Introduction
Nonalcoholic fatty liver disease (NAFLD) is defined
as the presence of increased fat in the liver that is not the
result of excessive alcohol consumption. It is comprised of a
morphological spectrum of liver injuries, ranging from simple
triglyceride (TG) accumulation in the cytoplasm of hepatocytes
(steatosis), to inflammatory and hepatocellular injury
(nonalcoholic steatohepatitis), which may lead to fibrosis and
cirrhosis (1–3). Due to its high prevalence in
association with obesity, diabetes and insulin resistance, NAFLD is
increasingly being considered as a hepatic manifestation of the
metabolic syndrome (4). The exact
cause of NAFLD remains unknown; however, both obesity and insulin
resistance have been shown to have a role in the disease process
(5,6). Fat accumulation in the liver results
from an imbalance between the uptake, synthesis, export and
oxidation of fatty acids. In normal circumstances, insulin inhibits
free fatty acid (FFA) release from adipose tissues. However, as
insulin resistance develops, the elevated plasma concentrations of
glucose and fatty acids promotes hepatic fatty acid synthesis and
impairs β-oxidation, resulting in the formation of hepatic
steatosis. Hepatic steatosis conversely exacerbates the degree of
insulin resistance, and accelerates the subsequent transition to
steatohepatitis and fibrosis (7,8).
There is currently no effective or generally
accepted treatment for NAFLD. Polydatin is a stilbenoid compound
derived from the rhizome of Polygonum cuspidatum (9). This plant has previously been used in
traditional Chinese medicine, to treat digestive disorders and
ischemia/reperfusion injury (9,10).
One of the main properties of polydatin is its hepatoprotective
activity, which has been reported to induce gallbladder
contraction, prevent biliary cholesterol-stone formation and
protect against tetrachloromethane and aflatoxin B1 hepatotoxicity
(9,11–13).
In addition, it has been demonstrated that polydatin suppresses the
oxidative and inflammatory damage in ischemic stroke (14). Furthermore, polydatin treatment has
been shown to significantly reduce the serum levels of total
cholesterol (TC) and TGs in hyperlipidemic hamsters and rabbits,
induced by a high-fat diet (15,16).
A previous study demonstrated that polydatin alleviated hepatic fat
accumulation, by reducing oxidative damage in the liver and
regulating the expression of genes associated with hepatic fatty
acid biosynthesis (17). Despite
these hypolipidemic and antioxidant activities, the extent to which
polydatin improves hepatic steatosis has yet to be elucidated.
In the present study, NAFLD with insulin resistance
was induced in male rats by feeding them a nutritionally complete
high-fat diet. The aim of the present study was to determine
whether polydatin, when administered orally with a high-fat diet,
would prevent hepatic lipid accumulation and reverse insulin
resistance.
Materials and methods
Ethical approval
All animal experiments undertaken in the present
study were performed in strict accordance with international
ethical guidelines and the National Institutes of Health (NIH)
Guide concerning the Care and Use of Laboratory Animals (NIH,
Bethesda, MA, USA). The experiments were performed with the
approval of the Committee of Experimental Animal Administration of
the Shaanxi University of Chinese Medicine (permit number: 10076;
Xianyang, China).
Animal model and experimental
protocol
Male Sprague Dawley rats, weighing 160–180 g, were
purchased from the Experimental Animal Center of Xi’an Jiaotong
University (Xi’an, China). The rats were housed at 24°C, with a 12
h light-dark cycle and free access to food and water. The animal
experiments were approved by the Committee of Experimental Animal
Administration of Shaanxi University of Chinese Medicine.
Initially, the rats were housed in conventional conditions and fed
a standard diet and water ad libitum at the animal facility
for one week prior to the experiments. Thereafter, the rats were
divided into four groups (n=8/group) housed in individual cages,
and administered one of four treatments: Control diet (Con),
control diet supplemented with polydatin (CP), high-fat diet (HF),
and high-fat diet supplemented with polydatin (HP). Both the
control (77.0% carbohydrate, 5.0% fat, 18.0% protein) and high-fat
pellet diet protocols (58.0% fat, 24.0% carbohydrate, 18.0%
protein) were obtained from the Experimental Animal Center of Xi’an
Jiaotong University (Xi’an, China). The lipids were in the form of
saturated (0.9 and 30.4 g/100 g diet) and unsaturated (4.6 and 5.3
g/100 g diet) fat in the control and high-fat diet groups,
respectively. Polydatin
(3,4′,5-trihydroxystilbene-3-β-mono-D-glucoside; purity, >99%)
was obtained from Suzhou Baozetang Biotechnology Co., Ltd. (Suzhou,
China). Polydatin was supplemented in the drinking water at a
concentration of 0.3% (wt/vol) and administered simultaneously with
the high-fat diet feeding. In all of the animals, body weight and
food intake were measured every other day. Food and water were
supplied ad libitum. Food intake was determined by measuring
the difference between the weight of the study diets provided, and
the weight of the food at the end of a 24 h period. Spillage was
checked throughout the experiment and was considered negligible.
The rats were maintained on the treatment regimes for 12 weeks,
prior to being sacrificed. At the end of the experiment, the rats
were sacrificed by CO2 inhalation following a 12 h
fasting period, and blood samples were collected from the femoral
artery and stored as plasma at −80°C, until further use. The intact
livers were harvested and weighed. The liver index was calculated
as the liver/body weight ratio. The epididymal fat was removed in
order to measure visceral adiposity. Sections from the right lobe
of the liver were washed in cold saline and placed in 10% formalin
solution for histopathological analysis. The other samples were
immediately frozen in liquid nitrogen, and stored at −80°C until
further use.
Histological analysis
The liver sections were paraffin-embedded, sliced
into 5 μm sections and stained with hematoxylin & eosin
(H&E), as described by previous methods (17). The pathological changes were
assessed and photographed using an Olympus BX-51 microscope
(Olympus Corporation, Tokyo, Japan). The liver biopsies were scored
according to Brunt et al (18), as follows: 0, no steatosis; 1,
fatty hepatocytes occupying <10% of the parenchyma; 2, between
10 and 30%; 3, between 30 and 60%; and 4, fatty hepatocytes
occupying >60% of the parenchyma. Liver pathology was assessed
in a blinded manner by two independent pathologists with expertise
in rodent liver histology.
Biochemical assays
The activities of alanine aminotransferase (ALT) and
aspartate aminotransferase (AST) were measured in the plasma using
commercially available enzyme assay kits (Wako Pure Chemical
Industries Ltd., Osaka, Japan). Plasma TG, TC, and FFA
concentrations were determined using enzymatic reagent kits from
Biosino Bio-Technology and Science Inc. (Beijing, China), according
to the manufacturer’s instructions. Hepatic lipids were extracted
using chloroform-methanol (2:1), as previously described by Folch
et al (19), and then
dissolved using Triton X-100. The hepatic concentrations of TG and
TC were determined using the enzymatic reagent kits (Biosino
Bio-Technology and Science Inc.), as previously described (17,20).
Glucose tolerance test
Glucose tolerance tests were conducted 1 wk prior to
the end of feeding. Following an 8 h fasting period, the rats were
anesthetized, and, after the collection of an unchallenged sample
(time 0), a solution of 50% glucose (2.0 g/kg body wt) was
administrated by oral gavage. During the test, blood was collected
from the tail vein at 30, 60 and 120 min following glucose
administration. All of the blood glucose measurements were
performed using a hand-held glucometer (Roche Diagnostics,
Shanghai, China).
Plasma insulin, leptin, and adiponectin
ELISA
Plasma insulin, leptin and adiponectin levels were
measured using commercially available ELISA kits (Crystal Chem
Inc., Downers Grove, IL, USA), according to the manufacturer’s
instructions.
The homeostasis model assessment of basal insulin
resistance (HOMA-IR) was used to calculate an index from the
product of the concentration of fasting plasma glucose (mmol/l) and
plasma insulin (μU/ml), divided by 22.5 (21). Lower HOMA-IR values were considered
to indicate a greater insulin sensitivity, whereas higher HOMA-IR
values were considered to indicate a lower insulin sensitivity, or
insulin resistance.
Quantitative polymerase chain reaction
(qPCR)
A qPCR was performed to detect changes in the mRNA
expression levels of insulin receptor (IR), insulin receptor
substrate-1 (IRS-1) and -2 (IRS-2) in the liver tissues, as
described by previous methods (17,22).
The extraction of total RNA, from the liver tissues, was performed
using the RNeasy Mini kit (Qiagen, Valencia, CA, USA), according to
the manufacturer’s instructions. The purity and concentration of
the RNA samples were determined spectrophotometrically (SpectraMax
M5 microplate spectrophotometer; Molecular Devices, LLC.,
Sunnyvale, CA, USA). First, 2 μg of total RNA was reverse
transcribed into cDNA using Reverse Transcriptase (Qiagen).
Contaminating genomic DNA was eliminated using DNase I (Qiagen).
The qPCR reactions were conducted by placing 10 μl cDNA into
96-well plates with the TaqMan PCR Master mix (Applied Biosystems
Life Technologies, Foster City, CA, USA). The specific primers and
probes used for detection of IR, IRS-1 and IRS-2 mRNA (Gene
Expression Products: IR, Rn00567070_m1; IRS1, Rn04244524_m1; IRS2,
Rn01482270_s1; and β-actin, Rn00667869_m1) were obtained from
Applied Biosystems Life Technologies, and the qPCR was performed
using an Applied Biosystems PRISM 7000 sequence detection system,
according to the manufacturer’s instructions (Applied Biosystems
Life Technologies). A fluorescent comparative cycle threshold (Ct)
method was used to quantify mRNA expression levels, with β-actin
used as the internal control. The results of the qPCR were
expressed as the ratio of the mRNA of interest to β-actin.
Western blotting
The liver tissues were homogenized in lysis buffer
containing 20 mM Tris·HCl (pH 6.8), 150 mM NaCl, 10% glycerol, 1%
NP-40, and 8 μl/ml inhibitor cocktail (125 mM phenylmethylsulfonyl
fluoride, 2.5 mg/ml aprotinin, 2.5 mg/ml leupeptin, 2.5 mg/ml
antipain, and 2.5 mg/ml chymostatin). The tissue lysates were
centrifuged at 15,000 × g for 10 mins at 4°C, and the supernatants
were collected. The protein concentration was determined using a
Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA). The
samples were boiled for 5 min, and separated by 8% SDS-PAGE (10 μg
of protein, 20 μl per well) using Bio-Rad Minigel apparatus, at 100
V for 60 min (Bio-Rad Laboratories. The fractioned proteins on the
gel were electrophoretically transferred to nitrocellulose
membranes (Millipore, Billerica, MA, USA), at 350 mA for 90 min.
Following blocking with 5% skimmed milk, the membranes then were
hybridized at 4°C overnight with the following primary antibodies:
polyclonal rabbit anti-IRS2 (1:500), polyclonal rabbit anti-p-AKT
(1:500), polyclonal rabbit anti-AKT (1:500) and polyclonal goat
anti-GAPDH (1:500), which were all purchased from Santa Cruz
Biotechnology (Dallas, TX, USA). The membranes were washed with
phosphate-buffered saline with Tween 20 (Sigma-Aldrich, St. Louis,
MO, USA) and further incubated with the secondary antibody,
horseradish peroxidase-conjugated immunoglobulin G (1:2,000; Santa
Cruz Biotechnology), at 37°C for 60 min. Immunoreactivity was
detected using a chemiluminescence reagent kit (GE Healthcare,
Little Chalfont, UK), for 5 min at room temperature. The exposed
bands were analyzed using the Quantity One Software (Bio-Rad
Laboratories).
Statistical analyses
All of the data are expressed as the means ±
standard error. Statistical analyses were performed using a one-way
analysis of variance, and further analyzed using the Newman-Keuls
test for statistical difference. A P<0.05 was considered to
indicate a statistically significant difference.
Results
Changes in body weight and food
consumption
Following the 12 week feeding regimes, the absolute
body weight of each rat from each group was measured (Table I). There were no significant
differences in the absolute body weight of the rats between the Con
and CP groups, at the end of the feeding period. The rats in the HF
group had a significantly larger body weight and greater body
weight gain, as compared with the Con group (P<0.05). The
increase in body weight, in response to a high-fat diet, was
associated with the development of visceral obesity, as shown by
the progressive increase in epididymal fat weight. Supplementation
of polydatin in the HF group (HP) had no significant effects on
body weight gain, but reduced the weight of epididymal fat, as
compared with the HF group (P<0.05). The consumption of food was
monitored every other day, and no significant differences were
observed in the daily food consumptions among the groups.
 | Table IBody weight, liver index and
biochemical parameters of the experimental rats. |
Table I
Body weight, liver index and
biochemical parameters of the experimental rats.
| Con | CP | HF | HP |
|---|
| Body Weight
(g) | 482.37±16.53 | 474.13±17.12 |
537.08±17.45a | 491.52±16.27 |
| Liver index | 3.12±0.27 | 2.97±0.24 | 5.04±0.32b | 3.68±0.26d |
| Epididymal fat | 1.43±0.12 | 1.39±0.11 | 2.25±0.13b | 1.78±0.09c |
| Plasma |
| ALT (U/l) | 52.11±3.13 | 49.73±3.81 | 82.37±5.49b | 57.83±4.25d |
| AST (U/l) | 63.51±4.31 | 56.83±5.72 | 134.38±8.02b | 76.32±3.66d |
| TG (mmol/l) | 0.68±0.08 | 0.73±0.09 | 1.28±0.08b | 0.98±0.06ac |
| TC (mmol/l) | 1.61±0.14 | 1.69±0.21 | 2.87±0.13b | 2.19±0.09ac |
| FFA (mmol/l) | 0.84±0.04 | 0.91±0.06 | 1.28±0.05b | 0.96±0.07d |
| Liver |
| TG (μmol/g) | 26.37±2.13 | 25.02±2.21 | 39.63±3.02b | 29.35±2.79d |
| TC(μmol/g) | 6.08±0.31 | 6.19±0.21 | 7.73±0.25b | 6.47±0.26d |
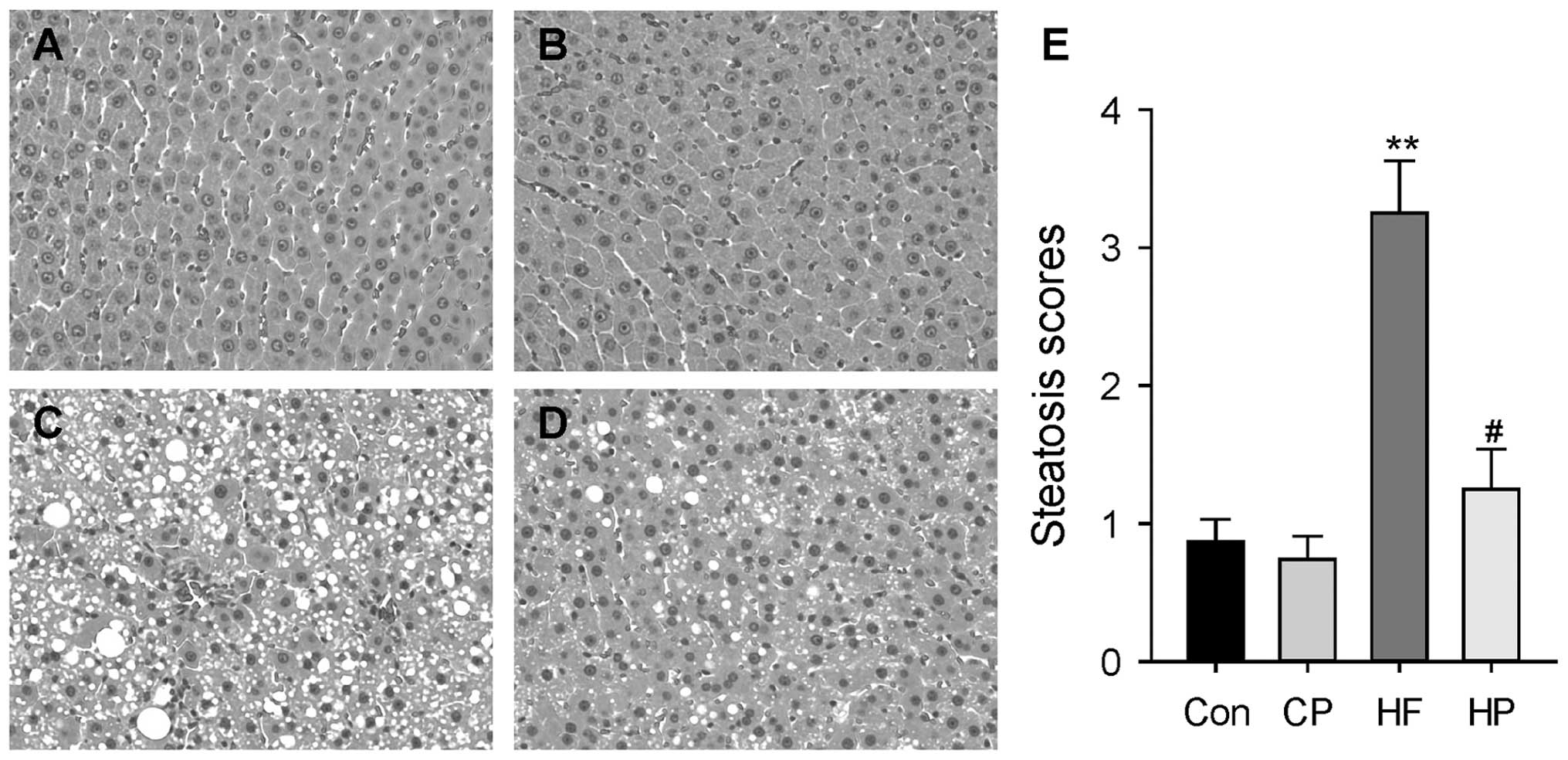
Polydatin supplementation alleviates
high-fat diet-induced fatty liver and liver injury
The hepatic pathological alterations from the four
groups were evaluated by measuring hepatic triglyceride contents
and circulating liver enzyme levels. A long-term high-fat diet
induced liver injury and fatty liver in the rats, which manifested
as increased plasma ALT and AST levels and hepatic triglyceride
contents (Table I). In response to
polydatin supplementation in the drinking water, both hepatic TG
accumulation and liver injury were significantly reduced in the
high-fat diet fed group (P<0.05). Furthermore, histological
sections of the liver from each experimental group were observed by
H&E staining. Following a 12 week high-fat diet, the rats
demonstrated severe hepatic microvesicular and macrovesicular fat,
whereas polydatin supplementation significantly eliminated hepatic
steatosis (P<0.05) (Fig.
1).
Polydatin supplementation alleviates
insulin resistance
To determine whether the hepatoprotective effects of
polydatin were associated with improved insulin resistance in the
high-fat diet fed rat model, the insulin sensitivity of the rats
was examined by measuring HOMA-IR, glucose tolerance, and plasma
FFA levels. High-fat diet feeding resulted in a significantly
higher HOMA-IR (P<0.01) (Fig.
2A), severe hyperglycemia upon glucose administration, and
impaired glucose tolerance (P<0.05)(Fig. 2B), as well as significantly
increased circulating FFA levels (P<0.05) (Table I), as compared with the control
group. However, polydatin supplementation markedly improved the
higher HOMA-IR, impaired glucose tolerance and increased FFA levels
in the rats, following a high-fat diet (P<0.05).
Polydatin improves adipokine
production
To determine the effects of polydatin on adipokine
production, the circulating levels of insulin, leptin and
adiponectin were measured. High-fat diet feeding resulted in a
significant increase in plasma insulin and leptin levels
(P<0.01) (Fig. 3A and B), as
compared with the Con group; whereas the adiponectin levels were
significantly decreased (P<0.01) (Fig. 3C). Polydatin supplementation in the
drinking water resulted in the substantial attenuation of the
increased insulin and leptin levels, as well as reversed the
abnormal adiponectin levels (P<0.05).
Polydatin improves IRS-2 expression
levels in the liver
The effects of polydatin supplementation were
examined on insulin-signaling pathways in the liver by qPCR and
western blotting. There were no marked differences in the IR and
IRS-1 mRNA expression levels in the rat livers, following high-fat
diet feeding (Fig. 4A and B).
However, high-fat diet feeding led to a significant decrease in the
hepatic mRNA and protein expression levels of IRS-2 (P<0.05)
(Fig. 4C and D). In response to
polydatin supplementation, the suppressive effects of the high-fat
diet on both mRNA and protein expression levels of IRS-2 were
attenuated. The effects of polydatin were also determined on Akt
phosphorylation status in the liver of rats fed a high-fat diet.
Polydatin supplementation significantly improved the Akt
phosphorylation in the rat liver, following a high-fat diet
(P<0.05) (Fig. 4E).
Discussion
The present study demonstrated that polydatin
supplementation alleviated insulin resistance and advanced hepatic
steatosis induced by a high-fat diet in rats. These beneficial
effects were shown to be associated with increased mRNA and protein
expression levels of IRS-2 in the liver, and improved abnormal
adipokine production. To the best of our knowledge, this is the
first study to report the effects of polydatin on insulin
resistance and hepatic steatosis.
Polydatin is one of the main compounds present in
Polygonum cuspidatum, a plant with both medicinal and
nutritional value. Zhang et al (13) previously reported that polydatin
possesses in vivo protective effects against CCl4-induced
liver injury in mice. Polydatin has also been shown to reduce body
weight and improve dyslipidemia in high-fat diet-fed hamsters and
rabbits (15,16). The results of the present study
demonstrated that polydatin has similarly beneficial effects on
rats with NAFLD induced by a high-fat diet. Polydatin significantly
decreased the plasma levels of TC, TG and FFA, and alleviated fatty
liver. In accordance with the alleviations observed in the
histological analyses of polydatin-treated rats, liver function was
also markedly improved by polydatin supplementation. Polydatin was
also shown to significantly decrease the weight of the epididymal
fat in the rats. These results support the notion that polydatin
effectively reduces visceral fat weight and hepatic fat content.
The mechanisms by which polydatin decreases serum and hepatic
lipids is yet to be fully elucidated. Usually, a reduction in fat
accumulation is accomplished through decreasing food consumption.
However, food consumption did not significantly differ among the
high-fat diet-fed groups, in the present study. These results
suggest that food and energy intake did not contribute to the
significant beneficial effects of dietary polydatin. It may be
hypothesized that the hypolipidemic effects of polydatin are caused
by altered hepatic lipid metabolism. Previous reports have shown
that polydatin may improve hyperlipidemia due to its effects on the
sterol regulatory element binding protein transcription factors,
which are important in the regulation of enzymes associated with
lipid metabolism in vivo (17). Consistent with this report, the
present study showed that polydatin decreased the weight of fat
tissue and reduced the accumulation of lipids in the liver induced
by NAFLD.
NAFLD is unique among the liver diseases since its
etiology is closely associated with metabolic syndromes. The
majority of the increased prevalence of NAFLD is due to obesity and
insulin resistance (5). It has
previously been reported that in the absence of obesity, even in
patients with total lipodistrophy, insulin resistance leads to
hepatic steatosis (8,23). The mechanisms underlying the
association of insulin resistance with hepatic steatosis remain
unclear; however, altered insulin sensitivity has been shown to
increase hepatic de novo lipogenesis and induce lipolysis of
adipocyte TGs and the flux of FFA to the liver (5,23).
In the present study, it was demonstrated that long-term high-fat
diet feeding resulted in a significant increase in hepatic fat
accumulation and plasma ALT and AST levels, which are key
indicators of liver injury. These changes were associated with an
increased gain in body weight and systemic insulin resistance,
which manifested as a significantly elevated HOMA-IR and
circulating FFA levels, as well as impaired blood glucose
clearance, as compared with the rats fed the control diet.
Polydatin supplementation was shown to alleviate hepatic steatosis
and lower plasma ALT and AST levels, as compared with the high-fat
group, which was accompanied by improved HOMA-IR, circulating FFA
levels and glucose tolerance test, without affecting body weight
changes and daily food consumptions. These results suggest that
polydatin may act as an insulin sensitizer.
In the liver, insulin has a crucial role in
mediating carbohydrate and lipid homeostasis by stimulating
glycogen synthesis, lipogenesis, lipoprotein synthesis, and
suppressing gluconeogenesis, and very low density lipoprotein
secretion in the fed state (24).
The binding of insulin to the extracellular domain of its receptor,
which is present on the plasma membrane of target cells, activates
its intrinsic cytoplasmic tyrosine kinase activity (25). The activated insulin receptor
phosphorylates tyrosine of various substrates, including IRS 1–3
and Shc (24,25). Tyrosine-phosphorylated IRS proteins
function as signaling molecules that propagate insulin action
through binding of Src homology 2 domain-containing proteins. These
include the p85 regulatory subunit of PI3K, Nck, Fyn, Grb-2, and
SHP2, which mediate various aspects of insulin action (26,27).
PI3K is a well-characterized downstream effector of the IRS
proteins (28). PI3K associates
with tyrosine-phosphorylated IRS proteins following insulin
stimulation, and catalyzes the formation of
phosphatidylinositol-3,4,5-trisphosphate, which in turn stimulates
phosphoinositide-dependent kinase activity. This results in the
initiation of the activation of the downstream effector Akt.
Activation of Akt leads to glucose transport and protein and
glycogen synthesis (29).
Hepatic insulin resistance is an important
pathophysiological feature of type II diabetes mellitus and the
metabolic syndrome (30).
Decreased IRS-2 expression levels, and the resultant impairment of
PI3K/Akt signalling, has previously been observed in the livers of
animal models for insulin resistance, including ob/ob and
lipodystrophic mice (31,32). Knockdown of IRS-2 gene expression
in mice resulted in the presentation of a phenotype resembling type
II diabetes in humans (33,34);
however, this phenotype was not induced in mice lacking IRS1
expression (35,36). It has also been reported that
insulin-induced PI3K and glycogen synthase activities were markedly
reduced in IRS-2 null hepatocytes, and reconstitution with IRS-2
led to the recovery of the response to insulin (37). These studies indicate that insulin
resistance may be mediated through inhibition of IRS-2. In the
present study, the effects of polydatin supplementation on insulin
sensitivity in the liver were determined by detecting the
expression of IRS and Akt kinase, which are major downstream
kinases activated by insulin binding to its receptors. High-fat
diet feeding decreased IRS-2 and phosphorylated-Akt protein
expression levels in the liver, which is indicative of dampened
insulin signal transduction in the liver. Notably, the present
study showed that the suppression of both kinases was rescued by
polydatin supplementation in the drinking water, suggesting that
polydatin may act directly on the liver tissue to prevent insulin
resistance induced by a high-fat diet.
As well as insulin resistance, another critical
mechanism implicated in the pathogenesis of NAFLD is the
dysregulated production of adipokines. Adipokines are associated
with the pathogenesis of NAFLD, through their metabolic and
pro-/anti-inflammatory activities (38,39).
Previous evidence showed that leptin is capable of promoting
insulin resistance and hepatocyte injury/fibrogenesis in both cell
cultures and animal models (40).
In animal models, decreased adiponectin levels are associated with
hepatic steatosis and inflammation in NAFLD (39,41).
In the present study, the circulating levels of leptin and
adiponectin were measured in rats that were fed a high-fat diet,
with or without polydatin supplementation. The long-term high-fat
diet feeding significantly increased the circulating levels of
leptin, whereas adiponectin levels were significantly decreased, as
compared with the control rats. Furthermore, the changes to
adipokine production following high-fat feeding were improved by
polydatin supplementation, implying that polydatin may not only
prevent early stage steatosis, but also the transition to
nonalcoholic steatohepatitis and fibrosis.
In conclusion, the present study was the first, to
the best of our knowledge, to demonstrate that long-term feeding of
a high-fat diet to rats induces fatty liver and liver injury, which
were associated with obesity, insulin resistance, and dysregulated
adipokine production. Polydatin supplementation in the drinking
water resulted in the alleviation of hepatic steatosis and liver
injury, which were associated with improved insulin resistance and
abnormal adipokine production. The results of the present study
suggest that polydatin may be an effective hepatoprotective agent,
and a potential candidate for the treatment of fatty liver disease
and insulin resistance.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 31171101 and 81100175)and
the Key Project of Chinese Ministry of Education (no. 212173).
References
|
1
|
Angulo P: Nonalcoholic fatty liver
disease. N Engl J Med. 346:1221–1231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clark JM, Brancati FL and Diehl AM:
Nonalcoholic fatty liver disease. Gastroenterology. 122:1649–1657.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Falck-Ytter Y, Younossi ZM, Marchesini G
and McCullough AJ: Clinical features and natural history of
nonalcoholic steatosis syndromes. Semin Liver Dis. 21:17–26. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guilherme A, Virbasius JV, Puri V and
Czech MP: Adipocyte dysfunctions linking obesity to insulin
resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 9:367–377.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gariani K, Philippe J and Jornayvaz FR:
Non-alcoholic fatty liver disease and insulin resistance: from
bench to bedside. Diabetes Metab. 39:16–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marchesini G, Bugianesi E, Forlani G, et
al: Nonalcoholic fatty liver, steatohepatitis, and the metabolic
syndrome. Hepatology. 37:917–923. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jou J, Choi SS and Diehl AM: Mechanisms of
disease progre ssion in nonalcoholic fatty liver disease. Semin
Liver Dis. 28:370–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tilg H and Moschen AR: Insulin resistance,
inflammation, and non-alcoholic fatty liver disease. Trends
Endocrinol Metab. 19:371–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kimura Y: Pharmacological studies on
resveratrol. Methods Find Exp Clin Pharmacol. 25:297–310. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue L: Progress in the pharmacological
study of Chinese herbal drug: Polygonum cuspidatum. Zhongguo
Zhong Yao Za Zhi. 25:651–653. 2000.(In Chinese).
|
|
11
|
Luper S: A review of plants used in the
treatment of liver disease: part two. Altern Med Rev. 4:178–188.
1999.PubMed/NCBI
|
|
12
|
Zhang H, Dou C and Gu F: Advances in the
study on pharmacological actions of Polygonum cuspidatum
Sieb. et Zucc.: clearing heat and detoxication. Zhong Yao Cai.
26:606–610. 2003.(In Chinese).
|
|
13
|
Zhang H, Yu CH, Jiang YP, et al:
Protective effects of polydatin from Polygonum cuspidatum
against carbon tetrachloride-induced liver injury in mice. PLoS
One. 7:e465742012.
|
|
14
|
Ji H, Zhang X, Du Y, Liu H, Li S and Li L:
Polydatin modulates inflammation by decreasing NF-κB activation and
oxidative stress by increasing Gli1, Ptch1, SOD1 expression and
ameliorates blood-brain barrier permeability for its
neuroprotective effect in pMCAO rat brain. Brain Res Bull.
87:50–59. 2012.PubMed/NCBI
|
|
15
|
Du J, Sun LN, Xing WW, et al:
Lipid-lowering effects of polydatin from Polygonum
cuspidatum in hyperlipidemic hamsters. Phytomedicine.
16:652–658. 2009. View Article : Google Scholar
|
|
16
|
Xing WW, Wu JZ, Jia M, Du J, Zhang H and
Qin LP: Effects of polydatin from Polygonum cuspidatum on
lipid profile in hyperlipidemic rabbits. Biomed Pharmacother.
63:457–462. 2009.
|
|
17
|
Zhang J, Tan Y, Yao F and Zhang Q:
Polydatin alleviates non-alcoholic fatty liver disease in rats by
inhibiting the expression of TNF-α and SREBP-1c. Mol Med Rep.
6:815–820. 2012.PubMed/NCBI
|
|
18
|
Brunt EM, Janney CG and Di Bisceglie AM:
Neuschwander-Tetri BA and Bacon BR: Nonalcoholic steatohepatitis: a
proposal for grading and staging the histological lesions. Am J
Gastroenterol. 94:2467–2474. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Folch J, Lees M and Sloane Starley GH: A
simple method for the isolation and purification of total lipides
from animal tissues. J Biol Chem. 226:497–509. 1957.PubMed/NCBI
|
|
20
|
Zhang Q, Zhao Y, Zhang DB and Sun LJ:
Effect of Sinai san decoction on the development of non-alcoholic
steatohepatitis in rats. World J Gastroenterol. 11:1392–1395. 2005.
View Article : Google Scholar
|
|
21
|
Matthews DR, Hosker JP, Rudenski AS,
Naylor BA, Treacher DF and Turner RC: Homeostasis model assessment:
insulin resistance and beta-cell function from fasting plasma
glucose and insulin concentrations in man. Diabetologia.
28:412–419. 1985. View Article : Google Scholar
|
|
22
|
Zhang Q, Yao F, Raizada MK, O’Rourke ST
and Sun C: Apelin gene transfer into the rostral ventrolateral
medulla induces chronic blood pressure elevation in normotensive
rats. Circ Res. 104:1421–1428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bugianesi E, McCullough AJ and Marchesini
G: Insulin resistance: a metabolic pathway to chronic liver
disease. Hepatology. 42:987–1000. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saltiel AR and Kahn CR: Insulin signalling
and the regulation of glucose and lipid metabolism. Nature.
414:799–806. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Youngren JF: Regulation of insulin
receptor function. Cell Mol Life Sci. 64:873–891. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khan AH and Pessin JE: Insulin regulation
of glucose uptake: a complex interplay of intracellular signalling
pathways. Diabetologia. 45:1475–1483. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Le Roith D and Zick Y: Recent advances in
our understanding of insulin action and insulin resistance.
Diabetes Care. 24:588–597. 2001.PubMed/NCBI
|
|
28
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Q, Somwar R, Bilan PJ, et al: Protein
kinase B/Akt participates in GLUT4 translocation by insulin in L6
myoblasts. Mol Cell Biol. 19:4008–4018. 1999.PubMed/NCBI
|
|
30
|
Boura-Halfon S and Zick Y: Phosphorylation
of IRS proteins, insulin action, and insulin resistance. Am J
Physiol Endocrinol Metab. 296:E581–E591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kerouz NJ, Hörsch D, Pons S and Kahn CR:
Differential regulation of insulin receptor substrates-1 and -2
(IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in
liver and muscle of the obese diabetic (ob/ob) mouse. J Clin
Invest. 100:3164–3172. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shimomura I, Matsuda M, Hammer RE, et al:
Decreased IRS-2 and increased SREBP-1c lead to mixed insulin
resistance and sensitivity in livers of lipodystrophic and ob/ob
mice. Mol Cell. 6:77–86. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Previs SF, Withers DJ, Ren JM, White MF
and Shulman GI: Contrasting effects of IRS-1 versus IRS-2 gene
disruption on carbohydrate and lipid metabolism in vivo. J Biol
Chem. 275:38990–38994. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Withers DJ, Gutierrez JS, Towery H, et al:
Disruption of IRS-2 causes type 2 diabetes in mice. Nature.
391:900–904. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rother KI, Imai Y, Caruso M, Beguinot F,
Formisano P and Accili D: Evidence that IRS-2 phosphorylation is
required for insulin action in hepatocytes. J Biol Chem.
273:17491–17497. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kido Y, Burks DJ, Withers D, et al:
Tissue-specific insulin resistance in mice with mutations in the
insulin receptor, IRS-1, and IRS-2. J Clin Invest. 105:199–205.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Valverde AM, Burks DJ, Fabregat I, et al:
Molecular mechanisms of insulin resistance in IRS-2-deficient
hepatocytes. Diabetes. 52:2239–2248. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Farrell GC and Larter CZ: Nonalcoholic
fatty liver disease: from steatosis to cirrhosis. Hepatology.
43:S99–S112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Musso G, Gambino R, Durazzo M, et al:
Adipokines in NASH: postprandial lipid metabolism as a link between
adiponectin and liver disease. Hepatology. 42:1175–1183. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gambino R, Cassader M, Pagano G, Durazzo M
and Musso G: Polymorphism in microsomal triglyceride transfer
protein: a link between liver disease and atherogenic postprandial
lipid profile in NASH? Hepatology. 45:1097–1107. 2007. View Article : Google Scholar
|
|
41
|
Wang Z, Yao T, Pini M, Zhou Z, Fantuzzi G
and Song Z: Betaine improved adipose tissue function in mice fed a
high-fat diet: a mechanism for hepatoprotective effect of betaine
in nonalcoholic fatty liver disease. Am J Physiol Gastrointest
Liver Physiol. 298:G634–G642. 2010. View Article : Google Scholar : PubMed/NCBI
|