Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune
disease that affects multiple tissues and organs, although synovial
joints are the main site of involvement (1). The basic pathology of RA has been
identified as a disorder of inflammation in the rheumatoid
synovium, in which the predominant cell type is the fibroblast-like
synovial (FLS) cell (2,3). RA-FLS cells in the synovium are
aggressively proliferative and invasive, and are known to attack
cartilage, resulting in joint damage. Thus, the phenotype of RA-FLS
cells is similar in a number of ways to that of tumor cells
(3).
Cysteine-rich angiogenic inducer 61 (Cyr61) is a
member of the CCN (also termed CTGF, Cyr61/cef10 and nov) gene
family in which the expression can be induced by growth factors,
cytokines, steroid hormones and certain drugs (4–6).
Recently, Cyr61 has been found to be associated with a number of
diseases related to chronic inflammation, such as RA,
atherosclerosis, diabetes-related nephropathy and retinopathy, and
several types of cancer (7). The
induction of Cyr61 expression in RA is well-documented. For
example, it has been shown that sirtuin-1/FoxO3a signaling is
crucial for the induction of Cyr61 expression in RA synovial
fibroblasts (8), and also that p53
is involved in the post-transcriptional regulation of Cyr61
expression via microRNA-22 (miR-22) (9). With regard to the role of Cyr61 in
RA, it has been demonstrated that Cyr61 promotes neutrophil
infiltration via upregulation of interleukin (IL)-8 production in
FLS cells (10). In addition,
Cyr61 has been shown to promote T helper 17 cell (Th17) development
in RA via upregulation of IL-6 production by FLS cells (11). Furthermore, Zhang et al
(12) demonstrated that Cyr61 is
critical in IL-17-mediated proliferation of RA-FLS cells and may
contribute to hyperplasia of the synovial lining cells and eventual
joint destruction in patients with RA. Therefore, Cyr61 appears to
be a crucial component of a vicious cycle formed by the interaction
between infiltrating neutrophils, proliferating FLS cells and
activated Th17 cells, in the development of RA (10).
Despite these recent advances, little is known about
the role of Cyr61 in the phenotype of RA-FLS cells. The present
study therefore explored the role of Cyr61 in FLS cell activities,
including cell proliferation, apoptosis and cell invasion.
Materials and methods
Synovial specimens and genechip
microarray
Synovial tissue specimens were obtained from four
patients with RA and three patients with osteoarthritis (OA) who
underwent arthroscopic surgery of the knee joint. All samples were
collected from Guangzhou General Hospital of Guangzhou Military
Command (Guangzhou, China). The tissue samples were stored in a
liquid nitrogen tank prior to the experiments. Total RNA was
extracted from all collected samples in order to synthesize cDNA.
Subsequently, cRNA was amplified and labeled with biotin. A human
genome-wide analysis was performed using the human genome U133 Plus
2.0 in a Genechip microarray hybridization oven 640 (Affymetrix
Inc., Santa Clara, CA, USA), as described previously (13). All study protocols and consent
forms were approved by the Institutional Medical Ethics Review
Board of Guangzhou General Hospital of Guangzhou Military
Command.
Cell lines and cell culture
Normal FLS, RA-FLS and HEK293T cell lines were
ordered from Cell Applications Inc. (San Diego, CA, USA). All cells
were cultured in Dulbecco’s modified Eagle’s medium supplemented
with 10% fetal bovine serum (FBS) at 37°C in a humidified
atmosphere containing 5% CO2.
Transient transfection of small
interfering RNA (siRNA)
FLS cells were seeded at a uniform density into a
6-well culture plate and then incubated overnight to allow cells to
attach to the plate. Cyr61-siRNA and non-target siRNA (control
siRNA) were ordered from GenePharma Company (Shanghai, China).
siRNA was transfected into the cells with the aid of Lipofectamine™
RNAiMAX reagents, according to the manufacturer’s instructions
(Invitrogen, Carlsbad, CA, USA). Following 4 h transfection, the
cells were recovered using fresh culture medium.
Construction and production of
lentivirus
The full-length human Cyr61 gene was cloned into the
pCDH-CMV-MCS-EF1-copGFP lentiviral vector (System Biosciences,
Mountain View, CA, USA) at the EcoRI and BamHI sites
using the following polymerase chain reaction (PCR) primers:
5′-TAGAGCTAGCGAATTCGCCACCATGAGCTCCCGCATCGC-3′, for CYR61-EcoRI-F
and 5′-TCGCGGCCGCGGATCCTTAGTCCCTAA ATTTGGAATGTC-3′, for
CYR61BamHI-R. Lentiviruses were produced in HEK293T cells using the
pCDH-CMV-MCS-EF1-copGFP lentiviral vector encased in viral capsid
encoded by three packaging plasmids (System Biosciences). The
supernatant containing the viruses was collected at 48 h
post-transfection and the viruses were concentrated as described
previously (14). Normal FLS cells
were infected with the lentiviruses at a multiplicity of infection
(MOI) of five, in the presence of 8 μg/ml polybrene (Sigma-Aldrich,
St. Louis, MO, USA).
MTS assays and apoptosis analysis
FLS cell proliferation was determined by CellTiter
96® AQueous One Solution Cell Proliferation assay (MTS
assay; Promega Corporation, Madison, WI, USA), which was performed
as previously described (15).
Apoptotic cells were measured by a DeadEnd™ Fluorometric terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling
(TUNEL system; Promega Corporation). Briefly, cells were seeded
onto glass cover slides previously coated with 1% gelatin in 24
wells plates and cultivated to reach 80% confluency. Following
siRNA or lentiviral vector transfection, culture medium was removed
and fixation was performed with 4% neutral formalin in
phosphate-buffered saline (PBS) for 25 min at 4°C. After washing
twice with PBS, the cells were maintained in 70% ethanol at −20°C
overnight. Cells were subsequently saturated and permeabilized with
0.2% Triton X-100 in PBS for 5 min. Subsequently, 100 μl buffer
including 45 μl equilibration buffer, 5 μl nucleotide mix and 1 μl
recombinant terminal deoxynucleotidyl transferase enzyme was added
for 10 min. After washing for 15 min with saline sodium citrate
buffer and twice with PBS, cells were incubated for DAPI staining
at room temperature for 15 min in darkness. Acquisition of the
images was performed with a fluorescence microscope (DMI6000B;
Leica Geosystems, St. Gallen, Switzerland). Cells on each slide
were counted in at least five fields, and the apoptosis ratio was
taken as the number of positive cells divided by the number of
DAPI-stained cells.
Transwell in vitro invasion assays
Cell invasion experiments were performed using
BioCoat Matrigel Invasion Chambers with 8 μm pores (BD Biosciences,
Bedford, MA, USA), according to the manufacturer’s instructions.
Briefly, cells were seeded at 2.5×104 cells per well in
serum-free medium overnight, and subsequently added to the upper
chamber of a 24-well transwell plate. The lower chamber contained
fresh culture media with 20% FBS as a chemoattractant. Cells were
allowed to invade for 24 h at 37°C in the 5% CO2
atmosphere, and the chambers were then washed with PBS. Cells that
did not invade through the membrane were removed, while the
invading cells on the lower surface of the membrane were fixed with
cold methanol, stained with 0.2% crystal violet and examined. The
number of invading cells in each chamber was counted in at least
five fields under a light microscope (OLYMPUS CKX41; Olympus Corp.,
Tokyo, Japan).
Enzyme-linked immunosorbent assay
(ELISA)
FLS cells were seeded at a uniform density into
6-well culture plates and incubated overnight. Following siRNA or
lentiviral vector transfection, the culture supernatant was
collected, centrifuged (2,000 xg for 10 min) and analyzed for the
secretion of Cyr61, matrix metalloproteinase (MMP)-1, MMP-3, MMP-10
and MMP-13 with ELISA kits, according to the manufacturer’s
instructions (Shanghai Westang Bio-Tech Co., Ltd., Shanghai,
China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen, Grand Island, NY, USA) according to the manufacturer’s
instructions. cDNA was synthesized from 1 μg total RNA using a high
capacity cDNA reverse transcription kit (GoScriptTM
Reverse Transcription System; Promega Corp.). Aliquots of cDNA were
used as the template for qPCR reactions containing gene-specific
primers and SYBR Green qPCR SuperMix (Invitrogen). The following
primer sequences were used: Forward: 5′-GGAAATCGTGCGTGACATT-3′ and
reverse: 5′-CAGGCAGCTCGTAGCTCTT-3′ for β-actin; and forward:
5′-CTGAAGCGGCTCCCTGTTTT-3′ and reverse: 5′-GCACCTCACAAATCCGGGTT-3′
for human Cyr61. RT-qPCR was performed using the CFX96 Touch Deep
Well™ Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.,
Berkeley, CA, USA) using the following steps: 10 min at 95°C,
followed by 40 cycles for 15 sec at 95°C and 15 sec at 60°C. The
expression of target genes in the treatment and control groups was
normalized using the reference gene β-actin, and the fold change in
the expression of each target gene was calculated using the
2−ΔΔCT method.
Western blot analysis
Cells were washed with ice-cold PBS, collected and
homogenized with radioimmunoprecipitation assay lysis buffer
containing 1X PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1%
SDS, and 1 mM phenylmethylsulfonyl fluoride (Beyotime, Beijing,
China). Total protein was extracted and measured by the Bio-Rad
protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Equal
quantities of protein (20 μg) were boiled for 10 min, separated by
SDS-PAGE, and transferred to polyvinylidene difluoride membranes
(Millipore, Billerica, MA, USA). The following antibodies were used
for the western blot analysis: Mouse monoclonal antibody against
Cyr61 (1:600) obtained from Santa Cruz Biotechnology (Santa Cruz,
CA, USA) and rabbit polyclonal antibody against GAPDH (1:1,000)
obtained from Cell Signaling Technology (Beverly, MA, USA). The
secondary antibodies, affinity purified goat anti-rabbit
immunoglobulin (Ig)G (H&L) and horse anti-mouse IgG (H&L)
antibodies, conjugated to horseradish peroxidase, were purchased
from Cell Signaling Technology (Beverly, MA, USA). Specific
proteins were detected by using an enhanced chemiluminescence
detection system (Clarity Western ECL Substrate; Bio-Rad
Laboratories, Inc., Berkeley, CA, USA).
Statistical analysis
For the human genome-wide analysis of synovial
tissues, raw data processing, normalization and data analysis were
performed with GeneSpring 7.31 software (Agilent, Santa Clara, CA,
USA). Welch’s t-test analysis was subsequently used to select genes
in which the expression varied at least 2.0-fold between RA and OA,
with P<0.05.
For the other experiments, Student’s t-test was used
to analyze the difference between two groups, whilst one-way
analysis of variance followed by Dunnett’s test was employed for
comparisons between three or more groups. Data are presented as the
mean ± standard deviation for all statistical tests. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS 16.0 statistical
software (SPSS Inc. Chicago, IL, USA).
Results
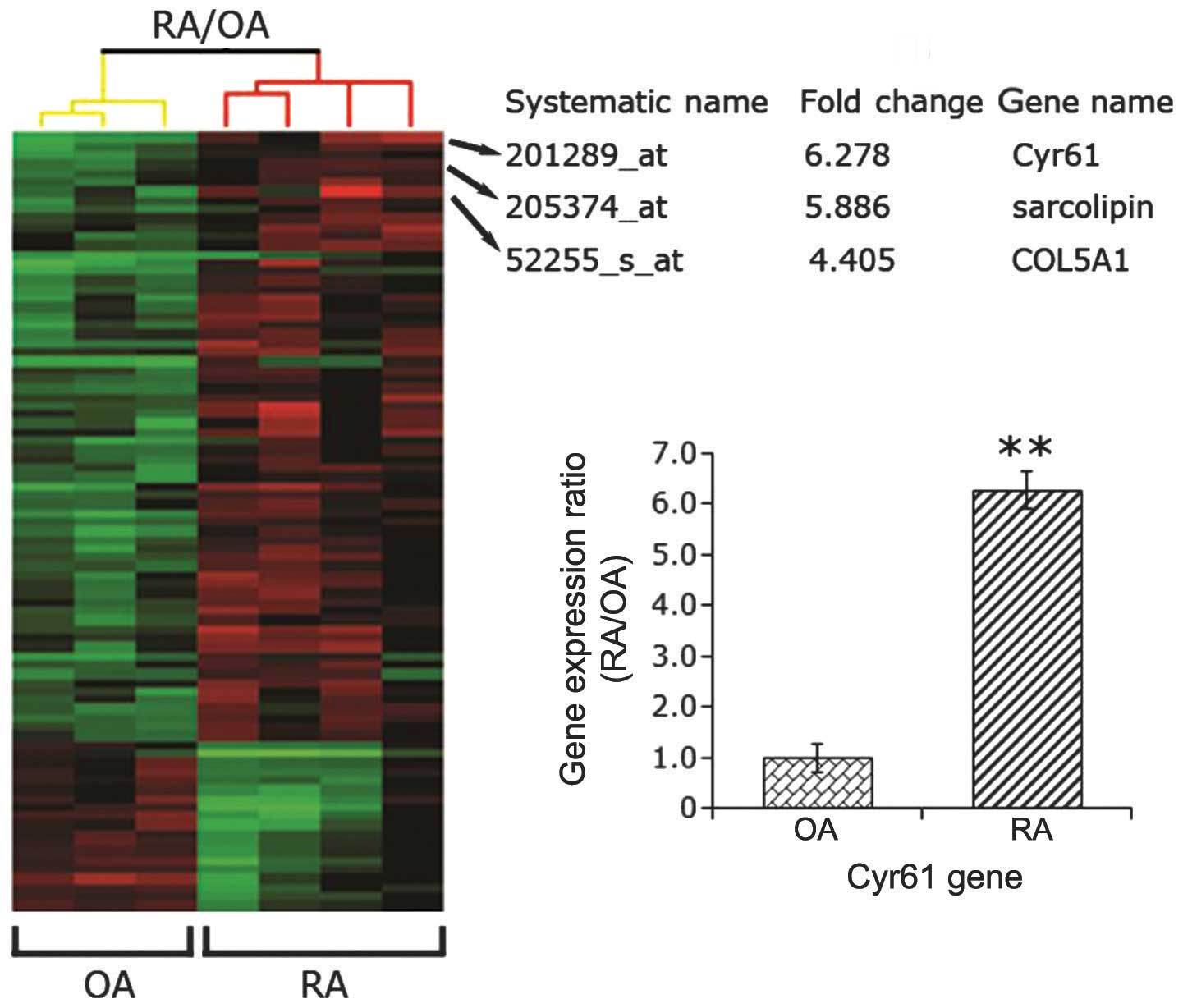
Cyr61 is highly expressed in RA synovial
tissues
A previous study (12) using immunohistochemistry, RT-qPCR
and western blot analysis found that Cyr61 is overexpressed in
synovial tissue and FLS cells from RA patients compared with
samples from disease-free control subjects. To confirm this result,
four synovial specimens from patients with RA and three samples
from patients with OA were collected. Using a human genome-wide
analysis, the present study found that the gene expression of Cyr61
in samples from patients with RA was 6.28-fold that of samples from
patients with OA (P<0.01; Fig.
1). In accordance with this result, Zhang et al
(12) found that the level of
Cyr61 was higher in synovial fluid samples from RA patients than
those from normal controls. These findings suggest that Cyr61 may
be involved in the pathogenesis of RA.
Cyr61 promotes FLS cell
proliferation
Due to the fact that Cyr61 was found to be
overexpressed in synovial tissues, its involvement in the
pathophysiological events associated with RA was investigated.
RA-FLS cells were transfected with Cyr61-siRNA or control-siRNA,
and effects on in vitro proliferation, apoptosis and
invasion were determined. In vitro experiments were
performed in RA-FLS cells without treatment and normal FLS cells.
In addition, normal FLS cells were transduced with lentivirus
vectors encoding Cyr61 cDNA or control lentivirus vectors.
siRNA-mediated downregulation of Cyr61 resulted in a >80%
reduction in Cyr61 mRNA expression in RA-FLS cells compared with
normal FLS cells (P<0.01). Conversely, transduction of
lentivirus vectors encoding Cyr61 led to a 304.43±24.14 fold
increase in Cyr61 mRNA expression in normal FLS cells compared with
normal FLS cells transfected with a control lentivirus
(P<0.0001; Fig. 2A). The
transfection efficacies were confirmed by western blot analysis
(Fig. 2B). To further examine
whether Cyr61 activity was affected by manipulation of its
expression, the levels of Cyr61 secreted into culture media were
assessed by ELISA assays. As hypothesized, the culture medium
collected from RA-FLS cells contained higher levels of Cyr61 than
normal FLS cells (P<0.0001; Fig.
2C). Levels of Cyr61 secretion in culture media were consistent
with the status of Cyr61 expression; the level of secreted Cyr61
was elevated in normal FLS cells transfected with Cyr61 cDNA,
whilst it was significantly decreased in RA-FLS cells transfected
with Cyr61-siRNA (P<0.0001 and P<0.05, respectively; Fig. 2C).
 | Figure 2Role of Cyr61 in FLS cell
proliferation. (A) Reverse transcription-quantitative polymerase
chain reaction evaluation for the efficacy of Cyr61 siRNA or cDNA
transfection in FLS cells. The mRNA level of Cyr61 in normal FLS
cells was used as a control. **P<0.01. (B) Western
blotting evaluation for the efficacy of Cyr61 siRNA or cDNA
transfection in FLS cells. Cyr61 (40 kDa) was detected using a
mouse IgG1 monoclonal antibody specific for Cyr61. GAPDH served as
the loading control. (C) Detection of levels of Cyr61 secreted into
the culture medium using enzyme-linked imunosorbent assays.
*P<0.05 and **P<0.01. (D) MTS assay
detection of cell proliferation. Data are presented as the mean ±
standard deviation of three independent experiments conducted in
triplicate. *P<0.05 and **P<0.01,
compared with RA-NC; #P<0.05 and
##P<0.01, compared with NOR-NC;
^P<0.05, compared with NOR-FLA. RA, rheumatoid
arthritis; FLS, fibroblast-like synoviocytes; siRNA, small
interfering RNA; NOR-FLS, normal FLS cells; NOR-CYR61, normal FLS
cells transduced with lentivirus vector encoding Cyr61 cDNA;
NOR-NC, normal FLS cells transduced with control lentivirus vector;
RA-NC, RA-FLS cells transfected with control siRNA; RA-siRNA,
RA-FLS cells transfected with Cyr61-siRNA. |
Cell proliferation was determined at the time points
after transfection indicated in Fig.
2D. The proliferation of RA-FLS cells was significantly greater
than that of normal FLS cells. The MTS assays showed that the
proliferation of RA-FLS cells for three, five and seven days was
105.9±0.2, 123.8±1.5 and 106.2±5.6% that of the normal FLS cells,
respectively (Fig. 2D). The
proliferative rates of RA-FLS cells transfected with siRNA were
74.3±0.9 and 56.2±7.4% those of RA-FLS cells transfected with
control siRNA at five and seven days respectively, suggesting that
the RA-FLS cell proliferative ability was significantly impaired by
the introduction of Cyr61-siRNA. However, normal FLS cells,
overexpressing Cyr61 showed enhanced cell proliferation at five and
seven days (130.3±0.6 and 143.3±0.3% that of FLS cells transduced
with control lentivirus vector, respectively; Fig. 2D).
Cyr61 suppresses apoptosis in FLS
cells
The data obtained by the MTS assay implied that
Cyr61 may have a pro-survival effect via promotion of FLS cell
proliferation. Subsequent experiments were conducted to examine
whether the status of Cyr61 expression in FLS cells affects
apoptosis. Analysis of the proportion of apoptotic cells in each
group was performed using the fluorometric TUNEL method. As shown
in Fig. 3A, knockdown of Cyr61 in
RA-FLS cells by siRNA led to increased cell apoptosis compared with
RA-FLS cells without transfection (2.8-fold) or RA-FLS cells
transfected with control siRNA (2.7-fold; P<0.0001 and
P<0.01, respectively; Fig. 3B).
The proportion of apoptotic RA-FLS cells was significantly less
than that of normal FLS cells (P<0.05; Fig. 3B). Transduction of normal FLS cells
with lentiviral vectors encoding Cyr61 cDNA decreased the fraction
of apoptotic cells by 86.5±0.01% compared with normal FLS cells,
and by 89.8±0.02% compared with normal FLS cells transduced with
control lentiviral vectors (P<0.05 and P<0.01, respectively;
Fig. 3B).
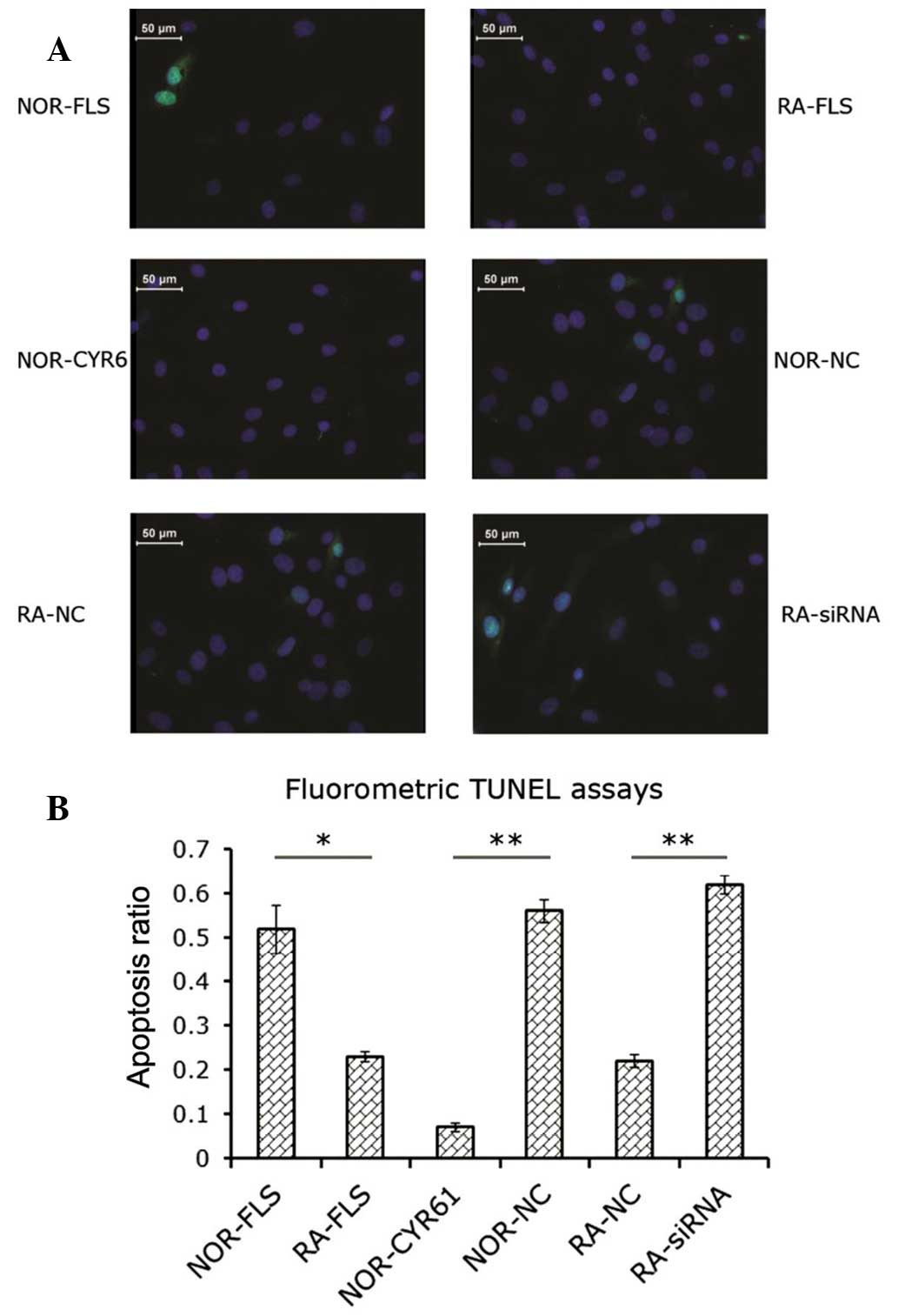 | Figure 3Role of Cyr61 in FLS cell apoptosis.
(A) Fluorometric image of apoptotic cells. Blue, DAPI staining;
Green, green fluorescent protein-positive staining for apoptosis.
(B) Apoptosis ratio was calculated as indicated in the Materials
and methods section. Data are expressed as the mean ± standard
deviation of three independent experiments. *P<0.05
and **P<0.01. RA, rheumatoid arthritis; FLS,
fibroblast-like synoviocytes; siRNA, small interfering RNA;
NOR-FLS, normal FLS cells; NOR-CYR61, normal FLS cells transduced
with lentivirus vector encoding Cyr61 cDNA; NOR-NC, normal FLS
cells transduced with control lentivirus vector; RA-NC, RA-FLS
cells transfected with control siRNA; RA-siRNA, RA-FLS cells
transfected with Cyr61-siRNA; TUNEL assay, terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling. |
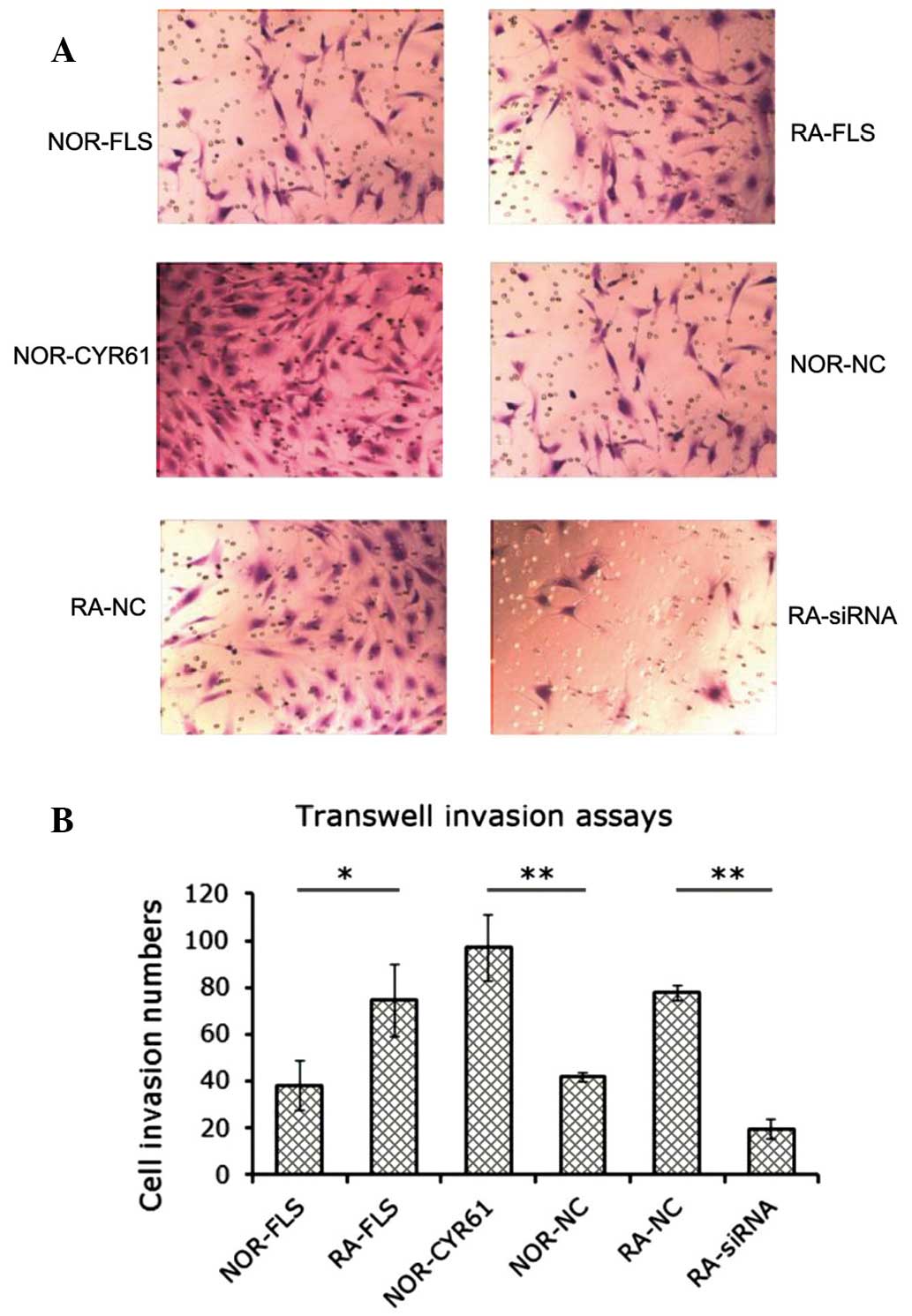
Cyr61 facilitates FLS cell invasion
The effect of Cyr61 on FLS cell invasion was
investigated using transwell in vitro invasion assays. As
shown in Fig. 4A, the number of
RA-FLS cells or normal FLS cells overexpressing Cyr61 that were
invasive was greater than that in the normal FLS cell group. In
addition, as hypothesized, the number of the RA-FLS cells
transfected with Cyr61-siRNA that were invasive was less that it
was in the RA-FLS cells transfected with control siRNA. The number
of invading cells was estimated as described, and is shown in
Fig. 4B. RA-FLS cells showed
markedly increased invasiveness compared with normal FLS cells,
supporting the hypothesis that RA synovial tissue possesses a
tumor-cell-like phenotype. Knockdown of Cyr61 in RA-FLS by siRNA
resulted in a 73.1% reduction in the number of invasive cells
(P<0.001), whereas overexpression of Cyr61 in normal FLS cells
promoted cell invasion by 133.3% (P<0.001). These findings
strongly suggest that Cyr61 leads to promotion of FLS cell
invasion.
Cyr61 regulates MMP-3 expression
MMPs are associated with cell proliferation,
migration and invasion. Therefore this study sought to determine
whether a change in Cyr61 expression affected the expression of
MMPS, thereby potentially impacting on these processes. The levels
of MMP-1, MMP-3, MMP-10 and MMP-13 secreted into the culture medium
were detected by ELISA assays. As shown in Fig. 5A and B, the levels of MMP-1 and
MMP-10 were not significantly altered by Cyr61. However, MMP-3
levels in RA-FLS cells were significantly higher than those in
normal FLS cells (P<0.001). This difference was abrogated by
downregulation of Cyr61 using siRNA (Fig. 5B). In addition, MMP-3 levels in
normal FLS cells over-expressing Cyr61 were 15.6-fold higher than
those in normal FLS cells and 10.9-fold higher than those in normal
FLS cells transduced with control lentivirus vectors (P<0.0001;
Fig. 5C). Furthermore, MMP-13
levels in RA-FLS transfected with Cyr61-siRNA cells were
significantly reduced by 50.1% compared with cells transfected with
control siRNA, and by 35.0% relative to RA-FLS cells without
treatment (Fig. 5D).
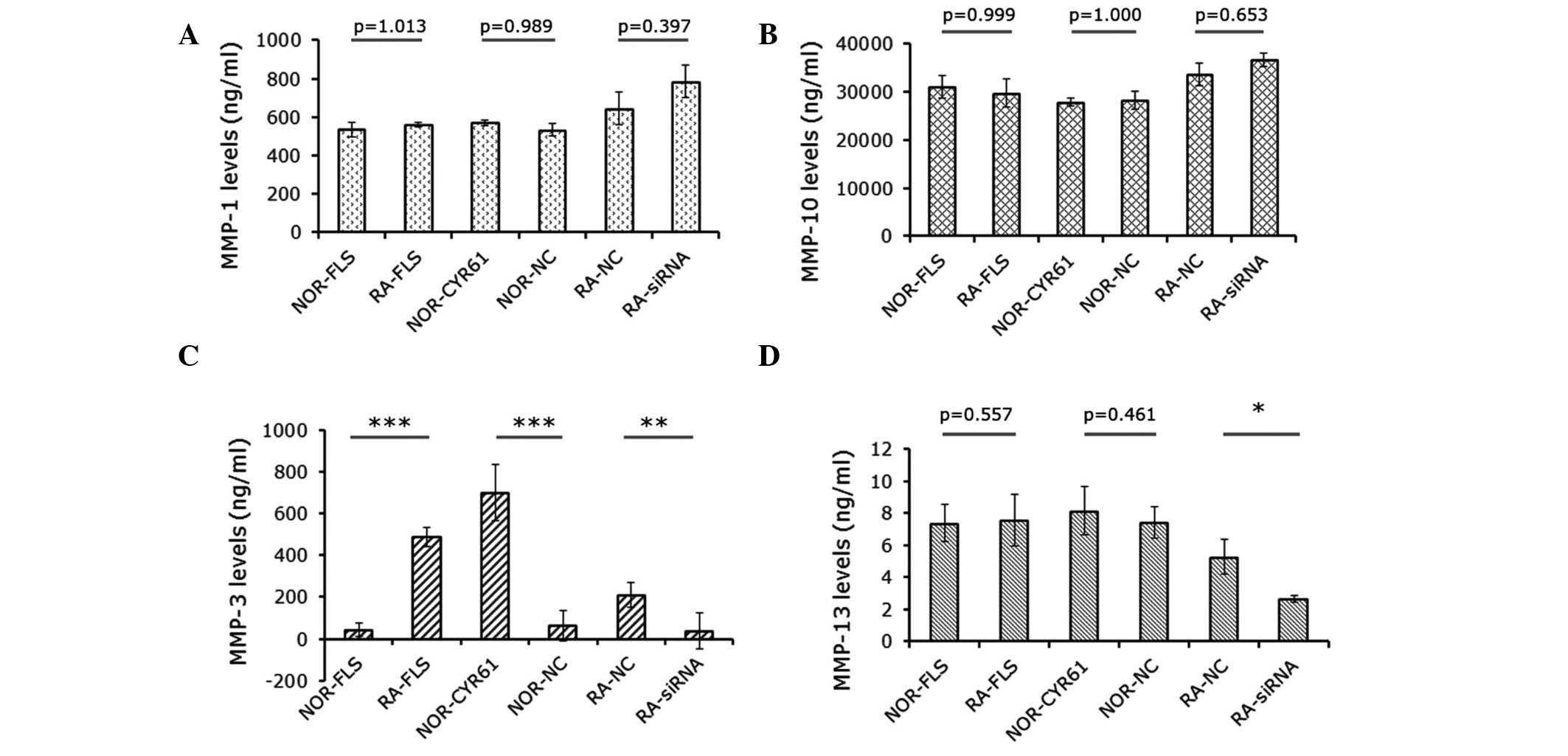 | Figure 5Effects of Cyr61 on expression of MMPs
in FLS cells. Levels of (A) MMP-1, (B) MMP-10, (C) MMP-3 and (D)
MMP-13. Data are presented as the mean ± standard deviation of
three independent experiments done in triplicate.
*P<0.05, **P<0.01 and
***P<0.001. MMP, matrix metalloproteinase; RA,
rheumatoid arthritis; FLS, fibroblast-like synoviocytes; siRNA,
small interfering RNA; NOR-FLS, normal FLS cells; NOR-CYR61, normal
FLS cells transduced with lentivirus vector encoding Cyr61 cDNA;
NOR-NC, normal FLS cells transduced with control lentivirus vector;
RA-NC, RA-FLS cells transfected with control siRNA; RA-siRNA,
RA-FLS cells transfected with Cyr61-siRNA. |
Discussion
The identification of unique and easily measurable
biomarkers for use in RA diagnosis is a predominant aim of
rheumatologists (16). Cyr61 was
shown in the current study to be overexpressed in RA synovial
tissue, synovial fluid and FLS cells (12) through the microarray and in
vitro experiments. Synovial tissue, which is comprised
predominantly of FLS cells, is the primary tissue targeted by the
pathological processes involved in RA (3). This study focused on the role of
Cyr61 in RA-FLS cells in terms of apoptosis, cell proliferation and
cell invasion, in order to explore whether it may be used as a
reliable marker in RA diagnosis.
The study used the dual approaches of ‘loss-of
function’ and ‘gain-of function’. The data demonstrated that cell
extracts and culture medium collected from RA-FLS cells contained
higher levels of Cyr61 than normal FLS cells from individuals
without RA. On the basis of these findings, Cyr61 expression was
knocked down using siRNA in RA-FLS cells, and overexpressed in
normal FLS cells by transducing lentivirus vectors encoding Cyr61
cDNA. A series of in vitro experiments were designed to
examine the role of Cyr61 in FLS cell activity. It was found that
RA-FLS cells had higher rates of proliferation and were more
invasive than normal FLS cells, supporting the hypothesis that the
phenotype of synovial cells is similar in a number of ways to that
of tumor cells (3).
The current study showed that downregulation of
Cyr61 in RA-FLS cells decreased cell proliferation, while
overexpression of Cyr61 in normal FLS cells significantly increased
cell proliferation. This suggests that Cyr61 is important in FLS
cell proliferation. This result is in accordance with another study
which showed that elevated levels of Cyr61 in RA synovial fluid
promotes the proliferation of FLS cells and that this effect was
abrogated by neutralizing Cyr61 (12). In addition, an analysis of
apoptosis in the present study demonstrated that this process was
induced by Cyr61-siRNA in RA-FLS cells, but was suppressed in
normal FLS cells overexpressing Cyr61. This implies that Cyr61
promotes FLS cell proliferation in part by suppressing
apoptosis.
Furthermore, the current study demonstrated that
Cyr61 promoted cell invasion in FLS cells, as shown by the
transwell invasion assays. The effects of Cyr61 on MMP expression
were also examined. The MMP family are involved in a number of
disease processes, including arthritis and tumor metastasis. They
are also understood to be involved in certain cellular processes,
such as cell proliferation, migration, differentiation,
angiogenesis and apoptosis (17).
MMP-3 is a key member of the MMP family. It is able to activate
other MMPs, meaning it is crucial in the connective tissue
remodeling process (18). During
RA progression, FLS cells secrete MMPs, which degrade cartilage and
bone. MMP-3 is the most important of these molecules (19). Measurement of active MMP-3 in
clinical samples may thus provide information regarding the
progression of rheumatoid diseases, and potentially also the
response to treatment (20).
Therefore, the data presented, showing that Cyr61 may significantly
affect the level of MMP-3 secreted into the culture medium of FLS
cells, suggests that Cyr61 promotes RA-FLS cell proliferation and
invasion at least in part through regulation of MMP-3 expression.
Notably, although MMP-13 levels were decreased by the introduction
of Cyr61-siRNA into RA-FLS cells, they were not affected by
overexpression of Cyr61 in normal FLS cells, suggesting that the
effect of Cyr61 on MMP-13 expression may be dependent on cell
context.
In conclusion, this study demonstrated increased
levels of Cyr61 in synovial tissues and FLS cells from patients
with RA. Cyr61 may act as a promoter for RA-FLS cell proliferation
and invasion via suppression of apoptosis as well as the regulation
of MMP-3 expression. Despite considerable efforts over a number of
years, current therapeutic strategies for RA treatment remain
unsatisfactory (21). Further
in vivo studies are required to determine whether Cyr61 may
be a candidate for therapeutic intervention.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81102688) and the
Postdoctoral Science Foundation of China (grant no. 2012M521584).
The authors would like to thank Land Huagene Biosciences Co. Ltd.
(Guangzhou, China.) for their technological assistance.
References
|
1
|
McInnes IB and Schett G: The pathogenesis
of rheumatoid arthritis. N Engl J Med. 365:2205–2219. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang RY, Huang QC and Burgering BM: Novel
insight into the role of α-actinin-1 in rheumatoid arthritis.
Discov Med. 17:75–80. 2014.PubMed/NCBI
|
|
4
|
Jun JI and Lau LF: Taking aim at the
extracellular matrix: CCN proteins as emerging therapeutic targets.
Nat Rev Drug Discov. 10:945–963. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hurvitz JR, Suwairi WM, Van Hul W, et al:
Mutations in the CCN gene family member WISP3 cause progressive
pseudorheumatoid dysplasia. Nat Genet. 23:94–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Y and Du XY: Functional properties
and intracellular signaling of CCN1/Cyr61. J Cell Biochem.
100:1337–1345. 2007. View Article : Google Scholar
|
|
7
|
Kular L, Pakradouni J, Kitabgi P, et al:
The CCN family: a new class of inflammation modulators? Biochimie.
93:377–388. 2011. View Article : Google Scholar
|
|
8
|
Kok SH, Lin LD, Hou KL, et al: Simvastatin
inhibits cysteine-rich protein 61 expression in rheumatoid
arthritis synovial fibroblasts through the regulation of
sirtuin-1/FoxO3a signaling. Arthritis Rheum. 65:639–649. 2013.
View Article : Google Scholar
|
|
9
|
Lin J, Huo R, Xiao L, et al: A novel
p53/microRNA-22/Cyr61 axis in synovial cells regulates inflammation
in rheumatoid arthritis. Arthritis Rheumatol. 66:49–59. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu X, Xiao L, Huo R, et al: Cyr61 is
involved in neutrophil infiltration in joints by inducing IL-8
production by fibroblast-like synoviocytes in rheumatoid arthritis.
Arthritis Res Ther. 15:R1872013. View
Article : Google Scholar
|
|
11
|
Lin J, Zhou Z, Huo R, et al: Cyr61 induces
IL-6 production by fibroblast-like synoviocytes promoting Th17
differentiation in rheumatoid arthritis. J Immunol. 188:5776–5784.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Q, Wu J, Cao Q, et al: A critical
role of Cyr61 in interleukin-17-dependent proliferation of
fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis
Rheum. 60:3602–3612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qingchun H, Runyue H, LiGang J, et al:
Comparison of the expression profile of apoptosis-associated genes
in rheumatoid arthritis and osteoarthritis. Rheumatol Int.
28:697–701. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang S, Xu JF, Cao WJ, et al: Human
decorin regulates proliferation and migration of human lung cancer
A549 cells. Chin Med J (Engl). 126:4736–4741. 2013.
|
|
15
|
Huang RY, Chu YL, Jiang ZB, et al:
Glycyrrhizin suppresses lung adenocarcinoma cell growth through
inhibition of thromboxane synthase. Cell Physiol Biochem.
33:375–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grassi W and Filippucci E: Rheumatoid
arthritis: Diagnosis of RA - we have a dream. Nat Rev Rheumatol.
9:202–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodríguez D, Morrison CJ and Overall CM:
Matrix metalloproteinases: what do they not do? New substrates and
biological roles identified by murine models and proteomics.
Biochim Biophys Acta. 1803.39–54. 2010.
|
|
18
|
Ghilardi G, Biondi ML, DeMonti M, et al:
Matrix metalloproteinase-1 and matrix metalloproteinase-3 gene
promoter polymorphisms are associated with carotid artery stenosis.
Stroke. 33:2408–2412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Knevel R, Klein K, Somers K, et al:
Identification of a genetic variant for joint damage progression in
autoantibody-positive rheumatoid arthritis. Ann Rheum Dis. Aug
16–2013.(Epub ahead of print). PubMed/NCBI
|
|
20
|
Sun S, Bay-Jensen AC, Karsdal MA, et al:
The active form of MMP-3 is a marker of synovial inflammation and
cartilage turnover in inflammatory joint diseases. BMC
Musculoskelet Disord. 15:932014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miossec P: Rheumatoid arthritis: still a
chronic disease. Lancet. 381:884–886. 2013. View Article : Google Scholar : PubMed/NCBI
|