Introduction
Myocardial infarction is regarded as one of the most
common types of ischemic heart disease and a major cause of
morbidity and mortality worldwide. It arises from a sudden and
persistent interruption of the myocardial blood supply, leading to
a marked loss of cardiomyocytes. Although certain western drugs are
used for treating myocardial ischemia, including
angiotensin-converting enzyme inhibitors, calcium channel blockers
and angiotensin II receptor antagonists, their application is
limited by their serious side effects, such as cardiac depression
or proarrhythmia (1). Thus, there
is an urgent requirement for the development of novel therapeutic
approaches to attenuate the damage caused by myocardial
infarction.
Substantial evidence has demonstrated that reactive
oxygen species (ROS) contribute to cell loss following myocardial
infarction. The high level of oxygen free radicals following
ischemia was reported to damage cellular structures, cause
mitochondrial dysfunction and activate apoptotic signaling cascades
(2). In addition, oxidative stress
is closely associated with the generation of myocardial ischemia.
Thus, the reduction of ROS may serve as an important therapeutic
target for attenuating the damage caused by acute myocardial
infarction (AMI).
Nuclear factor (NF)-κB is a vital cellular
transcription factor that is present in myocytes. Changes in its
expression level have been demonstrated to reflect pathological
alterations during AMI (3,4). Activation of NF-κB may trigger the
release of proinflammatory cytokines, such as TNF-α and IL-1β. In
cells undergoing myocardial infarction, high levels of NF-κB p65,
TNF-α and IL-1β facilitate myocardial necrosis and cellular
apoptosis, subsequently inducing heart failure. Therefore, drugs
that inhibit the NF-κB signaling pathway and the release of
proinflammatory factors may serve as potential treatments for a
variety of cardiovascular disorders.
It is well established that apoptosis participates
in the pathogenesis of myocardial injury during myocardial
infarction. Caspase-3 is the largest subgroup of the caspase family
and acts to induce apoptosis in response to apoptotic stimuli
(5). A previous investigation
demonstrated a marked elevation in the levels of caspase-3 in
response to isoproterenol-induced AMI in Wistar rats (6). These results suggest that modulating
caspase-3 may reduce cardiac injury during myocardial
infarction.
A number of traditional Chinese medicines have been
indicated to exert a protective role against AMI in animals
(7,8). Oxysophoridine (OSR) is a natural
alkaloid purified from Sophora alopecuroides L. and has been
demonstrated to possess diverse pharmacological actions. A previous
study by Wang et al (9)
demonstrated that OSR was able to perform a protective role against
ischemic damage following middle cerebral artery occlusion in mice.
They indicated that this neuroprotection may be associated with the
suppression of oxidative stress and apoptosis. However, whether OSR
can protect against AMI in rats has yet to elucidated. Therefore,
the current study was performed to investigate the cardioprotective
effects of OSR against AMI in rats and to clarify the underlying
molecular mechanisms.
Materials and methods
Ethical disclosure
All surgical procedures conducted in the present
study were approved by the animal ethics committee of the Huangdao
Branch of the Affiliated Hospital of Qingdao University Medical
College (Qingdao, China).
Animals and AMI production
Adult male Wistar rats (250–300 g) were supplied by
the Beijing Laboratory Animal Research Center (Beijing, China). All
animals were maintained in individual cages under a standard
environment (12/12 h light/dark cycle, 50–70% humidity, 24°C) and
provided with free access to food and water. The induction of rat
AMI was performed according to the methods of a previous study
(10). Briefly, animals were
anesthetized intraperitoneally (i.p.) with 40 mg/kg sodium
pentobarbitone. They were then intubated and artificially
ventilated with a respirator (Chengdu Taimeng Technology Co.,
Chengdu, China). The standard electrocardiogram II (Chengdu Taimeng
Technology Co.) was procured by a transducer attached to a
multi-channel recorder (BL-420F Data Acquisition & Analysis
System, Chengdu Technology & Market Co., Ltd., Chengdu, China)
following subcutaneous penetration of electrodes into the four
limbs. A 5.0 silk suture 1–2 mm in diameter was used to encircle
the left anterior descending coronary artery under the left atrial
appendage. Sham-operated animals were treated with the same
surgical procedures without the coronary artery ligation. Efforts
were made to reduce the number of animals used and minimize their
suffering. Successful ligation was verified by the observation of
ST-segment elevation and regional cyanosis of the myocardial
surface.
Drug administration
Oxysophoridine (OSR; purity, 98%), supplied by the
Institution of Chemistry and Chemical Engineering (Beijing, China)
was dissolved in physiological saline. The rats were randomly
assigned to five groups as follows: i) The sham-operated group,
which was injected with physiological saline (0.1 ml/100 g, i.p.)
and underwent the surgery without the coronary artery ligation; ii)
the vehicle group, which was injected with physiological saline
(0.1 ml/100 g, i.p.) and underwent occlusion of the left coronary
artery; iii) The OSR groups, which were subjected to the occlusion
of the left coronary artery and treated with OSR at concentrations
of 62.5, 125 or 250 mg/kg. Physiological saline or OSR were
administered for seven consecutive days, then 30 min after the
final treatment, rats underwent surgery.
Measurement of infarct size
Six hours after the occlusion of the coronary
artery, the hearts were swiftly excised and the left ventricles
sectioned into transverse slices (2-mm thick) from the apex to the
atrioventricular groove. The slices were then incubated in 1%
triphenyltetrazolium chloride (TTC; Sigma-Aldrich, St. Louis, MO,
USA) solution at 37°C for 30 min. Images were captured with a
digital camera (Canon 500D; Canon, Inc., Tokyo, Japan) and weighed.
Normal myocardium was stained brick red, while areas without color
indicated the ischemic heart muscle. The volume and weight as a
percentage of the left ventricle were used to calculate the size of
the infarcted area.
Determination of cardiac marker
enzymes
Six hours after occlusion of the coronary artery,
blood samples were collected in order to determine
myocardium-specific enzymes, including creatine kinase (CK), MB
isoenzyme of creatine kinase (CK-MB), lactate dehydrogenase (LDH)
and cardiac troponin T (cTnT). The colorimetric method was employed
to analyze the activity levels of CK, CK-MB and LDH according to
the manufacturer’s instructions (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). Serum levels of cTnT were measured
using a Diagnostics Elecsys 2010 Immunoassay system (Roche
Diagnostics, Basel, Switzerland).
Measurement of malondialdehyde (MDA),
superoxide dismutase (SOD), glutathione (GSH) and glutathione
peroxidase (GSH-PX) activity
The level of MDA and the enzymatic activity of
catalase (CAT), Cu/Zn-SOD, Mn-SOD and GSH-PX together with the
non-enzymatic GSH were measured in the heart homogenate using the
GSH-PX assay kit (colorimetric method) according to the
manufacturer’s instructions of this commercial assay kits (Nanjing
Jiancheng Bioengineering Institute).
Measurement of NF-κB p65 unit, TNF-α,
IL-1β, IL-6 and IL-10 levels
The p65 subunit may be positively correlated with
the activation of the NF-κB pathway; thus, the levels of NF-κB p65
were measured (Cayman Chemical, Ann Arbor, MI, USA), in addition to
the levels of TNF-α, IL-1β, IL-6 and IL-10, with commercial
immunoassay kits (KCB7226, RTA00, RLB00, R6000B and R1000,
respectively; R&D Systems, Inc., Minneapolis, MN, USA)
according to the manufacturer’s instructions.
Assay of caspase-3 activity
The levels of the cleavage product of the
chromogenic caspase substrate, Ac-DEVD-pNA
(acetyl-Asp-Glu-Val-Asp p-nitroanilide), were measured to quantify
the activity of caspase-3. The quantity of caspase-3 was measured
using the colorimetric approach with the Caspase-3 Activity Assay
Kit (Beyotime Institute of Biotechnology, Haimen, China). The
protein samples from the heart were obtained by centrifugation at
13,500 × g for 20 min. Approximately 50 μg protein was added to a
reaction buffer containing Ac-DEVD-pNA (2 mM), incubated at
37°C for 4 h, and the absorbance of yellow pNA (the cleavage
product) was calculated with a spectrometer (Shanghai Chenguang
Medical Technologies Co., Ltd., Shanghai, China) at a wavelength of
405 nm. The specific activity of caspase-3, which was normalized to
the total protein in the heart tissue, was then expressed as fold
of the baseline caspase-3 activity of the control group.
Statistical analysis
Data were expressed as the mean ± standard
deviation, with n=6 in each group. Experimental results were
analyzed using one-way analysis of variance, followed by Dunnett’s
test for individual comparisons between each group. All statistical
analyses were conducted using SPSS software, version 13.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to represent a
statistically significant difference.
Results
OSR treatment reduces myocardial infarct
size in an AMI rat model
The chemical structure of OSR is presented in
Fig. 1. The infarction size in the
infarcted group was 41.66±2.56%. Subsequent to treatment with OSR
at a dose of 62.5, 125 and 250 mg/kg, the infarcted area was
significantly reduced to 35.43±3.54%, 30.68±3.42% and 28.59±2.98%,
respectively (P<0.01 vs. the infarcted group; Fig. 2).
OSR reduces activity of CK, CK-MB and
LDH, and the level of cTnT in an AMI rat model
The measured levels of serum CK, CK-MB, LDH and cTnT
are shown in Fig. 3. An increase
in the levels of all the measured proteins (P<0.01) was observed
in infarcted rats compared with the sham controls. Treatment with
OSR reduced the levels of the proteins in a dose-dependent manner,
and all were significantly reduced compared with the
vehicle-treated group (P<0.01).
 | Figure 3Effects of OSR on serum (A) CK, (B)
CK-MB and (C) LDH activities together with (D) cTnT level in a rat
model of acute myocardial infarction (the mean ± standard
deviation, n=6). Groups were as follows: Sham, sham-operated;
Vehicle, vehicle-treated; OSR, OSR-treated (dose, mg/kg). As cTnT
serum level positively correlates with cTnT activity, cTnT serum
can directly reflect cTnT activity. **P<0.01 vs. the
sham group, ##P<0.01 vs. the vehicle group. OSR,
oxysophoridine; CK, creatine kinase; CK-MB, MB isoenzyme of CK;
LDH, lactate dehydrogenase; cTnT, cardiac troponin T. |
Effects of OSR treatment on the content
of MDA and activity levels of CAT, Cu/Zn-SOD, Mn-SOD, GSH and
GSH-PX in a rat model of AMI
To explore whether OSR exerted cardioprotective
effects against AMI via an antioxidant mechanism, the level of MDA
and the activity levels of CAT, Cu/Zn-SOD, Mn-SOD, GSH and GSH-PX
in heart homogenate were determined, as illustrated in Fig. 4. A marked increase was observed in
the level of MDA (an index of lipid peroxidation) in infarcted rats
(P<0.01 vs. sham operation group). Pretreatment with OSR at
different doses (62.5, 125 and 250 mg/kg) significantly diminished
the infarction-mediated lipid peroxidation (P<0.01 vs. the
vehicle group) in a dose-dependent manner. The activity levels of
antioxidants and anti-oxidative enzymes were also analyzed in the
heart homogenate of control and experimental groups. Rats with AMI
exhibited significant reductions in levels of CAT, Cu/Zn-SOD,
Mn-SOD, GSH and GSH-PX (P<0.01 vs. the sham-operated rats). OSR
treatment significantly augmented levels of CAT, Cu/Zn-SOD, Mn-SOD,
GSH and GSH-PX in the hearts of infarcted rats compared with levels
in the vehicle group.
 | Figure 4Effects of OSR on the content of (A)
MDA and activities of (B) CAT, (C) Cu/Zn-SOD, (D) Mn-SOD, (E) GSH
and (F) GSH-PX in a rat model of acute myocardial infarction (the
mean ± standard deviation, n=6). Groups were as follows: Sham,
sham-operated; Vehicle, vehicle-treated; OSR, OSR-treated (dose,
mg/kg). **P<0.01 vs. the sham group,
##P<0.01 vs. the vehicle group. OSR, oxysophoridine;
MDA, malondialdehyde; CAT, catalase; Cu/Zn-SOD,
copper/zinc-superoxide dismutase; Mn-SOD, manganese-SOD; GSH,
glutathione; GSH-PX, GSH-peroxidase. |
OSR reduces activity levels of NF-κB p65,
TNF-α, IL-1β, IL-6 and IL-10 in a rat model of AMI
Fig. 5 displays the
effects of OSR on molecules involved in the inflammatory response
(including NF-κB p65, TNF-α, IL-1β, IL-6 and IL-10) in a rat model
of AMI. It was noted that the levels of NF-κB p65, TNF-α, IL-1β,
IL-6 and IL-10 (P<0.01) were significantly increased in the
vehicle-treated myocardial infarction group. Treatment with OSR led
to clear reductions in levels of NF-κB p65, TNF-α, IL-1β, IL-6 and
IL-10 (P<0.01) in a dose-dependent manner, compared with the
vehicle-treated group.
 | Figure 5Effects of OSR on the activities of
(A) NF κB p65, (B) TNF-α, (C) IL-1β, (D) IL-6 and (E) IL-10 in a
rat model of acute myocardial infarction (the mean ± standard
deviation, n=6). Groups were as follows: Sham, sham-operated;
Vehicle, vehicle-treated; OSR, OSR-treated (dose, mg/kg).
**P<0.01 vs. the sham group, ##P<0.01
vs. the vehicle group. OSR, oxysophoridine; NF, nuclear factor;
TNF, tumor necrosis factor; IL, interleukin. |
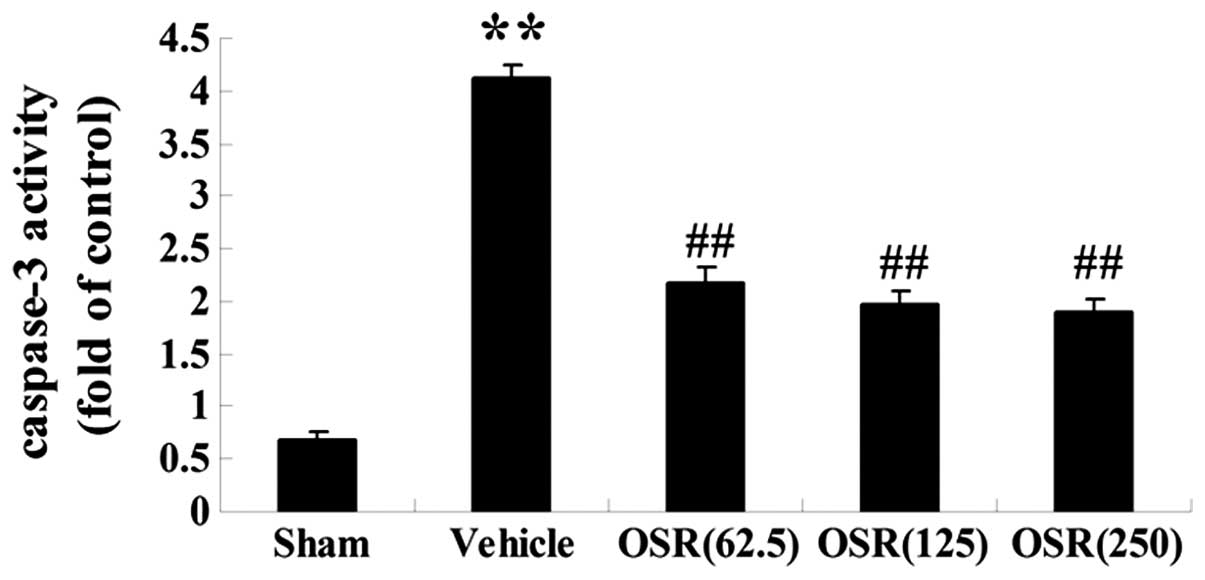
Effects of OSR on the caspase-3 activity
in a rat model of AMI
In order to determine whether OSR could attenuate
the apoptotic damage induced by myocardial infarction, the activity
level of caspase-3, an executioner molecule in the apoptotic
signaling pathway, was determined by colorimetric analysis. As
presented in Fig. 6, caspase-3
activity in the vehicle group was significantly enhanced
(P<0.01) compared with the sham group. In the OSR pretreatment
(62.5, 125 and 250 mg/kg) groups, there was a significant reduction
in caspase-3 activity (P<0.01) compared with that of the vehicle
group.
Discussion
AMI is a predominant ischemic disease, for which TTC
staining and enhanced cardiac magnetic resonance imaging can be
used to delineate the acute necrotic infarct zone directly. The
major findings of the present study in AMI rat models clarified
that: (i) OSR was able to diminish the myocardial infarct size and
the activity levels of serum CK, CK-MB and LDH together with levels
of cTnT protein; (ii) OSR significantly suppressed lipid
peroxidation (quantified by the level of MDA production) but
increased endogenous antioxidant enzymes (CAT, Cu/Zn-SOD, Mn-SOD
and GSH) in addition to the non-enzymatic scavenger (GSH-PX); (iii)
OSR inhibited the activities of a number of major inflammatory
factors, including NF-κB p65, TNF-α, IL-1β, IL-6 and IL-10; and
(iv) OSR treatment resulted in reduced activity of caspase-3. These
findings support the hypothesis that OSR exerts a cardioprotective
effect against injury resulting from AMI, through anti-oxidative,
anti-inflammatory and anti-apoptotic mechanisms.
OSR, which is derived from the natural alkaloid,
Sophora alopecuroides L. has been indicated to produce
various pharmacological effects. It has been previously reported
that OSR was able to ameliorate focal brain ischemia in mice via
inhibiting oxidative stress and apoptosis (9). The present study extended the
therapeutic profile of OSR and disclosed a potent cardioprotective
role against AMI in rats.
The infarct size, and levels of myocardium-specific
enzymes, including CK, CK-MB, LDH and cTnT are regarded as key
factors for the evaluation of cardiac injury resulting from
ischemic heart disease. Significant elevations in infarct size and
the activity of these myocardium-specific enzymes in rats were
identified during AMI (6,11). In addition, cTnT, a specific and
sensitive indicator of ischemic insults, was reported to be
produced when myocardial necrosis occurs (12). Consistent with these previous
studies, the present study revealed that the infarct size and the
activity levels of CK, CK-MB, LDH and cTnT were significantly
elevated in infarcted rats. Furthermore, these indices were all
reduced following OSR treatment in AMI-induced rats, demonstrating
the cardioprotective effect of OSR.
Excessive production of oxygen free radicals is
involved in the generation of ischemic impairments. Under normal
physiological conditions, the generation of oxygen free radicals is
controlled by endogenous antioxidant enzymes, such as CAT,
Cu/Zn-SOD, Mn-SOD, GSH-PX and low-molecular weight antioxidants,
such as non-enzymic GSH. Treatment with antioxidants and radical
scavengers is beneficial for ischemic stroke therapy (13,14).
In addition, MDA serves as a sensitive index for evaluating lipid
peroxidation, as it is composed of products of the decomposition of
fatty acids of myocardial membranes. Results from the current study
demonstrated that OSR significantly diminished the content of MDA
and increased the activity of Cu/Zn-SOD, Mn-SOD, GSH and GSH-PX in
the infarcted rats. This implies that the cardioprotection of OSR
against AMI in rats was associated with its anti-oxidative
properties.
The inflammatory response also participates in the
pathogenesis of ischemic heart diseases (15,16).
It was reported that the activation of NF-κB led to the marked
release of proinflammatory mediators, such as TNF-α and IL-1β. In
fact, inhibition of the NF-κB pathway may improve adverse left
ventricular remodeling and cardiac dysfunction during myocardial
infarction (17). The present
study demonstrated that OSR suppressed the increase of NF-κB p65
and reduced the activity levels of TNF-α, IL-1β, IL-6 and IL-10 in
rats subjected to myocardial infarction. Ruan et al
(18) noted that OSR exerted
anti-inflammatory effects against D-galactose-treated rats
(18), which is in agreement with
the findings of the current study. OSR was also demonstrated to
inhibit nuclear translocation of NF-κB and reduce the increased
levels of proinflammatory factors during transient focal cerebral
ischemia (19). These findings
suggest that OSR exerts cardioprotective effects subsequent to AMI
through its anti-inflammatory profile.
Oxidative damage may facilitate mitochondrial
dysfunction and subsequently activate an apoptotic cascade
following cardiac impairments. To further explore the amelioration
of the cardiac injuries resulting from AMI subsequent to OSR
treatment, the activity of apoptosis-related proteins was assessed
in the hearts of the rats. As evolutionarily conserved cysteinyl
proteases, caspases serve a crucial function in cellular apoptosis,
and caspase-3 is a critical molecule in the caspase-dependent
apoptotic cascade. It has been previously reported that caspase-3
activates diverse substrates that lead to DNA fragmentation and
cell death (20), and upregulation
of caspase-3 was observed following AMI (6,10).
In the present study, OSR significantly reduced caspase-3 activity
in infarcted rats. Consistent with these results, Wang and Tang
(7) indicated that OSR
significantly reduced the activity of caspase-3 in Alzheimer’s
disease. Furthermore, the overexpression of caspase-3 was also
suppressed in mice subjected to focal cerebral ischemic injury
(9). The present investigation
revealed a reduced level of caspase-3 activity in the AMI-induced
rats treated with OSR, suggesting that its cardioprotective ability
was acting through its anti-apoptotic profile.
The results of the current study demonstrated that
OSR is able to attenuate the AMI-induced injury in rats and that
this cardioprotection may be linked with its anti-oxidative,
anti-inflammatory and anti-apoptotic properties. These findings
imply that OSR has potential as a novel cardioprotective agent for
treating AMI, but further studies are required in order to verify
the results.
References
|
1
|
Yang B, Lin H, Xiao J, et al: The
muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic
potential by targeting GJA1 and KCNJ2. Nat Med. 13:486–491. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan PH: Mitochondria and neuronal
death/survival signaling pathways in cerebral ischemia. Neurochem
Res. 29:1943–1949. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Müller DN, Mervaala EM, Dechend R, et al:
Angiotensin II (AT(1)) receptor blockade reduces vascular tissue
factor in angiotensin II-induced cardiac vasculopathy. Am J Pathol.
157:111–122. 2000.PubMed/NCBI
|
|
4
|
Speir E: Cytomegalovirus gene regulation
by reactive oxygen species. Agents in atherosclerosis. Ann NY Acad
Sci. 899:363–374. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka M, Mokhtari GK, Terry RD, et al:
Overexpression of human copper/zinc superoxide dismutase (SOD1)
suppresses ischemia-reperfusion injury and subsequent development
of graft coronary artery disease in murine cardiac grafts.
Circulation. 110(Suppl 1): II200–II206. 2004. View Article : Google Scholar
|
|
6
|
Guo J, Li HZ, Wang LC, et al: Increased
expression of calcium-sensing receptors in atherosclerosis confers
hypersensitivity to acute myocardial infarction in rats. Mol Cell
Biochem. 366:345–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang R and Tang XC: Neuroprotective
effects of huperzine A. A natural cholinesterase inhibitor for the
treatment of Alzheimer’s disease. Neurosignals. 14:71–82. 2005.
|
|
8
|
Yu W, Liu Q and Zhu S: Carvacrol protects
against acute myocardial infarction of rats via anti-oxidative and
anti-apoptotic pathways. Biol Pharm Bull. 36:579–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang TF, Lei Z, Li YX, et al:
Oxysophoridine protects against focal cerebral ischemic injury by
inhibiting oxidative stress and apoptosis in mice. Neurochem Res.
38:2408–2417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong-Li S, Lei L, Lei S, et al:
Cardioprotective effects and underlying mechanisms of oxymatrine
against ischemic myocardial injuries of rats. Phytother Res.
22:985–989. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ming X, Tongshen W, Delin W and Ronghua Z:
Cardioprotective effect of the compound yangshen granule in rat
models with acute myocardial infarction. Evid Based Complement
Alternat Med. 2012:7171232012.PubMed/NCBI
|
|
12
|
Katus HA, Remppis A, Scheffold T,
Diederich KW and Kuebler W: Intracellular compartmentation of
cardiac troponin T and its release kinetics in patients with
reperfused and nonreperfused myocardial infarction. Am J Cardiol.
67:1360–1367. 1991. View Article : Google Scholar
|
|
13
|
Yamada J, Yoshimura S, Yamakawa H, et al:
Cell permeable ROS scavengers, Tiron and Tempol, rescue PC12 cell
death caused by pyrogallol or hypoxia/reoxygenation. Neurosci Res.
45:1–8. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Bao Y, Jiang B, et al: Catalpol
protects primary cultured astrocytes from in vitro ischemia-induced
damage. Int J Dev Neurosci. 26:309–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Frantz S, Fraccarollo D, Wagner H, et al:
Sustained activation of nuclear factor kappa B and activator
protein 1 in chronic heart failure. Cardiovasc Res. 57:749–756.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wong SC, Fukuchi M, Melnyk P, Rodger I and
Giaid A: Induction of cyclooxygenase-2 and activation of nuclear
factor-kappaB in myocardium of patients with congestive heart
failure. Circulation. 98:100–103. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshiyama M, Omura T, Takeuchi K, et al:
Angiotensin blockade inhibits increased JNKs, AP-1 and NF- kappa B
DNA-binding activities in myocardial infarcted rats. J Mol Cell
Cardiol. 33:799–810. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruan Q, Liu F, Gao Z, et al: The
anti-inflamm-aging and hepatoprotective effects of huperzine A in
D-galactose-treated rats. Mech Ageing Dev. 134:89–97. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang ZF, Wang J, Zhang HY and Tang XC:
Huperzine A exhibits anti-inflammatory and neuroprotective effects
in a rat model of transient focal cerebral ischemia. J Neurochem.
106:1594–1603. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manabat C, Han BH, Wendland M, et al:
Reperfusion differentially induces caspase-3 activation in ischemic
core and penumbra after stroke in immature brain. Stroke.
34:207–213. 2003. View Article : Google Scholar
|