Introduction
Atherosclerosis (AS) is a key component of
cardiovascular and cerebrovascular diseases, it is one of the most
prevalent and severe diseases in the world resulting in high rates
of mortality. The pathological mechanism of AS is complex (1). The widely accepted damage-reaction
hypothesis postulates that AS is initiated due to endothelial
damage (2–4). Endothelial barrier function
regulation is known to be associated with cellular signal
transduction mechanisms and previous studies have demonstrated that
endothelial cell (EC) signal transduction was initiated by
increased concentrations of Ca2+ (5,6). G
proteins activate phospholipase C (PLC), which stimulates the
release of Ca2+, which further activates protein kinase
C (PKC) and myosin light chain kinase (MLCK). MLKC phospharylates
MLC, which leads to the rearrangement of EC F-actin and results in
increased EC permeability (7–9). The
quantity of phosphorylated (p)MLC produced depends on MLCK
activity; increased MLCK activity stimulates the contraction of
ECs, enlarging the intracellular space, allowing for the
infiltration of lipids into the subendothelial layer and therefore
accelerating AS pathogenesis (10).
Previous studies have demonstrated that activation
of MLCK by extracellular regulated protein kinases (ERK) was
crucial for cell migration, which was accelerated by growth factors
and integrins (11). Hu et
al (12) demonstrated that
advanced glycation end product (AGE)-induced autophagy via ERK
signaling pathways contributed to vascular smooth muscle cell
(VSMC) proliferation, which was associated with atherosclerosis in
diabetes. Another study indicated that superoxide
anion-mitogen-activated protein kinase kinase (MEK)-ERK-MLCK-MLC
signaling mediated indoxyl sulfate-induced junctional dispersal of
bovine pulmonary artery endothelial cells (13).
Melatonin (MLT) is an endogeneous indole compound
associated with numerous biological activities, including circadian
rhythm regulation, seasonal changes, sleep, reproduction and
cardiovascular functions (14,15).
MLT was reported to have marked dose-dependent anti-oxidative
effects, acting as a free radical scavenger (16,17).
AS is an important disease process associated with the effect of
free radicals and chronic inflammatory processes (18,19).
Based on the data available, MLT was suggested to have
cardioprotective properties via its direct free radical scavenger
activity and indirect antioxidant activity (18,20).
MLT was reported to contribute to the amelioration of the early
phases of AS, including monocyte rolling and invasion of the
subendothelial space as well as inhibition of cyclophilin A
expression (21).
Previous studies have demonstrated that expression
and activity of MLCK were increased in oxidized low density
lipoprotein (ox-LDL)-treated human umbilical vein endothelial cells
(HUVECs); however, MLCK expression and activity were decreased
following treatment with MLT and the ERK1/2 inhibitor PD98059. This
study suggested that ox-LDL-induced MLCK expression and activity
were associated with phosphorylation of ERK (22). The aim of the present study was to
analyze the effects of MLT on EC permeability as well as the
activity and expression of MLCK using rabbit AS models. In
addition, the role of the MAPK signaling pathway in the association
between MLT and MLCK was investigated in order to provide a novel
therapeutic target for the treatment of AS.
Materials and methods
Animals and groups
New Zealand male purebred white rabbits (four weeks
old, 2.0–2.5 kg) were purchased from the Nanjing Rabbit Breeding
Farm (Nanjing, China). Rabbits were housed individually in
screen-bottomed plastic cages and kept in a temperature-controlled
room (25°C) with a standard 12-h light/dark cycle. All experimental
and surgical procedures were approved by the Animal Ethics
Committee in accordance with the National Guidelines for animal
welfare of Anhui Medical University. Rabbits were randomly
distributed into three groups: Group I (n=20) was the normal
control group in which rabbits were fed a standard diet, group II
(n=20) was the AS model group in which rabbits were fed a high-fat
diet (standard diet with 5% lard and 2% cholesterol) for 12 weeks,
and group III (n=20) was the MLT treatment group in which rabbits
were fed the high-fat diet for 12 weeks. From week nine, rabbits in
group III were administered 20 mg/kg MLT daily for four weeks
(Institute of Clinical Pharmacology, Anhui Medical University,
Anhui, China). At the end of the experiment, after 12 weeks all
rabbits were anesthetized with an intravenous injection of 3%
pentobarbital (Shanghai Healing Biotechnology Co., Shanghai,
China), and aortas were then excised and removed. One part of the
aorta was fixed in 4% formalin for further analysis through
immunohistochemical (IHC) and hematoxylin and eosin (HE) staining.
Another part of the aorta was embedded in optimum cutting
temperature compound (OCT) (Solarbio Bioscience and Technology Co.,
Shanghai, China) to produce frozen sections and the remaining
portions of the aortas were stored at −80°C for further use.
Reagents and antibodies
MLT was provided by the Institute of Clinical
Pharmacology, Anhui Medical University (Anhui, China). OCT was
purchased from Solarbio Bioscience and Technology Co. and
γ-32P-adenosine triphosphate (γ-32P-ATP) was
obtained from Yahui Biomedical Engineering Co. (Beijing, China).
The IHC kit (streptavidin-peroxidase 9000) was purchased from
Zhongshan Jinqiao Biotechnology Co. (Beijing, China). Anti-MLCK
antibody (monoclonal antibody produced in mouse; dilution, 1:1,000)
was purchased from Sigma-Aldrich (St. Louis, MO, USA), and other
antibodies were from Cell Signaling Technology, Inc. (Beverly, MA,
USA). All chemicals used were of the highest purity possible.
Endothelial permeability analysis
Endothelial permeability was detected using surface
biotinylation by sulfosuccinimidyl-6-(biotinamido) Hexanoate
(NHS-LC-biotin) (Pierce Chemical Co., Rockford, IL, USA) and
XRITC-avidin (Pierce Chemical Co.). Frozen aorta sections were
incubated with NHSLC-biotin for 30 min. Slides were washed three
times (10 min each) with phosphate-buffered saline (PBS) (Shanghai
Healing Biotechnology Co.) and air dried, slides were then blocked
with 5% (w/v) non-fat milk at 4°C overnight. After washing three
times with blocking buffer, sections were then incubated with
TRITC-avidin, diluted 1:500 in blocking buffer. Following removal
of excess staining solution, slides were washed three times (10 min
each) with PBS and air dried. Aortas were then mounted and
endothelial permeability was evaluated. Images were captured using
an Olympus Provis AX70 system fluorescence microscope (Olympus,
Shinjuku, Tokyo).
MLCK activity assays
Rabbit aortas were homogenized using lysis buffer [1
mM dithiothreitol; 14.5 mM NaCl; 0.5 mM KCl; 0.5 mM
MgSO4; 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; 0.5 mM glucose;
0.1 mM phenylmethylsulfonyl fluoride and 1 mM ethylene glycol
tetraacetic acid (Shanghai Healing Biotechnology Co.)] on ice. The
homogenate was then subjected to three cycles of freeze-thawing to
release MLCK and supernatants were harvested following
centrifugation at 21,920 × g for 30 min. MLCK activity was
calculated using the rate of γ-32P-ATP incorporation
into MLC. MLCK supernatant (5 μl) was added to 50 μl reaction
buffer [20 mM morpholinepropanesulfonic acid, pH 7.4; 2 mM
MgCl2; 0.25 mM CaCl2; 0.2 μM calmodulin
(Shanghai Healing Biotechnology Co.); 2 mM γ-32P-ATP;
and 5 μM recombinant MLC (Yahui Biomedical Engineering Co.)] which
was incubated at 25°C for 20 min. The reaction was terminated by
pipetting aliquots (40 μl) onto Whatman filter paper (GE
Healthcare, Little Chalfont, UK), allowing the paper to dry
naturally, rinsing with 75 mM H3PO4 (Shanghai
Healing Biotechnology Co.) three times and then washing several
times (5 min per wash) with 95% (v/v) ethanol (Shanghai Healing
Biotechnology Co.). Filter papers were then placed in scintillation
fluid and measured using a scintillation counter (Tri-Carb.
PerkinElmer, Waltham MA, USA). Samples without a substrate were
used as blank controls and all experiments were conducted three
times.
Immunohistochemical (IHC) and hematoxylin
and eosin (HE) staining
Slices from rabbit aortas were prepared and analyzed
using IHC and HE staining as previously described (23,4).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) assays
Total RNA from mouse tissues was extracted using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) and 1 μg isolated total RNA was converted into
complementary DNA (cDNA) using a First-Strand cDNA Synthesis kit
(Toyobo Co., Ltd., Osaka, Japan). Power SYBR green master mix
(Applied Biosystems, Foster City, CA, USA) was added to cDNA
samples, which were then subjected to qPCR using the
StepOneTM Real time PCR system (Applied Biosystems).
Relative MLCK messenger RNA (mRNA) levels were normalized to
β-actin, the reference gene. The following primers were used: MLCK
mRNA forward, 5′-GAG AGA CTG GAA ACC GAA GAA G-3′ and reverse,
5′-CAG GTC ACG AAT GGT CTT AGA G-3′; and β-actin forward, 5′-CCC
AGC ACC ATG AAG ATC AA-3′ and reverse, 5′-CTG CTT GCT GAT CCA CAT
CT-3′.
Western blot analysis
Aortas were homogenized in 1× SDS lysis buffer
containing 50 mM Tris-HCl (pH 6.8), 10% glycerol and 2% SDS
(Shanghai Healing Biotechnology Co.). The homogenates were boiled
for 10 min and then centrifuged at 16,060 × g for 20 min at room
temperature. The total protein concentration of each sample was
measured using a MicroBCATM Protein Assay Reagent kit
(Pierce Biotechnology, Inc.). Samples were separated using 10%
SDS-PAGE and then transferred to polyvinylidene difluoride
membranes (GE Healthcare, Piscataway, NJ, USA). Membranes were
blocked using 5% (w/v) bovine serum albumin (Amresco, Solon, OH,
USA) for 2 h, followed by a 4°C overnight incubation with primary
antibodies. Antibodies were purchased from Cell Signaling
Technology, Inc. except anti-MLCK antibody. Anti-p38 and anti-JNK
antibodies were polyclonal antibodies produced in rabbit.
Anti-pp38, anti-pJNK and anti-β-actin antibodies were monoclonal
antibodies produced in mouse. The dilution of anti-p38 and anti-JNK
antibodies was 1:1,000. The dilution of anti-pp38 and anti-pJNK
antibodies was 1:500. The dilution of anti-β-actin antibody was
1:5,000. Primary antibodies were detected using corresponding
horseradish peroxidase-conjugated secondary antibodies (Zhongshan
Jinqiao) coupled with enhanced chemiluminescence reagents (Engreen
Biosystems, Beijing, China). Three independent experiments were
conducted to confirm the reproducibility of the results.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analyses were conducted using multiple
comparisons between groups assuming population variances were equal
and with normal distributions. Comparisons between two groups were
based on least significant differences. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Atheromatous plaques and endothelial
permeability are reduced in MLT-treated AS model rabbits
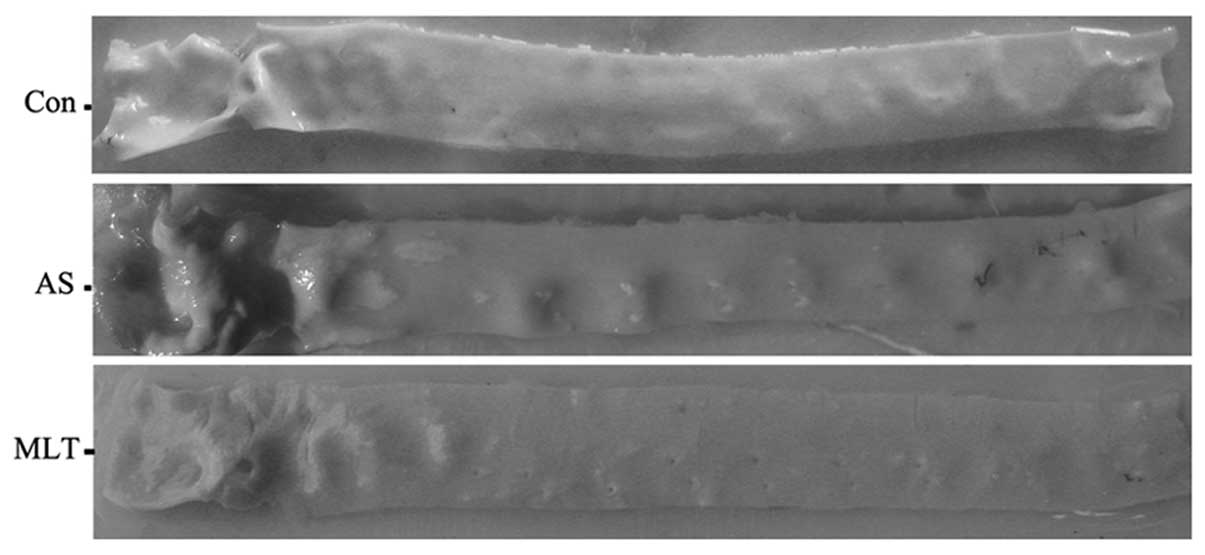
As shown in Fig. 1,
MLT treatment attenuated the number and area of atheromatous
plaques in the aortic walls of rabbits on high cholesterol rabbits
compared to those of the AS model rabbits. In order to further
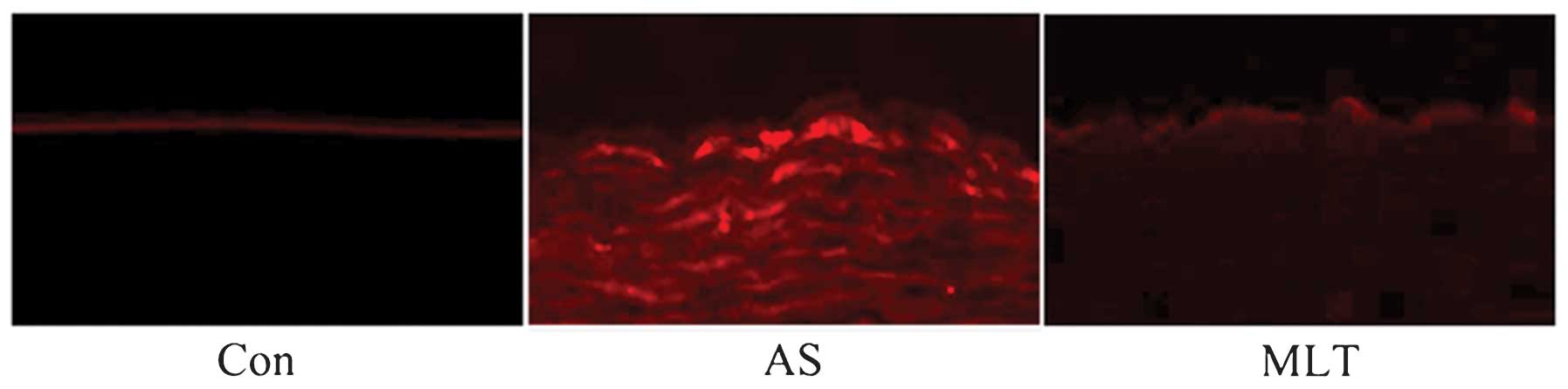
assess the cause of atheromatous plaque formation, endothelial
permeability was determined using rhodamine phalloidin fluorescence
staining. The results demonstrated that endothelial permeability
was markedly increased in the AS group compared with that of the
control group; in addition, the hyperpermeability of AS rabbits was
reduced following treatment with MLT (Fig. 2).
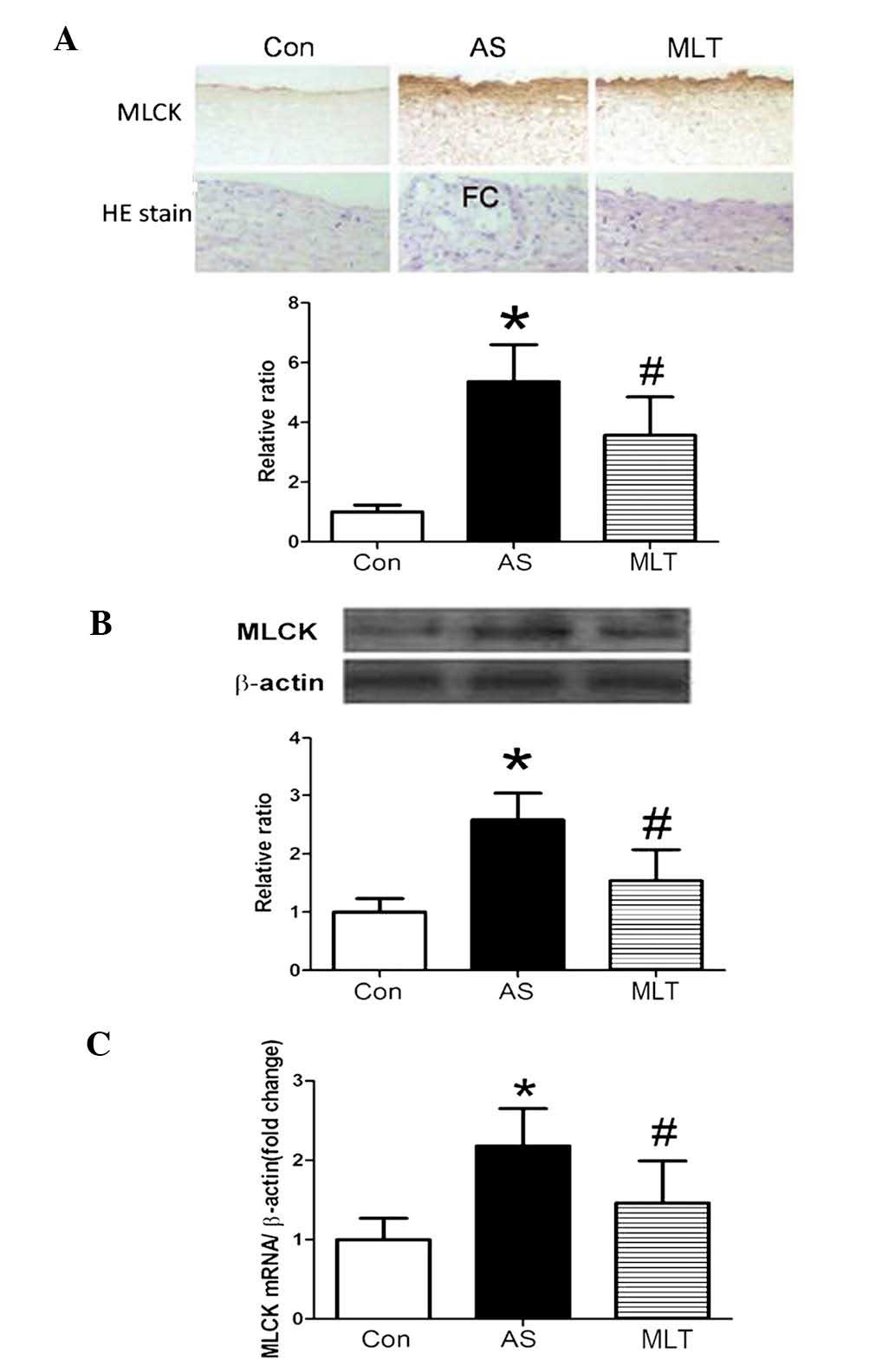
MLT treatment attenuated MLCK protein and
mRNA expression levels, morphological abnormalities and MLCK
activity in AS aortas
MLCK protein and mRNA expression levels were
demonstrated to be significantly higher in the AS model group
compared with those of the control group; however, the MLT
treatment group expressed significantly decreased levels of MLCK
protein and mRNA compared to those of the AS group (Fig. 3). HE staining showed that normal
aortas had tight junctions between cells and a complete tunica
intima; however, damage to the tunica intima and the formation of
fibrous caps on the surface of plaques were observed in the AS
group. Furthermore, following treatment with MLT, these
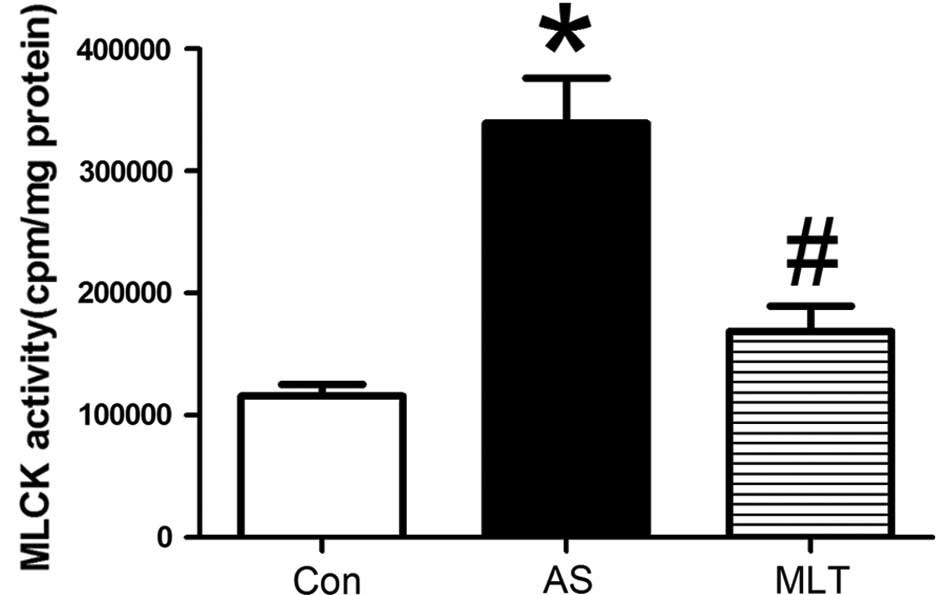
abnormalities in the AS group were less evident (Fig. 3A). As shown in Fig. 4, MLCK activity was significantly
increased in the AS group compared to that of the control group; by
contrast, following MLT treatment, MLCK activity was significantly
reduced compared to that of the AS group (P<0.05) (Fig. 4).
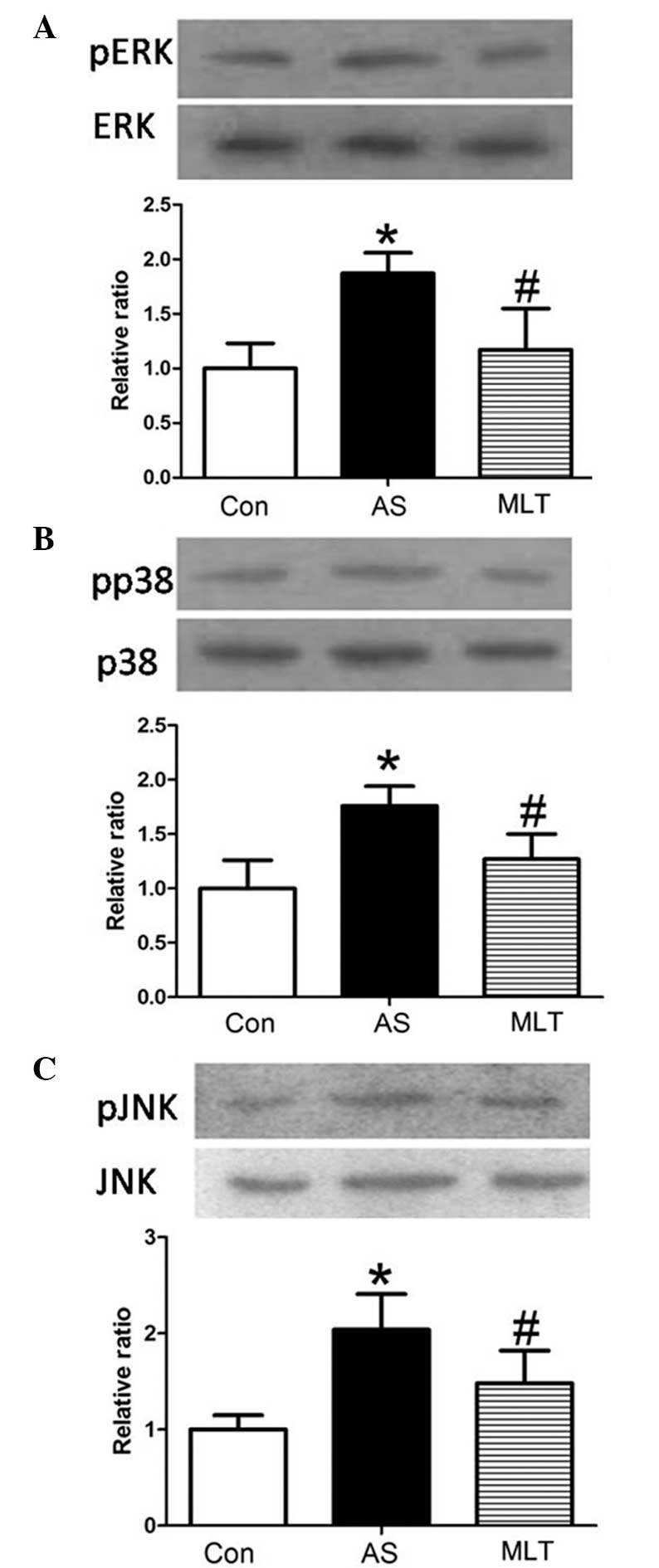
MLT treatment decreases levels of MLCK,
p-extracellular regulated protein kinase (pERK), pp38 and p-c-Jun
N-terminal kinase (pJNK)
Western blot analysis of rabbit aortas revealed that
protein expression levels of pERK, pp38 and pJNK were markedly
increased in the AS group compared with those of the control group
(P<0.05), whereas MLT treatment significantly decreased the
levels of these phosphorylated proteins compared with those of the
AS group (P<0.05) (Fig. 5).
Changes in levels of MLCK, pERK, pp38 and pJNK were all comparable,
which therefore indicated that hyper-permeability of the aortic
endothelium was associated with the expression and activity of
MLCK, which may be attenuated by MLT via the MAPK signaling
pathway.
Discussion
Vascular endothelial cells are the first
permeability barrier between vascular tissue and blood, which were
reported to be important for maintaining normal biological
homeostasis (24). It is widely
accepted that endothelial injury in arteries initiates the
formation of AS lesions. MLCK is a protein kinase that has been
reported to have an important role in the reorganization of the
cytoskeleton, leading to the disruption of vascular barrier
integrity (25); in addition, the
mechanism of MLCK action was reported to proceed via the catalysis
of MLC phosphorylation, which may lead to cytoskeletal
rearrangements (26). Endothelial
cell concentric contraction and gap formation are followed by
cytoskeletal changes, which facillitates the infiltration of lipids
into the arterial intima and accumulation in arterial walls,
ultimately leading to atheromatous plaque formation (27).
The results of the present study demonstrated that
MLT anti-AS effects were associated with the expression and
activity of MLCK regulated via MAPK phosphorylation. A rabbit model
of AS was established through a sustained high-fat diet.
Atheromatous plaques were found to be deposited on the arterial
walls of these rabbits. The lipid infiltration and endothelial
injury hypothesis are widely used to explain the pathogenesis of AS
(28). In the present study,
Rhodamine phalloidin fluorescence staining demonstrated that
endothelial permeability was significantly increased in AS.
Permeability was previously reported to be regulated via complex
interactions between signaling molecules and structural proteins in
the endothelium, including adhesive cell-cell and cell-matrix
contacts, which occurred between junctional proteins and focal
adhesion complexes in the cytoskeleton (8). At the beginning of the present study
it was hypothesized that the change in arterial intima integrity
may be associated with increased activity and expression of MLCK.
The results of the present study confirmed that MLCK expression and
activity were significantly increased in the AS group compared with
those of the controls. These results therefore indicated that MLCK
activity and expression may have a crucial role in AS.
Previous studies have demonstrated that following
ERK pathway suppression, MLCK and downstream pMLC expression levels
were decreased in leptomeniges carcinomatosis-MCF-7 cells,
indicating that ERK1/2 may regulate MLCK and its phosphorylation
(29). In addition, a previous
study revealed that the expression and activity of MLCK induced by
ox-LDLs were associated with ERK phosphorylation (22). ERK is a member of the MAPK family,
which also includes the highly conserved Ser/Thr protein kinase,
p38 and JNK. Studies have demonstrated that p38 MAPK had an
important role in regulating burn-induced intestinal permeability
via the activation of MLCK; in addition, p38 inhibition was
reported to be a potential therapeutic target for the attenuation
of the breakdown of the intestinal barrier through the prevention
of burn-induced modifications of tight junction proteins (30). Phosphorylation cascades were
reported to trigger biochemical and conformational changes in the
barrier structure of the endothelium, which may lead to the
induction of unwanted paracellular pathways (8). The results of the present study
demonstrated that phosphorylation levels of ERK, p38 and JNK were
markedly increased in the AS group; in addition, the changes in
expression of pERK, pp38, pJNK and MLCK were comparable. This
therefore indicated the use of protein kinase inhibitors as
potential therapeutic agents for the prevention and treatment of
endothelial hyperpermeability and vascular barrier dysfunction.
MLT is an endogeneous indole compound, which is
synthesized and secreted by the pineal body in vertebrates.
Previous studies have shown that MLT had potent antioxidant
properties that may prevent the development of AS (31). It has also been reported that the
expression and activity of MLCK, induced by ox-LDL, was
significantly decreased by MLT. The results of the present study
demonstrated that MLT attenuated the formation of atheromatous
plaques and reversed the increase of endothelial hyperpermeability
as well as MLCK expression and activity in AS model rabbits. This
therefore indicated that AS may be associated with MLCK expression
and activity, which may be reduced by MLT via the MAPK signal
transduction pathway.
In conclusion, the results of the present study
provided evidence from animal models that changes to the
endothelial cytoskeleton affected endothelial function.
MLCK-mediated MLC phosphorylation was suggested to have an
important role in the development of AS. MLT served as a switch for
modulating the activity and expression of MLCK via the MAPK pathway
involving its downstream signaling molecules ERK, p38 and JNK.
However, further studies are required to localize components of the
pathways and to examine their interactions and mobility in order to
elucidate the exact regulatory mechanisms involved in the effect of
MAPK cascades on MLCK.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81070232, 81270372 and
30971226), the Key Project of Chinese Ministry of Education (no.
212077) and the Grants for Scientific Research of BSKY from Anhui
Medical University. (nos. XJ201107 and XJ2008015).
References
|
1
|
Daffu G, del Pozo CH, O’Shea KM, et al:
Radical roles for RAGE in the pathogenesis of oxidative stress in
cardiovascular diseases and beyond. Int J Mol Sci. 14:19891–19910.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tengattini S, Reiter RJ, Tan DX, et al:
Cardiovascular diseases: protective effects of melatonin. J Pineal
Res. 44:16–25. 2008.PubMed/NCBI
|
|
3
|
Liao JK: Linking endothelial dysfunction
with endothelial cell activation. J Clin Invest. 123:540–541. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan DX, Manchester LC, Liu X, et al:
Mitochondria and chloroplasts as the original sites of melatonin
synthesis: a hypothesis related to melatonin’s primary function and
evolution in eukaryotes. J Pineal Res. 54:127–138. 2013.PubMed/NCBI
|
|
5
|
Chen HI, Huang YC, Su WH and Jen CJ:
Endothelial calcium signaling in rabbit arteries and its local
alterations in early-stage atherosclerosis. J Biomed Sci.
14:145–153. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nausch LW, Bonev AD, Heppner TJ, et al:
Sympathetic nerve stimulation induces local endothelial
Ca2+ signals to oppose vasoconstriction of mouse
mesenteric arteries. Am J Physiol Heart Circ Physiol.
302:H594–H602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Turner JR, Angle JM, Black ED, et al:
PKC-dependent regulation of transepithelial resistance: roles of
MLC and MLC kinase. Am J Physiol. 277(3 Pt 1): C554–C562.
1999.PubMed/NCBI
|
|
8
|
Yuan SY: Protein kinase signaling in the
modulation of microvascular permeability. Vascul Pharmacol.
39:213–223. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rashid G, Bernheim J, Green J and
Benchetrit S: Parathyroid hormone stimulates endothelial expression
of atherosclerotic parameters through protein kinase pathways. Am J
Physiol Renal Physiol. 292:F1215–F1218. 2007. View Article : Google Scholar
|
|
10
|
Shen Q, Rigor RR, Pivetti CD, et al:
Myosin light chain kinase in microvascular endothelial barrier
function. Cardiovasc Res. 87:272–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong T and Grabel LB: Migration of F9
parietal endoderm cells is regulated by the ERK pathway. J Cell
Biochem. 97:1339–1349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu P, Lai D, Lu P, et al: ERK and Akt
signaling pathways are involved in advanced glycation end
product-induced autophagy in rat vascular smooth muscle cells. Int
J Mol Med. 29:613–618. 2012.PubMed/NCBI
|
|
13
|
Peng YS, Lin YT, Chen Y, et al: Effects of
indoxyl sulfate on adherens junctions of endothelial cells and the
underlying signaling mechanism. J Cell Biochem. 113:1034–1043.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Korkmaz A, Tamura H, Manchester LC, et al:
Combination of melatonin and a peroxisome proliferator-activated
receptor-gamma agonist induces apoptosis in a breast cancer cell
line. J Pineal Res. 46:115–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu C, Wu A, Zhu H, et al: Melatonin is
involved in the apoptosis and necrosis of pancreatic cancer cell
line SW-1990 via modulating of Bcl-2/Bax balance. Biomed
Pharmacother. 67:133–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reiter RJ and Korkmaz A: Clinical aspects
of melatonin. Saudi Med J. 29:1537–1547. 2008.
|
|
17
|
Wilhelm EA, Jesse CR, Bortolatto CF and
Nogueira CW: Correlations between behavioural and oxidative
parameters in a rat quinolinic acid model of Huntington’s disease:
protective effect of melatonin. Eur J Pharmacol. 701:65–72.
2013.PubMed/NCBI
|
|
18
|
Galano A, Tan DX and Reiter RJ: Melatonin
as a natural ally against oxidative stress: a physicochemical
examination. J Pineal Res. 51:1–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Broncel M, Koziróg-Kołacińska M and
Chojnowska-Jezierska J: Melatonin in the treatment of
atherosclerosis. Pol Merkur Lekarski. 23:124–127. 2007.(In
Polish).
|
|
20
|
Rodella LF, Favero G, Foglio E, et al:
Vascular endothelial cells and dysfunctions: role of melatonin.
Front Biosci (Elite Ed). 5:119–129. 2013.PubMed/NCBI
|
|
21
|
Maldonado MD, Murillo-Cabezas F, Terron
MP, et al: The potential of melatonin in reducing
morbidity-mortality after craniocerebral trauma. J Pineal Res.
42:1–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu HQ, Cheng XW, Xiao LL, et al:
Melatonin prevents oxidized low-density lipoprotein-induced
increase of myosin light chain kinase activation and expression in
HUVEC through ERK/MAPK signal transduction. J Pineal Res.
45:328–334. 2008. View Article : Google Scholar
|
|
23
|
Zhang N, Liang J, Tian Y, et al: A novel
testis-specific GTPase serves as a link to proteasome biogenesis:
functional characterization of RhoS/RSA-14–44 in spermatogenesis.
Mol Biol Cell. 21:4312–4324. 2010.PubMed/NCBI
|
|
24
|
Hirase T and Node K: Endothelial
dysfunction as a cellular mechanism for vascular failure. Am J
Physiol Heart Circ Physiol. 302:H499–H505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rossi JL, Ralay Ranaivo H, Patel F, et al:
Albumin causes increased myosin light chain kinase expression in
astrocytes via p38 mitogen-activated protein kinase. J Neurosci
Res. 89:852–861. 2011. View Article : Google Scholar
|
|
26
|
Tinsley JH, Teasdale NR and Yuan SY:
Myosin light chain phosphorylation and pulmonary endothelial cell
hyperpermeability in burns. Am J Physiol Lung Cell Mol Physiol.
286:L841–L847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anssari-Benam A and Korakianitis T:
Atherosclerotic plaques:is endothelial shear stress the only
factor? Med Hypotheses. 81:235–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahashi M: Inflammatory cytokines in the
pathogenesis of atherosclerosis. Nihon Rinsho. 69:30–33. 2011.(In
Japanese).
|
|
29
|
You JZ, Wang HB, Yang ZW, et al: Signal
transduction pathways involved in promotion of proliferation and
migration via p-ERK in high metastasis potential breast cancer
cells. Chin J Biochem Mol Biol. 22:1007–1013. 2006.
|
|
30
|
Costantini TW, Peterson CY, Kroll L, et
al: Role of p38 MAPK in burn-induced intestinal barrier breakdown.
J Surg Res. 156:64–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sewwrynek E: Melatonin and the
cardiovascular system. Neuro Endocrinol Lett. 23:79–83. 2002.
|