. In the
present study, the sequence analysis demonstrated that CVB-3 and
CVB-1 are the most implicated in heart infections. Similarly,
RT-PCR as well as immunohistochemistry revealed an association
between enterovirus infection, in particular the cardiotropic
coxsackie B enterovirus, serotypes B1-B5 and myocarditis (46). In the diagnostic approach, the
positive results were confirmed using a variety of techniques. The
frequency and identification of infectious agents with infectious
heart diseases, including myocarditis, have varied widely between
10 and 100% (27,47–49).
These marked variations between studies are due to several factors,
including the studied samples (blood, cardiac puncture, pericardial
fluid samples, heart biopsies), the methods used to detect the
infectious agent, result confirmation, correlation with clinical
and histological findings, and the time during the illness when the
samples were obtained.
In the present study, the series of 51 myocarditis,
31 pericarditis, 12 myopericarditis and 8 dilated cardiomyopathy
cases suspected clinically, blood, pericardial fluid samples and
heart biopsies were used according to a pre-set protocol. RT-PCR
complemented with sequencing and histopathology supplemented with
immunohistochemistry were the methods that could be performed to
detect the infectious agents.
Enterovirus isolation in cell culture and detection
of their cytopathic effect were adopted in the present study.
Enterovirus was cultured in the pericardial fluid sample from one
pericarditis patient. Of higher significance, 25–35% of the
specimens from the patients with characteristic enterovirus
infection of any serotype will be negative by cell culture due to
antibody neutralization in situ, inadequate sample
collection, handling or processing of the samples and cell line
intrinsic insensitivity (50). The
failure to detect enteroviral infection markers is not infrequent
and has generated several hypotheses, including antigen mimicry and
efficient clearing of the organism by the time of testing and
autoimmune reactions (27,47,51).
It may be hypothesized that a number of the microorganism-negative
cases, particularly in adults, are non-infectious in nature and are
due to drugs and toxins. Furthermore, limitations of the assays may
also be responsible for the inability to make an organism-specific
diagnosis in every myocarditis case (47). However, a higher prevalence of
viral cardiopathy may be revealed by the application of a
comprehensive combination of molecular pathological analysis for
the detection of enterovirus RNA from blood and pericardial fluid
samples, and immunohistochemical techniques for the detection of
the enteroviral capsid protein VP1 from formalin-fixed,
paraffin-embedded heart tissue.
The present epidemiological study has provided some
new results of marked interest. Age, gender and seasonal variation
appear to be important factors affecting the CV-B prevalence in
human infectious heart diseases. Although enterovirus infections in
Tunisia generally reach a peak in autumn (52), this seasonal variation was also
found to progress until winter, as demonstrated in the present
study. According to age, CV-B specifically affects young male
adults, possibly because they have a more intense and active
lifestyle. Finally, males appear to be more susceptible to these
infections, which may raise the hypothesis of different hormone
secretions. To the best of our knowledge, the present study is the
first report of the epidemiology of CV-B heart infections in
Tunisia. These viruses are associated with various diseases and
epidemiological data may help to clarify their impact on human
health.
In conclusion, the present study suggests that the
pathogenesis of myocardial and/or pericardial diseases is highly
associated with CV-B infection. The combined investigations using
molecular pathological techniques and immunohistochemical methods
has improved our ability to diagnose viral heart infections in a
rapid and specific way. Such a prompt diagnosis has implications in
the treatment of patients. These findings may be of major interest
for the development of further therapies or preventive strategies
in the prevention of the development of cardiovascular infections
and inflammations. Further studies are required to determine other
virus serotypes involved in these heart infections. Prospective
murine experimental myocarditis may be desirable and may have a
marked impact on the quality of future investigations to improve
the understanding and elucidate the physio and immuno-pathogenesis
of such infectious heart diseases.
Acknowledgements
The present study was supported by the National
Institute of Health grant (HL108371). The authors are grateful to
Mr. Adel Rdissi for proof-reading the paper.
References
|
1
|
Ben-Haim S, Gacinovic S and Israel O:
Cardiovascular infection and inflammation. Semin Nucl Med.
39:103–114. 2009. View Article : Google Scholar
|
|
2
|
Mattingly TW: Changing concepts of
myocardial diseases. JAMA. 191:33–37. 1965. View Article : Google Scholar
|
|
3
|
Kaski JP and Burch M: Viral myocarditis in
childhood. Paediatr Child Health. 17:11–17. 2007. View Article : Google Scholar
|
|
4
|
Dörner A, Pauschinger M, Schwimmbeck PL,
Kühl U and Schultheiss HP: The shift in the myocardial adenine
nucleotide translocator isoform expression pattern is associated
with an enteroviral infection in the absence of an active T-cell
dependent immune response in human inflammatory heart disease. J Am
Coll Cardiol. 35:1778–1784. 2000.
|
|
5
|
Zhang H, Li Y, Peng T, et al: Localization
of enteroviral antigen in myocardium and other tissues from
patients with heart muscle disease by an improved
immunohistochemical technique. J Histochem Cytochem. 48:579–584.
2000. View Article : Google Scholar
|
|
6
|
Peng T, Li Y, Yang Y, et al:
Characterization of enterovirus isolates from patients with heart
muscle disease in a selenium-deficient area of China. J Clin
Microbiol. 38:3538–3543. 2000.PubMed/NCBI
|
|
7
|
Bowles NE, Olsen EGJ, Richardson PJ and
Archarda LC: Detection of Coxsackie-B-virus-specific RNA sequences
in myocardial biopsy samples from patients with myocarditis and
dilated cardiomyopathy. Lancet. 1:1120–1123. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pauschinger M, Phan MD, Doerner A, Kuehl
U, et al: Enteroviral RNA replication in the myocardium of patients
with left ventricular dysfunction and clinically suspected
myocarditis. Circulation. 99:889–895. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andreoletti L, Hober D, Decoene C, et al:
Detection of enterovirus RNA by polymerase chain reaction in
endomyocardial tissue of patients with chronic diseases. J Med
Virol. 48:53–59. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maekawa Y, Ouzounian M, Opavsky MA and Liu
PP: Connecting the missing link between dilated cardiomyopathy and
viral myocarditis: virus, cytoskeleton, and innate immunity.
Circulation. 115:5–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Petitjean J, Freymuth F, Kopecka H, et al:
Entérovirus et cardiomyopathies. Méd Mal Infect. 24:102–107.
1994.(In French).
|
|
12
|
Kaski JP and Burch M: Viral myocarditis in
Childhood. Pediatr Child Health. 17:11–18. 2007. View Article : Google Scholar
|
|
13
|
Dan M and Chantler JK: A genetically
engineered attenuated coxsackievirus B3 strain protects mice
against lethal infection. J Virol. 79:9285–9295. 2005. View Article : Google Scholar
|
|
14
|
Mariani M, Petronio AS, Manes MT, et al:
Detection of enteroviral infection in myocardial tissues by
polymerase chain reaction (PCR). Clin Microbiol Infect. 2:109–114.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kandolf R, Klingel K, Zell R, et al:
Molecular mechanisms in the pathogenesis of enteroviral heart
disease: acute and persistent infections. Clin Immunol
Immunopathol. 68:153–158. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muir P: The association of enteroviruses
with chronic heart disease. Med Virol. 2:9–18. 1992. View Article : Google Scholar
|
|
17
|
Li Y, Bourlet T, Andreoletti L, et al:
Enteroviral capsid protein VP1 is present in myocardial tissues
from some patients with myocarditis or dilated cardiomyopathy.
Circulation. 101:231–234. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pauschinger M, Chandrasekharan K, Noutsias
M, et al: Viral heart disease: molecular diagnosis, clinical
prognosis, and treatment strategies. Med Microbiol Immunol.
193:65–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
D’Ambrosio A, Patti G, Manzoli A, et al:
The fate of acute myocarditis between spontaneous improvement and
evolution to dilated cardiomyopathy: a review. Heart. 85:499–504.
2001.PubMed/NCBI
|
|
20
|
Goyle KK and Walling AD: Diagnosing
pericarditis. Am Fam Physician. 66:1695–1702. 2002.PubMed/NCBI
|
|
21
|
Ferreira AG Júnior, Ferreira SM, Gomes ML
and Linhares AC: Enteroviruses as a possible cause of myocarditis,
pericarditis and dilated cardiomyopathy in Belém, Brazil. Braz J
Med Biol Res. 28:869–874. 1995.PubMed/NCBI
|
|
22
|
Imazio M and Trinchero R: Myopericarditis:
Etiology, management, and prognosis. Int J Cardiol. 127:17–26.
2008. View Article : Google Scholar
|
|
23
|
Smith WG: Coxsackie B myopericarditis in
adults. Am Heart J. 80:34–46. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Imazio M and Trinchero R: The spectrum of
inflammatory myopericardial diseases. Int J Cardiol. 144:124–134.
2010. View Article : Google Scholar
|
|
25
|
Imazio M, Cecchi E, Demichelis B, et al:
Myopericarditis versus viral or idiopathic acute pericarditis.
Heart. 94:498–501. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leonard EG: Viral myocarditis. Pediatr
Infect Dis J. 23:665–666. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feldman AM and McNamara D: Myocarditis. N
Engl J Med. 343:1388–1398. 2000. View Article : Google Scholar
|
|
28
|
Kandolf R, Ameis D, Kirschner P and Canu
A: In situ detection of enteroviral genomes in myocardial cells by
nucleic acid hybridization: an approach to the diagnosis of viral
heart disease. Proc Natl Acad Sci USA. 84:6272–6276. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Klingel K, Rieger P, Mall G, et al:
Visualization of enteroviral replication in myocardial tissue by
ultrastructural in situ hybridization: identification of
target cells and cytopathic effects. Lab Invest. 78:1227–1237.
1998.PubMed/NCBI
|
|
30
|
Rueckert RR: Picornaviridae: The viruses
and their replication. Fields Virology. Fields BM, Knipe DM and
Howley PM: 3rd edition. Lippincott-Raven Publishers; Philadelphia:
pp. 609–654. 1996
|
|
31
|
Dettmeyer R, Baasner A, Schlamann M, Haag
C and Madea B: Coxsackie B3 myocarditis in 4 cases of suspected
sudden infant death syndrome: diagnosis by immunohistochemical and
molecular-pathologic investigations. Pathol Res Pract. 198:689–696.
2002. View Article : Google Scholar
|
|
32
|
Esfandiarei M, Suarez A, Amaral A, et al:
Novel role for integrin-linked kinase in modulation of
coxsackievirus B3 replication and virus-induced cardiomyocyte
injury. Circ Res. 99:354–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ahn J, Joo CH, Seo I, et al: All CVB,
serotypes and clinical isolates induce irreversible cytopathic
effects in primary cardiomyocytes. J Med Virol. 75:290–294. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Treacy A, Carr MJ, Dunford L, et al: First
report of sudden death due to myocarditis caused by adenovirus
serotype. J Clin Microbiol. 48:642–645. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang H, Soteriou B, Knowlson S,
Theodoridou A and Archard LC: Characterisation of genomic RNA of
Coxsackievirus B3 in murine myocarditis: reliability of direct
sequencing of reverse transcription-nested polymerase chain
reaction products. J Virol Methods. 69:7–17. 1997. View Article : Google Scholar
|
|
36
|
Zoll GJ, Melchers WJ, Kopecka H, et al:
General primer-mediated polymerase chain reaction for detection of
enteroviruses: application for diagnostic routine and persistent
infections. J Clin Microbiol. 30:160–165. 1992.
|
|
37
|
Altschul SF, Gish W, Miller W, Myers EW
and Lipman DJ: Basic local alignment search tool. J Mol Biol.
215:403–410. 1990. View Article : Google Scholar
|
|
38
|
Oberste MS, Maher K, Flemister MR, et al:
Comparison of classic and molecular approaches for the
identification of untypeable enteroviruses. J Clin Microbiol.
38:1170–1174. 2000.PubMed/NCBI
|
|
39
|
Cioc AM and Nuovo GJ: Histologic and in
situ viral findings in the myocardium in cases of sudden,
unexpected death. Mod Pathol. 15:914–922. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dettmeyer R, Kandolf R, Schmidt P,
Schlamann M and Madea B: Lympho monocytic enteroviral myocarditis
traditional, immunohistological and molecularpathological methods
for diagnosis in a case of suspected sudden infant death syndrome
(SIDS). Forensic Sci Int. 119:141–144. 2001. View Article : Google Scholar
|
|
41
|
Guarner J, Bhatnagar J, Shieh WJ, et al:
Histopathologic, immunohistochemical, and polymerase chain reaction
assays in the study of cases with fatal sporadic myocarditis. Hum
Pathol. 38:1412–1419. 2007. View Article : Google Scholar
|
|
42
|
Chimenti C and Frustaci A: Histopathology
of myocarditis. Diagn Histopathol. 14:401–407. 2008. View Article : Google Scholar
|
|
43
|
Shi SR, Key ME and Kalra KL: Antigen
retrieval in formalinfixed paraffin-embedded tissues: an
enhancement method for immunohistochemical staining based on
microwave oven heating of tissue sections. J Histochem Cytochem.
39:741–748. 1991. View Article : Google Scholar
|
|
44
|
Shi SR, Cote RJ and Taylor CR: Antigen
retrieval immunohistochemistry: past, present and future. J
Histochem Cytochem. 45:327–343. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sabattini E, Bisgard K, Ascani SS, Poggi,
et al: The EnVision++ system: a new immunohistochemical
method for diagnostics and research: critical comparison with the
APAAP, ChemMate, CSA, LABC and SABC techniques. J Clin Pathol.
51:506–511. 1998.
|
|
46
|
Jin O, Sole MJ, Butany JW, et al:
Detection of enterovirus RNA in myocardial biopsies from patients
with myocarditis and cardiomyopathy using gene amplification by
polymerase chain reaction. Circulation. 82:8–16. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guarner J, Bhatnagar J, Shieh WJ, et al:
Histopathologic, immunohistochemical, and polymerase chain reaction
assays in the study of cases with fatal sporadic myocarditis. Hum
Pathol. 38:1412–1419. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bowles NE, Ni J, Kearney DL, et al:
Detection of viruses in myocardial tissues by polymerase chain
reaction. Evidence of adenovirus as a common cause of myocarditis
in children and adults. J Am Coll Cardiol. 42:466–472. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cambridge M, MacArthur C, Waterson A,
Goodwin J and Oakley C: Antibodies to Coxsackie B viruses in
congestive cardiomyopathy. Br Heart J. 41:692–696. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Abzug MJ and Rotbart HA: Enterovirus
infections of neonates and infants. Semin Pediatr Infect Dis.
10:169–176. 1999. View Article : Google Scholar
|
|
51
|
Peters NS and Poole-Wilson PA: Myocarditis
- continuing clinical and pathologic confusion. Am Heart J.
121:942–946. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bahri O, Rezig D, Ben Nejma-Oueslati B, et
al: Enteroviruses in Tunisia: virological surveillance over 12
years (1992–2003). J Med Microbiol. 54:63–69. 2005.PubMed/NCBI
|