Introduction
Glaucoma is the second leading cause of blindness
worldwide (1). During its
pathogenesis, optic nerve axons are continuously damaged at the
optic nerve head, a process that leads to axonal degeneration,
apoptosis of retinal ganglion cells and, finally, characteristic
defects in the visual field of the affected patients (2,3).
Glaucoma occurs in the presence of imbalances between the
production and drainage of the aqueous humor (AH), due to
obstruction of the outflow pathways of the trabecular meshwork
(TM).
The most critical risk factor for the progression of
glaucomatous optic nerve damage has been identified in several
studies as intraocular pressure (IOP) (4,5). IOP
is generated and maintained by the AH circulatory system in the
anterior eye (4). AH is actively
secreted by the epithelial layers of the ciliary body into the
posterior chamber of the eye and leaves the eye through the
iridocorneal angle and Schlemm’s canal (SC) (5). The vast majority of the AH leaves the
eye through the TM, across the SC and finally enters the general
circulation. When AH has passed through the trabecular outflow
pathways, it drains into the episcleral venous system (5). The TM pathways are critical in
providing resistance to AH outflow. The decisive factor causing
hypertonicity may be an alteration in the thickness of the TM. An
increase in the thickness of the TM may alter the drainage of AH
due to the presence of higher resistance. A thickened TM may be
correlated with the lamellar deposition of collagen and the
accumulation of amorphous material that reduces the interlamellar
spaces and the lumen of juxtacanalicular pathways. IOP builds up in
response to this resistance until it is high enough to allow AH to
flow across the TM into SC (6).
The TM consists of three regions that differ in
structure: The inner uveal meshwork, the deeper corneoscleral
meshwork and the juxtacanalicular tissue (JCT), or cribriform
region, which is localized directly adjacent to the inner
endothelial wall of the SC. The uveal meshwork, which originates
from the anterior chamber of the ciliary body, consists of one to
three layers of trabecular beams or lamellae. The corneoscleral
meshwork forms 8–15 trabecular layers, which are thicker compared
with those of the uveal TM and originate from the scleral spur. The
JCT, which is localized directly in the endothelial lining of the
SC, is the smallest part of the TM with a thickness of only 2–20
μm. The JCT does not form trabecular lamellae or connective tissue
beams, but rather represents a typical loose connective tissue with
2–5 layers of scattered cells that are embedded in a loosely
arranged fibrillar ECM (5).
In primary open-angle glaucoma (POAG), there is a
gradual obstruction of the outflow channels, and in the more rare
narrow-angle glaucoma, drainage systems suddenly become blocked.
The increased pressure within the eye compresses the small blood
vessels and the fibers of the optic nerve, causing a progressive
loss of vision that may result in blindness.
In the majority of cases the molecular changes that
determine POAG are unclear. However, it is hypothesized that it is
due to the significant increase of fibrillary bands of the ECM in
the TM. This hypothesis is supported by the observation that
treatment with metalloproteinases degrades components of the ECM
and leads to an improvement in the AH outflow.
The TM cells have contractile properties, thus an
increased tone of the TM modifies the resistance to outflow.
Consequently, increased contraction of the TM may lead to increased
rigidity. In fact, the destruction of actin filaments diminishes
resistance to AH outflow (7).
The pathological processes that determine resistance
to AH outflow in the TM, and consequently increase of IOP, are
constituted by an acute inflammatory phase, in which there is
infiltration of inflammatory cells and upregulation of various
cytokines; a proliferative phase in which there is migration of
fibroblasts and vascular endothelial cells from the surrounding
tissues, and transformation of fibroblasts into myofibroblasts; and
a remodelling phase, which leads to the formation of a fibrous scar
(8).
In addition, numerous fibroangiogenic growth factors
have been implicated in POAG pathogenesis, including tumor necrosis
factor-α (TNF-α) and transforming growth factor-β1 (TGF-β1).
Angiogenesis is defined as the formation of novel
blood vessels from pre-existing vasculature and underlies a large
number of physiological processes, including growth and
differentiation, wound healing and pathological conditions, such as
neoplasia and complicated ocular diseases, which result in severe
loss of vision.
The processes of vascularization involve the
activation of cell-derived angiogenic factors and the appropriate
synthesis of extracellular matrix (ECM) components, required for
anchorage of the endothelium. One of the most potent and specific
angiogenic factors is vascular endothelial growth factor (VEGF)
(9). Additionally,
pro-inflammatory cytokines, including interleukin (IL)-6 and IL-1β,
may be involved in mediating the inflammation associated with
different ocular diseases.
IL-6, IL-1β and TNF-α have numerous characteristics
similar to VEGF and are also reported to be induced by hypoxia.
Increased IL-6 in aqueous humor was noted in intraocular
inflammatory conditions, such as uveitis and ophthalmitis.
Furthermore, Cohen et al (10) demonstrated that pro-inflammatory
cytokines regulate VEGF expression. In previous studies, increased
levels of VEGF were reported in samples of vitreous humour and AH
of patients with POAG (11). In
the present study the concentrations of IL-6, IL-1β, TGF-β1, VEGF
and TNF-α were measured in TM samples taken from patients with
POAG.
Materials and methods
Ethical considerations
A total of seven adult patients affected by POAG who
had undergone a trabeculectomy were studied, together with three
female autoptic specimens (from patients without ocular and/or
inflammatory pathologies) harvested as control cases. The patients,
aged between 45 and 69 years, were females (n=2) and males (n=5).
The uncorrected baseline IOP in POAG eyes ranged from 26 to 30 mm
Hg. The protocol and informed written consent forms were approved
by the Ethical Committee of the Sapienza University of Rome (Rome,
Italy) and G.B. Bietti Eye Foundation (Rome, Italy). Prior to
signing the informed consent form, the patients were informed about
the study in detail by a physician providing them with ample time
to ask possible questions. The study was conducted in accordance
with the Declaration of Helsinki. Each clinical unit selected
specimens and assigned a progressive number to each sample followed
by a letter indicative of the participating unit. For each case, a
report was prepared indicating the age and gender of the patients
and their general clinical characteristics. The control
morphological sections were stained with hematoxylin and eosin. The
following factors were investigated: VEGF, TGF-β1, IL-1β, IL-6 and
TNF-α.
Immunohistochemical analysis
The samples were washed in phosphate-buffered saline
(PBS), fixed in 10% formalin and embedded in paraffin according to
a standard procedure. The method employed for immunohistochemical
tests was the ABC/HRP technique (avidin complexed with biotinylated
peroxidase). Serial 3-μm thick sections were cut using a rotative
microtome, mounted on gelatin-coated slides and processed for
immunohistochemistry. These sections were deparaffinized in xylene,
dehydrated, immersed in citrate buffer (pH 6.0) and subjected to
microwave irradiation twice for 5 mins. Subsequently, all the
sections were treated for 30 mins with 0.3% hydrogen peroxide in
methanol to quench endogenous peroxidase activity. In order to
prevent non-specific binding, the slides were incubated in 3%
normal goat serum in PBS for 30 mins at room temperature. The
slides were incubated overnight at 4°C with the following
antibodies: i) Rabbit anti-IL-1β polyclonal antibody; ii) rabbit
anti-IL-6 polyclonal antibody; iii) mouse anti-TNF-α monoclonal
antibody; iv) mouse anti-VEGF monoclonal antibody; and v) rabbit
anti-TGF-β1 polyclonal antibody (all Santa Cruz Biotechnology,
Santa Cruz, CA, USA). Optimal antisera dilutions and incubation
times were assessed in a series of preliminary experiments.
Following exposure to the primary antibodies, the slides were
rinsed twice in phosphate buffer and incubated for 1 h at room
temperature with the appropriate secondary biotinylated goat
anti-mouse or anti-rabbit IgG antibodies (Vectastain Elite ABC Kit
Standard* PK-100; Vector Laboratories Burlingame, CA,
USA). Following a further wash with phosphate buffer, the slides
were treated with 0.05% 3,3-diaminobenzidine and 0.1%
H2O2. Finally, the sections were
counterstained with Mayer’s hematoxylin and observed using a light
microscope (Zeiss photomicroscope III; Carl Zeiss AG, Jena,
Germany). Negative control experiments were performed by omitting
the primary antibody; by substituting the primary antibody with an
equivalent quantity of non-specific immunoglobulin; or by
pre-incubating the primary antibody with the specific blocking
peptide. The staining assessment was conducted by two experienced
histologists. The intensity of the immune reaction was assessed
microdensitometrically using an IAS 2000 image analyzer (Delta
Sistemi, Rome, Italy) connected via a TV camera to the microscope.
The system was calibrated taking zero as the background obtained in
sections exposed to non-immune serum. A total of 10
100-μm2 areas were delineated in each section using a
measuring diaphragm. The quantitative data regarding the intensity
of immune staining were statistically analyzed using analysis of
variance (ANOVA) followed by Duncan’s multiple range as a post hoc
test.
Transmission electron microscopy
(TEM)
A standard trabeculectomy was performed. A
limbus-based conjunctival flap was raised, and a 4×4-mm
half-thickness scleral flap was dissected. A window was formed by
removing a block of trabecular tissue (~1×2 mm). The trabecular
tissues were fixed immediately in buffer containing 2%
glutaraldehyde for 2 h, washed, postfixed in buffer with 2% osmium
tetroxide for 2 h, dehydrated and embedded in araldite. Ultrathin
sections were cut using a Reichert Ultra-microtome (Reichert
Technologies, Munich, Germany). These sections were counterstained
with uranyl acetate and lead citrate and observed under a Zeiss EM
109 electron microscope (Carl Zeiss AG).
Statistical analysis
Quantitative data related to immune staining were
analyzed statistically by ANOVA followed by Duncan’s multiple range
as a post hoc test. The comparison of the expression levels of
VEGF, TGF-β, TNF-α, IL-1β and IL-6 in the TM from glaucomatous eyes
and eyes of control patients was performed by a t-test. Statistical
analyses were performed using the SPSS statistical software package
version 12.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was used to
indicate a statistically significant difference.
Results
TEM
The morphological changes that occurred in the TM of
glaucomatous eyes were analyzed using TEM (Figs. 1 and 2). Changes, such as increased cellular
content, macrophages, fibrosis and accumulation of neutrophils were
evaluated.
In all the cases, proliferation of fibrous
connective tissue was observed at the inner wall of SC.
Accumulation of collagen fibers, irregular striations and
agglomerations of microfibrillar material with the structure of
collagen microfibrils were observed. The AH outflow pathways of the
inner TM were obstructed by cellular fragments.
The outer TM was characterized by deposition of
extracellular material and collagen, presence of degenerative cells
and collapse of the trabecular lamellae.
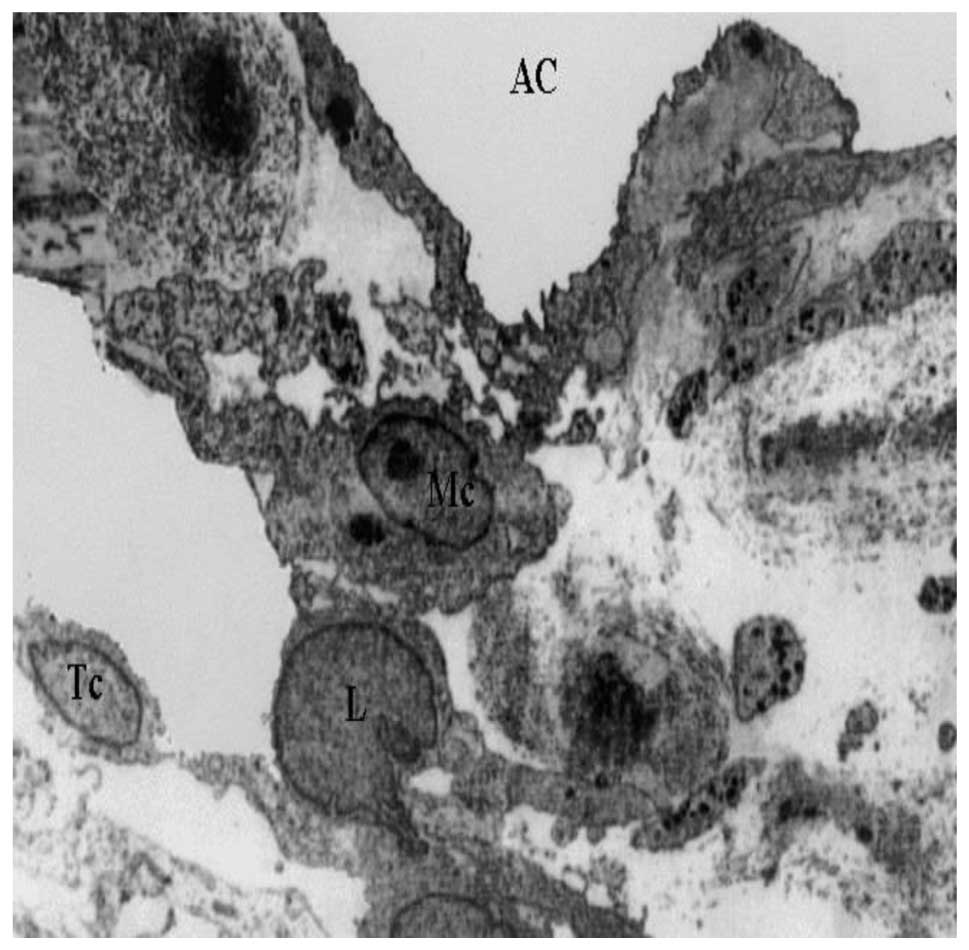
TEM images of the innermost section of the uveal
meshwork (Fig. 1) revealed
distribution of macrophages, lymphocytes and melanocytes. In the
anterior uveal compartment the connective tissue possessed a rich
population of resident tissue macrophages. Inflammatory changes,
including the presence of lymphocytes or plasma cells in the TM and
the iridocorneal angle were also visible.
The pattern of distribution of resident tissue
macrophages close to vascular beds indicates a guardian role at the
blood-tissue interface. It is known that uveal tract macrophages
produce pro-inflammatory substances (including nitric oxide and
cytokines) in conditions, such as acute anterior or endogenous
posterior uveitis. This could be of particular significance in
light of their close proximity to the blood-ocular barriers
(12). Neovascularization,
associated with infiltration of lymphocytes and melanocytes, was
also observed in the TM. The initiation of immune responses is
dependent on the presentation of antigens to T-helper lymphocytes.
T cells are the predominant inflammatory cell type (13) in the TM.
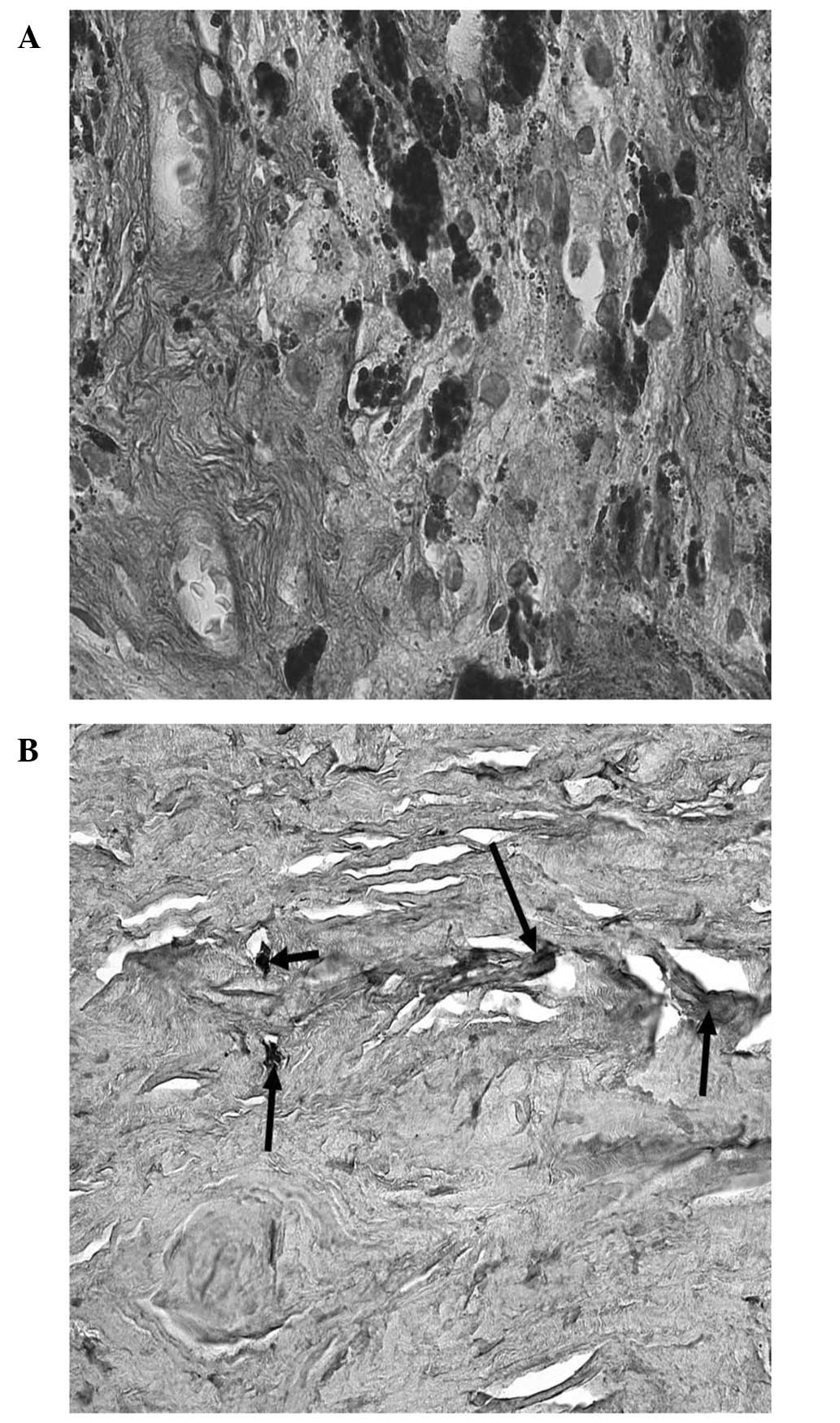
Cells of all regions of the TM, have thick bundles
of actin filaments along their basal cytoplasm that are oriented in
parallel, indicated as attachment plaques of microfilament at the
plasma membrane (Fig. 2). In POAG
the oxidative stress may trigger degeneration in human TM, which
affects the cytoskeleton and the adhesive properties in TM cells,
leading to increased IOP. Elevated pressure induced the disruption
of tight junctions, which may result in changes in the
microenvironment and damage the TM cells, thus modifying the normal
outflow of AH. The effects of elevated IOP may also influence cell
adhesion to collagen beams, which may partially explain the
presence of fewer TM cells in POAG patients when compared with
normal subjects (14).
Immunohistochemical analysis
Sections of TM samples were exposed to
primary/secondary antibodies developing a dark-brown (intense),
yellow-brown (slight) or no immune staining. The expression levels
of IL-6, IL-1β, TNF-α, TGF-β1 and VEGF are shown in Table I. Immunoreactivity was specific
since no immunostaining was obtained in the control sections
incubated with each primary antibody absorbed with the specific
peptide or with pre-immune serum (data not shown). It was revealed
that IL-6 is markedly expressed in human trabecular meshwork
(Fig. 3). The concentration of
IL-6 in the TM of patients with POAG was significantly higher
compared with that of the control subjects. Normally, the cells do
not synthesize and secrete IL-6 unless they are stimulated by other
cytokines or by certain physiological events. It has been
demonstrated that a wide range of ocular tissues can produce IL-6
under pathological conditions, such as cytokine-stimulated vascular
endothelial cells and vascular smooth muscle cells (15). Inflammatory cells, particularly
mast cells, are known to be able to stimulate IL-6 secretion from
leukocytes and human vascular endothelial cells in ischemic and
inflammatory conditions. The present study demonstrated that
expression of other inflammatory cytokines in the trabecular
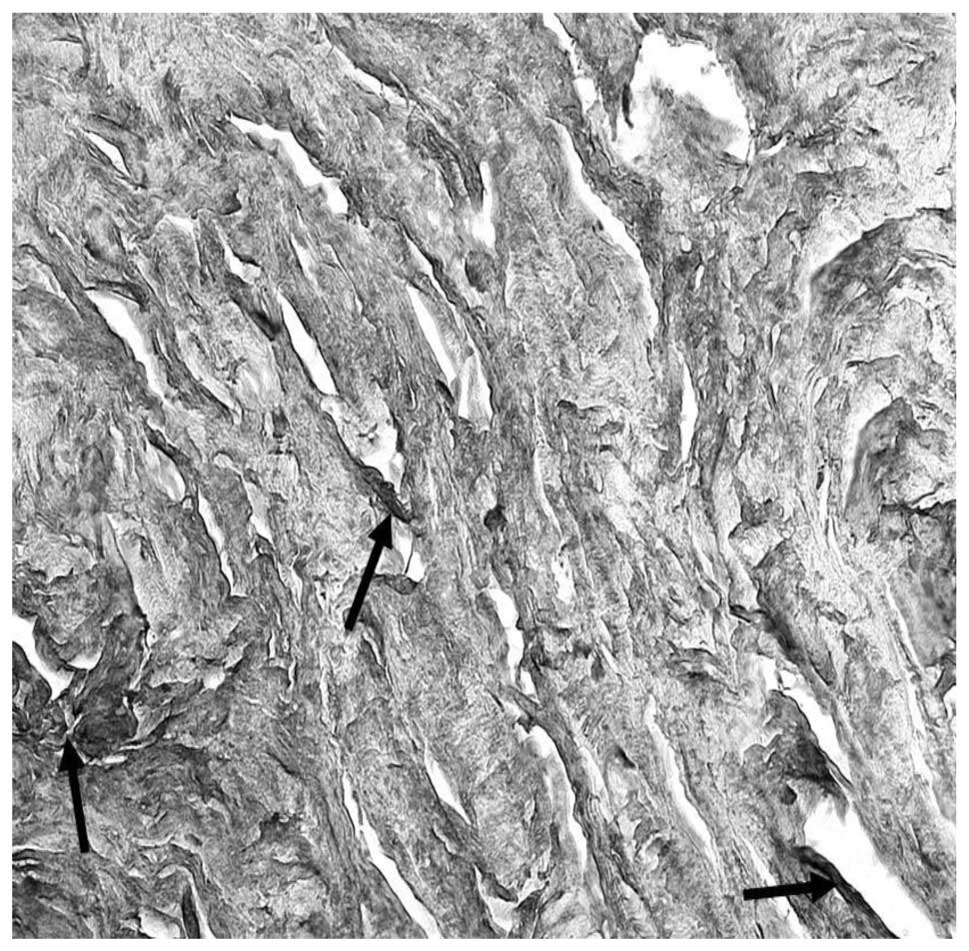
meshwork, including IL-1β (Fig. 5A and
B) and TNF-α (Fig. 4), that
are significant components of the pro-inflammatory response and
intraocular inflammation. IL-1β systemically regulates the
metabolic, immuno-inflammatory and reparative properties of
tissues, and can be a mediator of a number of diseases. It is
secreted by monocytes and tissue macrophages, and is involved in
the activation of T cells. In human TM tissues, evident
immunoreactivity for IL-1β was observed in the macrophages of all
the patients (all specimens) and moderate immunoreactivity was
present in the blood vessels. Evident immunoreactivity for TNF-α
was observed in uveal meshwork, particularly in the macrophages and
lymphocytes of the samples analyzed. TNF-α is a cytokine secreted
by lymphocytes and reticuloendothelial cells in numerous acute and
chronic inflammatory diseases. TNF-α, like IL-6, is also a
pro-inflammatory cytokine and is involved in the blood-retinal
barrier breakdown by opening tight junctions of retinal vascular
endothelial cells (16). In the
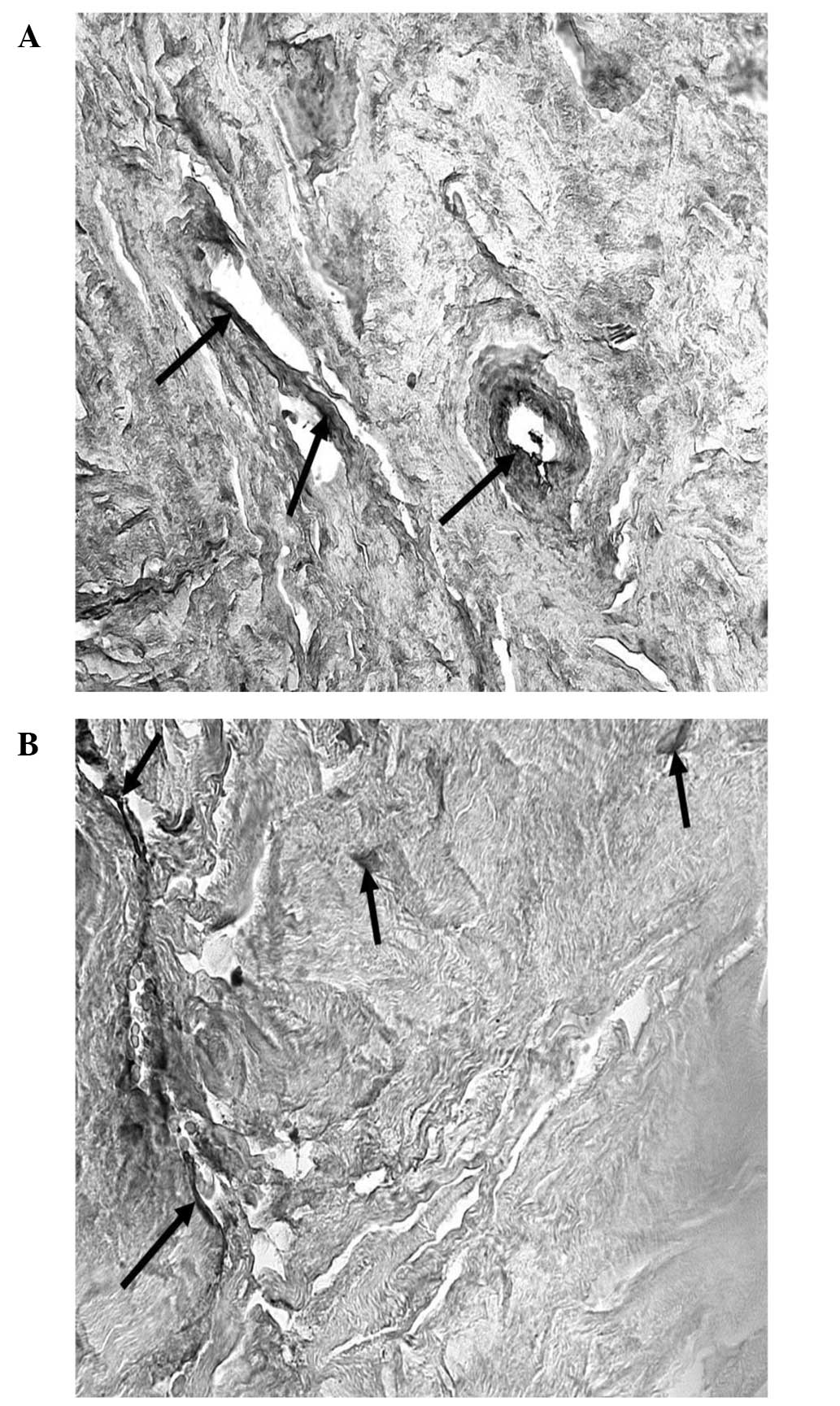
majority of the eyes, TGF-β1 immunolabeling was predominantly
observed in extracellular areas of the juxtacanalicular
(cribriform) section of the TM. An extent of focal staining was
observed in the corneoscleral and uveal regions of the TM. In the
eyes of six glaucoma patients, TGF-β1 immunoreactivity was
considerably more intense and all the regions of the TM were
positively labelled (Fig. 6A and
B). In human TM, VEGF revealed a moderate expression in
extracellular areas of the juxtacanalicular (cribriform) region,
while evident immunoreactivity was observed in the iris, in vessel
endothelial cells and in fibroblasts (Fig. 7).
 | Table IExpression levels of IL-6, IL-1β,
TNF-α, TGF-β1 and VEGF in POAG and control specimens, and
respective levels of statistical significance (t-test). |
Table I
Expression levels of IL-6, IL-1β,
TNF-α, TGF-β1 and VEGF in POAG and control specimens, and
respective levels of statistical significance (t-test).
| Factor | Cases with POAG
(%)a | Controls (%)b | P-value |
|---|
| IL-6 |
| Extracellular matrix
cells | 76.14 | 53.00 | 0.0065 |
| Vascular endothelial
cells | 85.42 | 36.00 | <0.0001 |
| Trabecular
endothelial cells | 55.14 | 24.33 | 0.0007 |
| IL-1β |
| Extracellular matrix
cells | 64.00 | 39.66 | 0.0002 |
| Vascular endothelial
cells | 70.42 | 38.33 | 0.0001 |
| Trabecular
endothelial cells | 57.55 | 33.66 | 0.0040 |
| TNF-α |
| Extracellular matrix
cells | 80.42 | 30.66 | <0.0001 |
| Vascular endothelial
cells | 60.43 | 41.00 | 0.0298 |
| Trabecular
endothelial cells | 66.57 | 35.33 | 0.0001 |
| TGF-β1 |
| Extracellular matrix
cells | 86.43 | 29.33 | <0.0001 |
| Vascular endothelial
cells | 81.57 | 31.33 | <0.0001 |
| Trabecular
endothelial cells | 73.28 | 40.00 | <0.0001 |
| VEGF |
| Extracellular matrix
cells | 85.57 | 30.33 | <0.0001 |
| Vascular endothelial
cells | 72.28 | 15.33 | <0.0001 |
| Trabecular
endothelial cells | 59.86 | 29.33 | <0.0001 |
Discussion
In POAG patients, sustained increases in the
trabecular outflow resistance commonly result in elevated IOP.
Elevated IOP is the primary risk factor for glaucomatous optic
neuropathy, which is associated with progressive vision loss. The
molecular changes that lead to an increase in outflow resistance in
POAG have not been clarified, and one hypothesis is based on the
observation that the exposure of trabecular cells to mechanical
stress, caused by ocular vascular changes, increases the expression
of TGF-β1 in the TM. The overexpression of TGF-β1 induces
proliferation of fibrotic tissue, which determines the remodelling
of the ECM, trabecular obstruction and increase in IOP (17). In patients with POAG, TGF-β1 is
widely expressed in the TM, in the AH, inside the anterior chamber,
in the neural retina and in retinal pigments. The increase of
TGF-β1 is due to the loss of the blood-eye barrier, thus the
concentration of plasma-derived TGF-β1 increases in the plasma,
contributing to the high levels in the eye. TGF-β1 reduces the
production of enzymes that are responsible for the degradation of
the ECM, is involved in fibrosis and induces the expression of
various components of the ECM, including collagen type I, III, IV
and VI, elastin, fibronectin and miociclin. Furthermore, TGF-β1
increases the expression of transglutaminase, which induces
irreversible cross-linking of fibronectin in trabecular bone.
Tissue transglutaminase is normally expressed in trabecular cells
and is found at high levels in patients with POAG (7).
TGF-β1 has been demonstrated to promote
transdifferentiation of fibroblasts into myofibroblasts that
exhibit contractile activity and increase the rigidity of the TM
(7).
The trabecular cells activate TGF-β1
cross-orientation proteolysis or via trompospondin-1, which is a
potent activator of TGF-β1 in vivo and in vitro. The
involvement of TGF-β1 in the remodelling of the ECM and
inflammation of glaucomatous eyes renders this protein a novel
target for the treatment of glaucoma (17). In addition, TGF-β1 regulates and
induces the expression of IL-6 in the TM (18).
TM endothelial cells secrete a number of factors and
cytokines that modulate the functions of the cells and the ECM of
the conventional outflow pathway. In the TM the macrophages produce
cytokines, such as IL-6, IL-1β and TNF-α, leading to an acute
inflammatory response and recruitment of other immune cells,
including T lymphocytes. T-helper cells produce Th17 stimulated by
IL-1β, IL-6 and TGF-β1.
Increased levels of IL-6, IL-1β and TNF-α were
observed in patients with POAG when compared with the control
subjects, indicating that these cytokines may be correlated with
disease activity. The continuous activation of macrophages and
production of TNF-α, IL-6 and IL-1β prolongs the inflammatory
response with production of large quantities of Th17. In fact, IL-6
and Il-1β have been implicated in the differentiation of Th cells
into Th17, which produce Il-17, and appear to be responsible for
the transformation from acute to chronic inflammatory responses. In
a normal patient, activation of fibroblasts, resulting in
deposition of ECM, leads to a downregulation of the inflammatory
reaction with transformation into a fibrotic reaction.
Pro-inflammatory cytokines, such as IL-6 and TNF-α,
which are significant in inflammation, were detected at elevated
levels in the AH of patients with neovascular glaucoma. In the
neovascularization processes, there is a correlation between IL-6
and VEGF. IL-6 indirectly induces angiogenesis through stimulation
of the expression of VEGF. A previous study revealed that
interactions between fibroblasts, myofibroblasts and vascular
endothelial cells occur at the site of the inflammatory lesion
(11).
VEGF not only induces vascular proliferation, but
also acts as a mediator in the process that leads to fibroblast
proliferation. As angiogenesis is fundamental in the formation of
granulation tissue (8), inhibition
of neovascularization induced by anti-VEGF agents may decrease
fibroblastic proliferation (19).
The pathogenesis of these disorders is a result of a
neuroinflammatory process (20).
Pro-inflammatory cytokines and oxidative stress contribute to
glaucomatous degeneration (21).
TNF-α is a pro-inflammatory cytokine secreted in
response to infection and trauma. TNF-α is involved in the
neurodegenerative process of glaucoma. In fact, this cytokine
mediates the cytotoxic effect of ocular hypertension through a
mechanism that involves activation of microglia and loss of
oligodendrocytes.
The increase in IOP determines increased levels of
TNF-α, together with a massive expansion of the macrophage
population. It is possible that the elevated IOP, with consequent
hypoxia, could be responsible for the loss of the ocular
blood-brain barrier, thus provoking an evident inflammatory
response (22).
In conclusion, the present results add support to
the evidence that growth factors and cytokines can induce ECM
remodelling and alter cytoskeletal interactions in the TM.
The changes described may aid in the treatment of
POAG. The generalized edema of the trabecular endothelium and the
presence of inflammatory cells in POAG eyes, associated with an
acute and marked rise of IOP could possibly respond better to a
combination of anti-inflammatory therapy and glaucoma therapy. The
gradual, chronic rise of IOP, is most likely due to the progressive
activation of the inflammatory response, gradually transformed into
a long-term fibrotic reaction. Therapy should therefore include
anti-inflammatory drugs administered topically and systemically
together with IOP-lowering agents, in order to control the
inflammatory cells and reduce trabecular edema.
References
|
1
|
Resnikoff S, Pascolini D, Etya’ale D,
Kocur I, Pararajasegaram R, Pokharel GP and Mariotti SP: Global
data on visual impairment in the year 2002. Bull World Health
Organ. 82:844–851. 2004.
|
|
2
|
Kwon YH, Fingert JH, Kuehn MH and Alward
WL: Primary open-angle glaucoma. N Engl J Med. 360:1113–1124. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quigley HA: Glaucoma. Lancet.
377:1367–1377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamm ER, Toris CB, Crowston JG, Sit A, Lim
S, Lambrou G and Alm A: Basic science of intraocular pressure.
Intraocular Pressure. Reports and Consensus Statements of the 4th
Global AIGS Consensus Meeting on Intraocular Pressure. Weinreb RN,
Brandt JD, Garway-Heath D and Medeiros F: Kugler Publications;
Amsterdam: pp. 1–14. 2007
|
|
5
|
Tamm ER: The trabecular meshwork outflow
pathways: structural and functional aspects. Exp Eye Res.
88:648–655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barany EH: In vitro studies on the
resistance to flow through the angle of the anterior chamber. Acta
Soc Med Ups. 59:260–276. 1954.PubMed/NCBI
|
|
7
|
Fuchshofer R and Tamm ER: The role of
TGF-β in the pathogenesis of primary open-angle glaucoma. Cell
Tissue Res. 347:279–290. 2012. View Article : Google Scholar
|
|
8
|
Takeuchi K, Nakazawa M and Ebina Y:
Effects of trehalose on VEGF-stimulated angiogenesis and
myofibroblasts proliferation: implications for glaucoma filtration
surgery. Invest Ophthalmol Vis Sci. 52:6987–6993. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bianchi E, Scarinci F, Grande C, Plateroti
R, Plateroti P, Plateroti AM, Fumagalli L, Capozzi P, Feher J and
Artico M: Immunohistochemical profile of VEGF, TGF-β and
PGE2 in human pterygium and normal conjunctiva:
experimental study and review of the literature. Int J Immunopathol
Pharmacol. 25:607–615. 2012.PubMed/NCBI
|
|
10
|
Cohen T, Nahari D, Cerem LW, Neufeld G and
Levi BZ: Interleukin 6 induces the expression of vascular
endothelial growth factor. J Biol Chem. 271:736–741. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen KH, Wu CC, Roy S, Lee SM and Liu JH:
Increased interleukin-6 in aqueous humour of neovascular glaucoma.
Invest Ophthalmol Vis Sci. 40:2627–2632. 1999.PubMed/NCBI
|
|
12
|
McMenamin PG: The distribution of immune
cells in the uveal tract of the normal eye. Eye (Lond). 11:183–193.
1997. View Article : Google Scholar
|
|
13
|
Romeike A, Brügmann M and Drommer W:
Immunohistochemical studies in equine recurrent uveitis (ERU). Vet
Pathol. 35:515–526. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang X, Liu B, Bai Y, Chen M, Li Y, Chen
M, Wei Y, Ge J and Zhuo Y: Elevated pressure downregulates ZO-1
expression and disrupts cytoskeleton and focal adhesion in human
trabecular meshwork cells. Mol Vis. 17:2978–2985. 2011.PubMed/NCBI
|
|
15
|
Yan SF, Tritto I, Pinsky D, et al:
Induction of interleukin-6 (IL-6) by hypoxia in vascular cells.
Central role of the binding site for nuclear factor-IL-6. J Biol
Chem. 270:11463–11471. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Evans CA, Jellis J, Hughes SP, Remick DG
and Friedland JS: Tumour necrosis factor-alpha, interleukin-6, and
interleukin-8 secretion and the acute-phase response in patients
with bacterial and tuberculous osteomyelitis. J Infect Dis.
177:1582–1587. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prendes MA, Harris A, Wirostko BR, Gerber
AL and Siesky B: The role of transforming growth factor β in
glaucoma and the therapeutic implications. Br J Ophthalmol.
97:680–686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liton PB, Li G, Luna C, Gonzalez P and
Epstein DL: Cross-talk between TGF-beta1 and IL-6 in human
trabecular meshwork cells. Mol Vis. 15:326–334. 2009.PubMed/NCBI
|
|
19
|
Horsley MB and Kahook MY: Anti-VEGF
therapy for glaucoma. Curr Opin Ophthalmol. 21:112–117. 2010.
View Article : Google Scholar
|
|
20
|
Ahmed F, Brown KM, Stephan DA, Morrison
JC, Johson EC and Tomarev SI: Microarray analysis of changes in
mRNA levels in the rat retina after experimental elevation of
intraocular pressure. Invest Ophthalmol Vis Sci. 45:1247–1258.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tezel G: Oxidative stress in glaucomatous
neurodegeneration: mechanisms and consequences. Prog Retin Eye Res.
25:490–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roh M, Zhang Y, Murakami Y, Thanos A, Lee
SC, Vavvas DG, Benowitz LI and Miller JW: Etanercept, a widely used
inhibitor of tumor necrosis factor-α (TNF-α), prevents retinal
ganglion cell loss in a rat model of glaucoma. PLoS One.
7:e400652012. View Article : Google Scholar
|