Introduction
Gastric cancer is a prevalent type of cancer with
high mortality rates throughout the world, which is often diagnosed
at an advanced stage (1,2). The five-year survival rate was
reported to be 70–75% for stage I disease, which drops to 35% for
stage II (2). Numerous efforts
have been taken to improve therapies and survival; at present,
chemotherapy is one of the primary treatments for gastric cancer
(3). However, chemotherapy
treatment is not always effective; hypoxia, a characteristic of
solid tumors, including gastric cancer, has been reported to induce
chemotherapy resistance (4).
5-fluorouracil (5-FU) is an antimetabolite
chemothrapeutic drug which targets thymidylate synthase, blocking
the transformation of deoxy-uridine monophosphate into
deoxy-thymidine acid. This results in cell death via decreased DNA
synthesis and S-phase arrest (5).
Clinical trials showed that regimens containing 5-FU improved the
survival rate of gastric cancer patients; however, local treatment
failure and distant metastases still occur (3,6).
Previous studies have demonstrated that hypoxic conditions induced
cancer cell resistance to 5-FU treatment in vitro (7,8).
Celecoxib is a non-steroidal anti-inflammatory drug
(NSAID) and a selective cyclooxygenase (COX)-2 inhibitor with
anti-inflammatory and analgesic effects (9). Previous studies indicated that
celecoxib may have a promising novel use in the treatment of
cancer; however, its mechanism of action remains to be elucidated
(10–12).
The aim of the present study was to assess the
effects of celecoxib on hypoxic gastric cancer SGC7901 cells and
determine whether celecoxib reduced the hypoxia-induced resistance
of these cells to 5-FU. Furthermore, the present study aimed to
elucidate the underlying mechanisms of action in order to improve
the treatment of gastric cancer and increase the survival rate of
patients.
Materials and methods
Materials
Human gastric cancer cells SGC7901 (Shandong Academy
of Sciences, Jinan, China)and cobalt chloride (CoCl2)
were provided by Professor Feng from the Affiliated Hospital of
Weifang Medical University (Weifang, China). The cells tested
negative for mycoplasmic infection. 5-FU was obtained from Zhenguo
Pharmaceutical Co., Ltd. (Jiangsu, China). RPMI 1640 medium was
purchased from Gibco-BRL (Carlsbad, CA, USA). MTT kits were
purchased from Sigma (St Louis, MO, USA). Fetal bovine serum (FBS)
was obtained from Hyclone (Thermo Fisher Scientific, Waltham, MA,
USA). Rabbit anti-hypoxia-inducible factor (HIF)-2α, anti-octamer
binding protein (Oct)-4 and anti-adenosine triphosphate-binding
cassette sub-family G member 2 (ABCG2) antibodies and
immunohistochemical kits were for purchased from Abcam (Cambridge,
MA, USA). TRIzol® reagent was purchased from Invitrogen
Life Technologies (Carlsbad, CA, USA). Oligo-deoxy-thymine(dT),
Moloney murine leukemia virus (M-MLV) reverse transcriptase, 5×
reverse transcription buffer and 10× polymerase chain reaction
(PCR) buffer were obtained from Fermentas (Waltham, MA, USA). A
protein extraction kit was purchased from Biyuntian Biotech, Co.
(Shanghai, China). Finally, the western blot enhanced
chemiluminescence (ECL) reagent kit was obtained from Thermo Fisher
Scientific (Waltham, MA, USA).
Cell culture
SGC7901 cells were inoculated in RPMI 1640 medium
containing FBS (100 ml/l), penicillin and streptomycin
(105 U/l). Cells were subcultured regularly at 37°C in a
5% CO2 incubator. The chemical hypoxia-inducing agent
CoCl2 (150 μmol/l) was used to simulate the hypoxic
microenvironment of solid tumors.
Proliferation inhibition rate
The proliferation inhibition rates of different
concentrations of 5-FU and celecoxib in gastric cancer cells under
hypoxia were determined by MTT assay. Cells in the logarithmic
growth phase were inoculated in 96-well culture plates at a cell
density of 2×104/l (200 μl). Cells were divided into
four groups: The hypoxia control group, 5-FU group, celecoxib group
and 5-FU/celecoxib combination group. CoCl2 was used to
simulate a hypoxic microenvironment following the cells becoming
adherent. The hypoxic control group was not treated with any drug.
Cells in the 5-FU group were exposed to numerous concentrations of
5-FU (25, 50, 100 and 200 mg/l). The celecoxib group was exposed to
different concentrations of celecoxib (50, 100, 200 and 300
μmol/l). Cells were cultured for 24, 48 or 72 h at 37°C in a 5%
CO2 incubator. Optical density (OD) for each well was
measured using a microplate reader (Bio-rad 680; Bio-rad
Laboratories, Inc., Hercules, CA, USA) at 490 nm. Cell growth
inhibition rates were calculated as: [(control OD-experimental
OD)/control OD] ×100%. The half inhibitory concentrations
(IC50) of 5-FU and celecoxib under hypoxic conditions
were calculated. The 5-FU/celecoxib combination group was subjected
to 5-FU and celecoxib using their respective IC50. Cell
growth inhibition rates were calculated following culturing the
cells for 24, 48 and 72 h at 37°C in a 5% CO2
incubator.
Immunohistochemical detection of HIF-2α,
ABCG2 and Oct-4
SGC7901 cells in the logarithmic growth phase were
prepared into a 4×104 cells/ml suspension (0.5 ml) and
added to 24-well plates with cover glasses. Cells were separated
into identical groups and subjected to identical conditions to
those of the proliferation inhibition rate experiment. The cover
glasses were removed following 48 h in culture. Cells were fixed
using cold acetone for 10–15 min and then washed with PBS.
Immunohistochemistry kits for HIF-2α, ABCG2 and Oct-4 were used
according to the manufacturer’s instructions. MDA-MB-231 breast
cancer cells (Shanghai Baili Biological Technology Co., Shanghai,
China) were used as the positive control and PBS in place of the
primary antibodies was used as the negative control. Cytoplasms
stained with yellowish brown pellets indicated a positive
result.
HIF-2α, ABCG2 and Oct-4 reverse
transcription quantitative PCR (RT-qPCR)
Cells were grouped and subjected to identical
conditions as in the proliferation inhibition rate and
immunohistochemistry experiments. TRIzol® was used to
extract total RNA from the cells, RNA was then dissolved in 30 μl
0.1% diethylpyrocarbonate water (Biyuntian Biotech, Co., Shanghai,
China). Reverse transcription was performed in 20 μl to obtain
cDNA: RNAase-free deionized water (9 μl), RNA template (2 μl),
Oligo-(dT)-18 (1 μl), 5× reaction buffer (4 μl), RNase inhibitor
(20 U/μl; 1 μl), dNTP mix (10 mmol/l; 2 μl), and M-MLV RT (1 μl).
Reaction conditions were: 70°C for 5 min, then immediately put on
ice for 5 min; 25°C for 5 min; 37°C for 60 min; and 70°C for 10
min. Samples were kept on ice if used immediately, or kept at
−150°C if used later.
Primer sequences for semi-quantitative PCR were:
HIF-2α forward, 5′-CTT GGA GGG TTT CAT TGC TGT GGT-3′ and reverse,
5′-GTG AAG TCA AAG ATG CTG TGT CCT-3′ (123 bp); ABCG2 forward,
5′-CCC TTA TGA TGG TGG CTT ATT C-3′ and reverse, 5′-GTG AGA TTG ACC
AAC AGA CCA T-3′ (132 bp); Oct-4 forward, 5′-CCC GAA AGA GAA AGC
GAA CC-3′ and reverse, 5′-CAG AAC CAC ACT CGG ACC AC-3′ (151 bp);
and GAPDH forward, 5′-GCA CCA CCA ACT GCT TAG CAC-3′ and reverse,
5′-GCA GCG CCA GTA GAG GCA GG-3′ (1143 bp). PCR reaction (50 μl)
was performed using cDNA template (1 μl), forward and reverse
primers (1 μl each), Taq DNA polymerase (1 μl), dioxynucleotide
triphosphates (2 mmol/l, 5 μl), MgCl2 (25 mmol/l, 2 μl),
10× PCR buffer (5 μl) and double distilled H2O (34 μl).
Conditions were as follows: 94°C for 5 min, 94°C for 30 sec, 50°C
for 30 sec and 72°C for 60 sec, for 40 cycles and then 72°C for 10
min. Fragments were separated using 1.5% agarose gel
electrophoresis. The MiniLumi digital photo gel imaging system (DNR
Bio-Imaging Systems Ltd., Jerusalem, Israel) and Image J 1.26t
(National Institutes of Health, Bethesda, MD, USA) were used to
capture images of the gels. HIF-2α, ABCG2 and Oct-4 messenger RNA
(mRNA) expression was determined based on the OD value using GAPDH
as reference.
Western blot analysis
Cells were grouped and treated as described above.
Cells were washed twice with chilled PBS following 24 h in culture.
Radio-immunoprecipitation assay cell lysis solution (Biyuntian
Biotech, Co.) was added, then kept in an ice bath for 30 min. Cells
were centrifuged (100 × g) for 10 min at 4°C. The supernatant was
collected and stored at −70°C. Proteins (25 μg) were separated
using SDS-PAGE, transferred to nitrocellulose membranes, and
incubated for 2 h with 5% skimmed milk powder at 37°C. Primary
rabbit anti-HIF-2α, Oct-4 and ABCG2 polyclonal antibodies (dilution
1:50) and GAPDH were added and incubated overnight at 4°C.
Secondary horseradish peroxidase-labeled antibodies were added and
incubated for 2 h at 37°C. The antibodies were purchased from Abcam
(Cambridge, MA, USA). Blots were quantified using an ECL reagent.
The ratio of the absorbance value of HIF-2α, ABCG2 and Oct-4 was
determined relative to GAPDH using the digital photo gel imaging
system (Image J).
Statistical analysis
SPSS 17.0 (IBM, Armonk, NY, USA) was used for data
processing and analysis. Continuous data are presented as the means
± standard deviation and analyzed using a one-way analysis of
variance, followed by a Dunnett’s post-hoc T3 test. P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
5-FU and celecoxib, alone or in
combination, inhibit the proliferation of hypoxic SGC7901
cells
The proliferation of hypoxic SGC7901 gastric cancer
cells was significantly inhibited in a dose-dependent manner by
5-FU (Table I) and celecoxib
(Table II), (P<0.05 for
comparisons of all concentrations for 5-FU as well as celecoxib).
Cells were in the logarithmic growth phase within 48 h following
inoculation. The IC50 of 5-FU was 200 mg/l, while the
IC50 of celecoxib was 100 μmol/l. The respective
IC50 of 5-FU and celecoxib were used in combination for
the treatment of the 5-FU/celecoxib combination group. The
combination treatment inhibited cell proliferation to a greater
extent at each time-point than each treatment alone (Table III).
 | Table IEffect of treatment time and 5-FU
concentration on proliferation of SGC7901 cells under hypoxic
conditions. |
Table I
Effect of treatment time and 5-FU
concentration on proliferation of SGC7901 cells under hypoxic
conditions.
| 24 h | 48 h | 72 h |
|---|
|
|
|
|
|---|
| Group | OD | Inhibition rate
(%) | OD | Inhibition rate
(%) | OD | Inhibition rate
(%) |
|---|
| Hypoxia | 0.531±0.020 | | 0.672±0.021 | | 0.860±0.026 | |
| 5-FU |
| 25 mmol/l | 0.416±0.017 | 21.66 | 0.517±0.019 | 23.07 | 0.652±0.025 | 24.19 |
| 50 mmol/l | 0.388±0.016 | 26.93 | 0.468±0.022 | 30.36 | 0.546±0.018 | 36.51 |
| 100 mmol/l | 0.349±0.015 | 34.27 | 0.403±0.015 | 40.03 | 0.459±0.016 | 46.63 |
| 200 mmol/l | 0.298±0.016 | 43.88 | 0.334±0.010 | 50.29 | 0.406±0.017 | 52.80 |
 | Table IIEffect of treatment time and
celecoxib concentration on proliferation of SGC7901 cells under
hypoxic conditions. |
Table II
Effect of treatment time and
celecoxib concentration on proliferation of SGC7901 cells under
hypoxic conditions.
| 24h | 48h | 72h |
|---|
|
|
|
|
|---|
| Group | OD | Inhibition rate
(%) | OD | Inhibition rate
(%) | OD | Inhibition rate
(%) |
|---|
| Hypoxia | 0.520±0.020 | | 0.678±0.023 | | 0.840±0.022 | |
| Celecoxib |
| 50 μmol/l | 0.476±0.018 | 8.46 | 0.534±0.019 | 21.24 | 0.737±0.019 | 12.26 |
| 100 μmol/l | 0.295±0.020 | 43.27 | 0.348±0.016 | 48.67 | 0.466±0.018 | 44.52 |
| 200 μmol/l | 0.244±0.017 | 53.08 | 0.284±0.017 | 58.11 | 0.385±0.014 | 54.17 |
| 300 μmol/l | 0.188±0.012 | 60.50 | 0.207±0.013 | 69.50 | 0.319±0.016 | 62.02 |
 | Table IIIInhibition ratio using half
inhibitory concentrations of each drug alone or in combination. |
Table III
Inhibition ratio using half
inhibitory concentrations of each drug alone or in combination.
| 24h | 48h | 72h |
|---|
|
|
|
|
|---|
| Group | OD | Inhibition rate
(%) | OD | Inhibition rate
(%) | OD | Inhibition rate
(%) |
|---|
| Hypoxia | 0.506±0.023 | | 0.690±0.022 | | 0.888±0.024 | |
| 5-FU | 0.272±0.018 | 46.25 | 0.358±0.019 | 52.61 | 0.467±0.022 | 47.41 |
| Celecoxib | 0.306±0.027 | 39.53 | 0.367±0.019 | 46.81 | 0.504±0.021 | 43.24 |
| Combination | 0.210±0.011 | 58.50 | 0.234±0.015 | 66.09 | 0.390±0.014 | 56.08 |
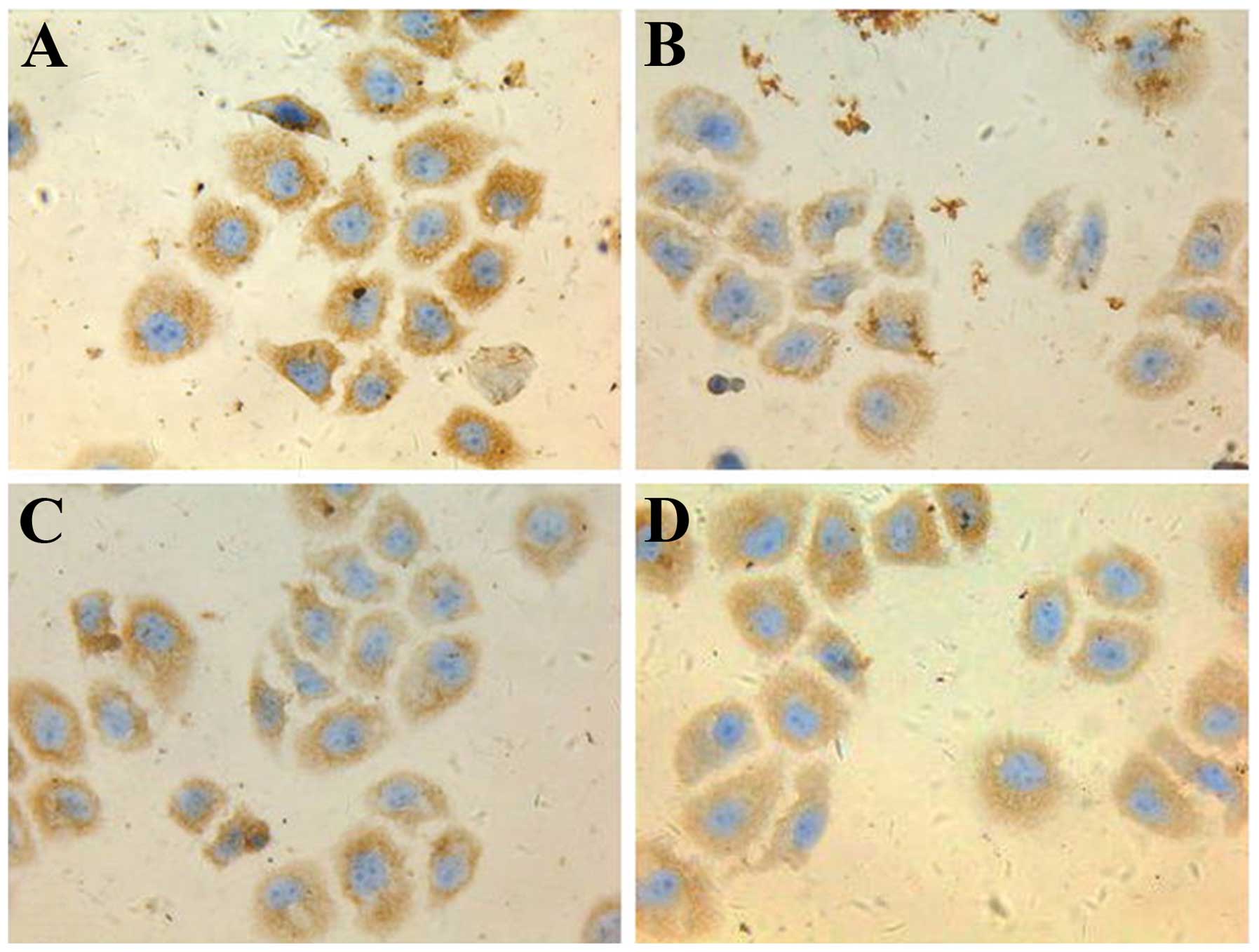
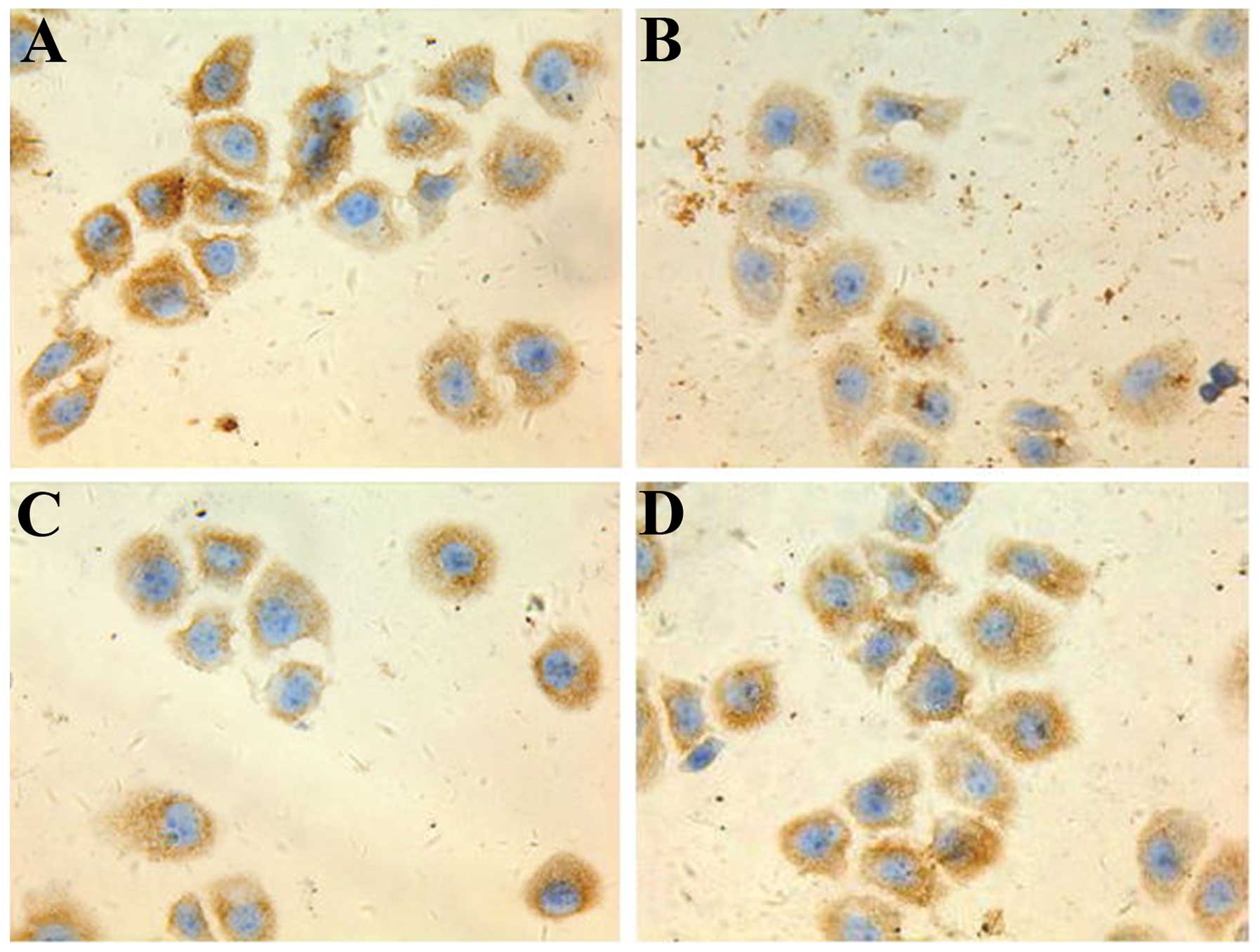
5-FU-treated cells express the highest
levels of HIF-2α, ABCG2 and Oct-4
Following 48 h in culture, immunohistochemical
analysis revealed that the expression of HIF-2α, ABCG2 and Oct-4
proteins were the highest in the 5-FU group, followed by the
hypoxia control group. The celecoxib and 5-FU/celecoxib combination
groups demonstrated the lowest expression of the proteins (Figs. 1–3).
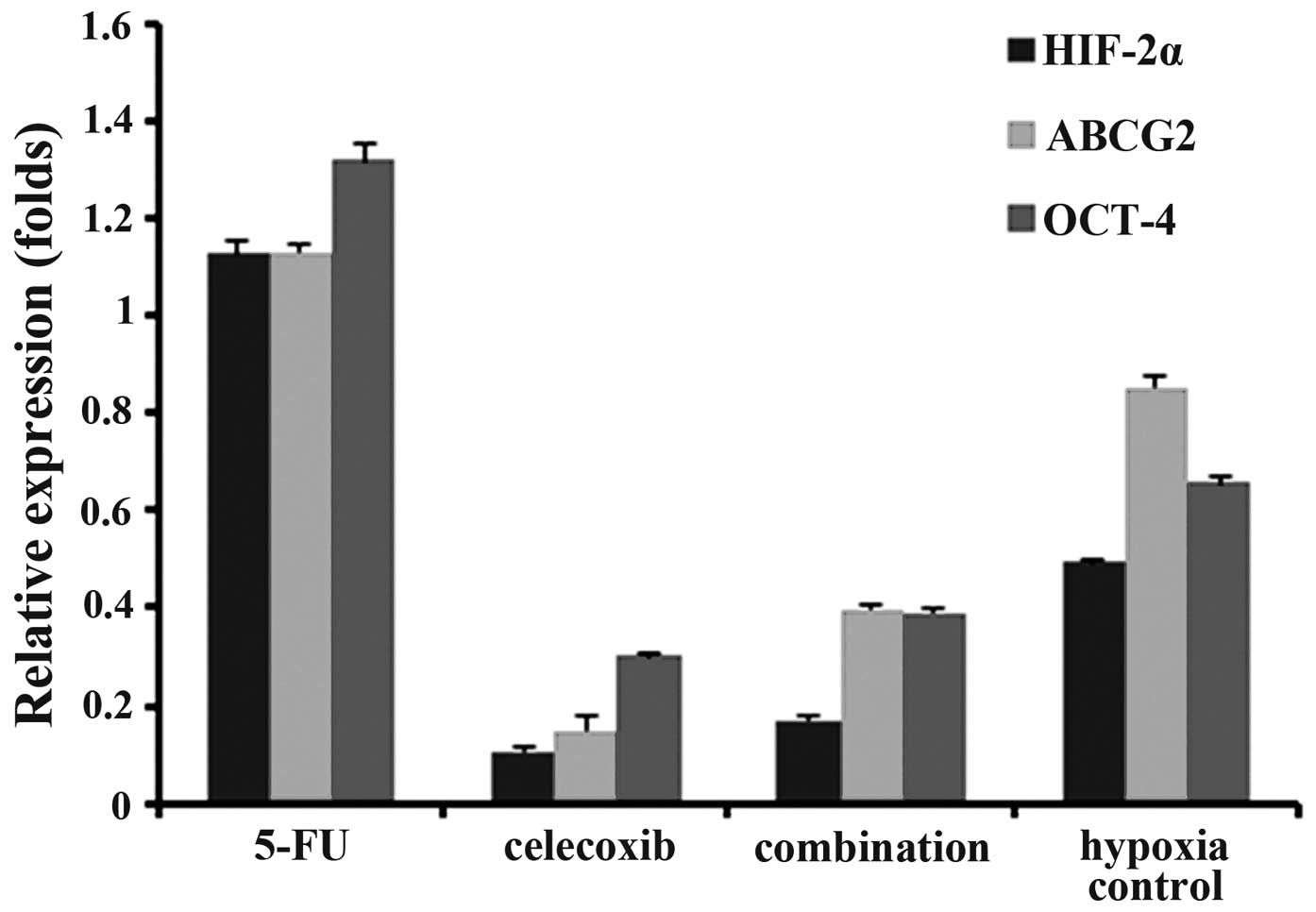
HIF-2α, ABCG2 and Oct-4 expression levels
are significantly reduced by celecoxib and 5-FU/celecoxib
combination treatments
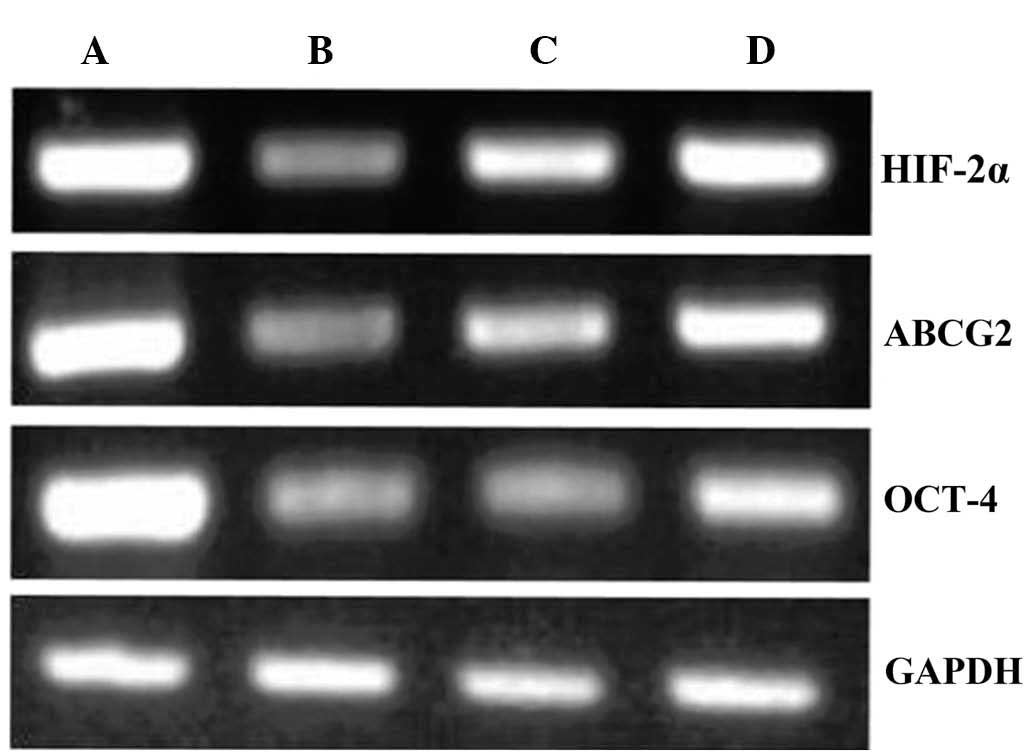
RT-qPCR was used to observe changes in HIF-2α, ABCG2
and Oct-4 mRNA expression in each group following 48 h in culture.
HIF-2α, ABCG2 and Oct-4 expression levels were the highest in the
5-FU group, followed by the hypoxia control group, and
significantly lower in the 5-FU/celecoxib combination and celecoxib
groups (P<0.01) (Figs. 4 and
5).
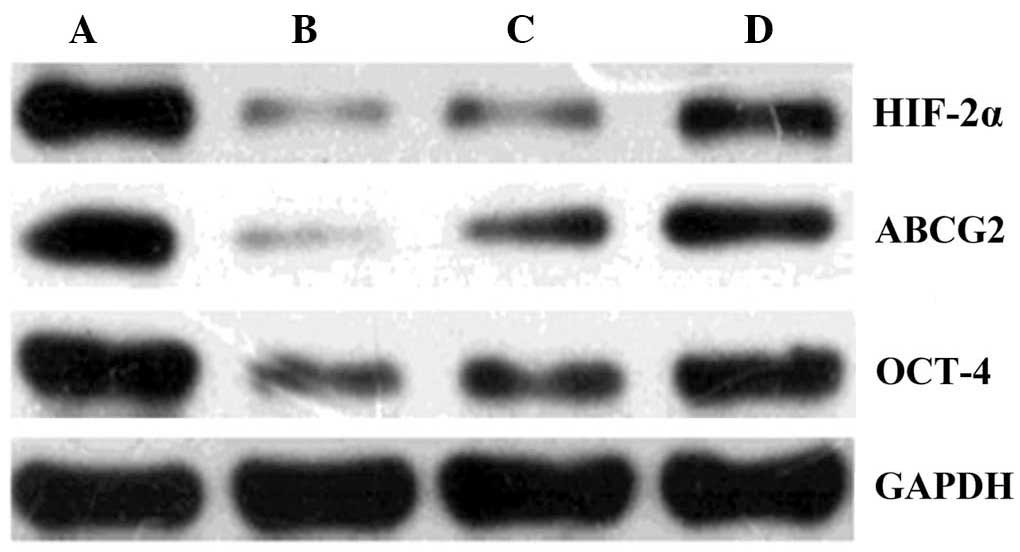
HIF-2α, ABCG2 and Oct-4 levels by western
blot
Western blot analysis was used to observe the
changes in HIF-2α, ABCG2 and Oct-4 expression following 48 h in
culture. HIF-2α, ABCG2 and Oct-4 expression were the highest in the
5-FU group, followed by the hypoxia control group, and
significantly lower in the 5-FU/celecoxib combination and celecoxib
groups (P<0.01) (Figs. 6 and
7).
Discussion
5-FU is one of the most commonly used
chemotherapeutic drugs, which is also employed to test the tumor
susceptibility of gastric cancer cells in vitro (7,8). The
mechanism of 5-FU proceeds through inducing apoptosis via blocking
DNA synthesis, which is done by restricting the progression of
cells in the S phase of the cell cycle (13). However, cells have been shown to
develop resistance to 5-FU, particularly solid tumor cells under
hypoxic conditions (4,7,8).
Therefore, determining novel strategies to overcome this resistance
is of prime importance.
The present study demonstrated that celecoxib or
5-FU alone were able to inhibit gastric cancer cell growth. Of
note, the combination of the two drugs had a synergistic effect,
further inhibiting the growth of tumor cells. The results also
revealed that the expression levels of HIF-2α, ABCG2 and Oct-4 were
involved in the growth suppression of these tumor cells.
Previous studies have reported that small amounts of
cancer stem cells were present in tumor tissues; these cells had an
unlimited self-renewal ability and unlimited differentiation
potential. Cancer stem cells are increasingly thought to be the
cause of metastases and tumor recurrence as well as drug and
radiation resistance (14,15). However, little is currently known
about these cells and their phenotypic marker profile has not yet
been defined; therefore, purification of cancer stem cells is
difficult.
HIF was reported to be closely associated with a
malignant phenotype, which was involved in tumor angiogenesis,
invasion and metastasis as well as drug and radiation resistance
(16). HIF-2α, in comparison to
HIF-1α, was shown to be more closely associated with cancer stem
cells and maintains the stem cell phenotype via the regulation of
several associated pathways (17).
ABCG2, a member of the superfamily of transport proteins, was
reported to be involved in the excretion of numerous
chemotherapeutic drugs from cells; therefore, high expression of
ABCG2 may be a significant contributing factor in tumor multi-drug
resistance (18). Studies have
shown that ABCG2 expression was high in numerous cancer stem cells,
and that ABCG2 was a direct target gene of HIF-2α. This therefore
indicated that high expression of HIF-2α and ABCG2 may lead to the
multi-drug resistance observed in tumor stem cells and hypoxic
cells (19). Oct-4, a member of
the Pit-Oct-Unc (POU) family of transcription factors, acts as a
marker of cancer stem cell pluripotency (20,21).
Covello et al (17)
demonstrated that Oct-4 was also a direct target gene of HIF-2α.
This therefore indicated that hypoxia may induce the retention of a
stem cell phenotype in tumor cells through activation of the
HIF-2α/Oct-4 pathway.
Dallas et al (22) reported a high expression of the
stem cell phenotype (CD133+/CD44+) in colon cancer cell lines
(HT29/5-FUR) resistant to 5-FU, therefore suggesting that the
cancer stem cells resistant to 5-FU may be the source of
chemotherapy resistance. A previous study showed that expression
levels of HIF-2α and ABCG2 were increased when 5-FU was added to
the SGC7901 gastric cancer cells under hypoxic conditions,
therefore indicating that this resistance may be associated with
the induction of the HIF-2α/ABCG2 pathway and promote the
maintenance of the stem cell phenotype (23).
Celecoxib is a selective COX-2 inhibitor which has
anti-inflammatory and analgesic effects (24). It is primarily used for the
treatment of acute or chronic osteoarthritis as well as rheumatoid
arthritis; in addition, celecoxib has fewer gastrointestinal side
effects than other NSAIDs (9).
Celecoxib was also reported to have certain anti-tumor effects
(10–12). Steinbach et al (25) showed that celecoxib significantly
reduced the occurrence of polyps in patients with familial
adenomatous polyposis. In addition, chronic NSAID therapy may be
able to reduce the risk of colon cancer by 50%, as well as the
incidence of esophageal and gastric cancer (26). Studies on animals showed that
celecoxib prevented and inhibited gastric cancer carcinogenesis
(27,28). The results of the present study
demonstrated that celecoxib inhibited the proliferation of SGC7901
gastric cancer cells. The inhibition rate of the combined
5-FU/celecoxib group was significantly increased compared with that
of the 5-FU group; these results were consistent with those of a
previous study using rofecoxib combined with anti-tumor drugs on
gastric cancer (29).
However, the mechanism of the anti-tumor effect of
NSAIDs remains to be elucidated. Previous experiments have
suggested that NSAIDs induce apoptosis of tumor cells through
inhibiting COX-2 activity, therefore reducing the synthesis of
prostaglandin E2 (30). However,
Ding et al (31) observed
that the mechanism of the anti-tumor effect of celecoxib occurs
prior to the deterioration of oral mucosa cells. Numerous studies
have suggested that celecoxib may promote tumor cell apoptosis via
COX-2-independent pathways, and its effects on apoptosis may be
achieved through the regulation of genes, including p21, Fas,
protein kinase B, glycogen synthase kinase 3β, forkhead homolog in
rhabdomyosarcoma, caspase-9, B cell lymphoma 2/B cell lymphoma
2-associated X protein, p53 and survivin (32–35).
However, further studies are required in order to elucidate the
exact mechanisms underlying the effects of celecoxib in tumor
cells.
In conclusion, the results of the present study
demonstrated that celecoxib reduced mRNA and protein expression of
HIF-2α, Oct-4 and ABCG2 in gastric cancer SGC7901 cells under
hypoxic conditions. This therefore indicated that elevated
expression of HIF-2α, ABCG2 and Oct-4 mRNA may lead to 5-FU
resistance. Furthermore, the results showed that celecoxib
increased the efficacy of 5-FU in gastric cancer by reducing 5-FU
resistance, therefore indicating its potential synergic use in
chemotherapy treatment. However, clinical trials are required to
confirm this hypothesis.
Acknowledgements
This study was supported by the Science and
Technology Innovation Foundation of Affiliated Hospital of Weifang
Medical University, and by a grant from the Shandong Province
Science and Technology Program (no. 2012YD18108).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Agboola O: Adjuvant treatment in gastric
cancer. Cancer Treat Rev. 20:217–240. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cunningham D, Allum WH, Stenning SP, et
al: Perioperative chemotherapy versus surgery alone for resectable
gastroesophageal cancer. N Engl J Med. 355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cosse JP and Michiels C: Tumour hypoxia
affects the responsiveness of cancer cells to chemotherapy and
promotes cancer progression. Anticancer Agents Med Chem. 8:790–797.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Griffiths EA, Pritchard SA, Welch IM,
Price PM and West CM: Is the hypoxia-inducible factor pathway
important in gastric cancer? Eur J Cancer. 41:2792–2805. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu L, Ning X, Sun L, et al:
Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced
chemoresistance in gastric cancer. Cancer Sci. 99:121–128.
2008.
|
|
8
|
Kang HC, Kim IJ, Park JH, Shin Y, Ku JL,
Jung MS, Yoo BC, Kim HK and Park JG: Identification of genes with
differential expression in acquired drug-resistant gastric cancer
cells using high-density oligonucleotide microarrays. Clin Cancer
Res. 10(1 Pt 1): 272–284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mathew ST, Devi SG, Prasanth VV and Vinod
B: Efficacy and safety of COX-2 inhibitors in the clinical
management of arthritis: Mini review. ISRN Pharmacol.
2011:4802912011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dannenberg AJ and Subbaramaiah K:
Targeting cyclooxygenase-2 in human neoplasia: rationale and
promise. Cancer Cell. 4:431–436. 2003. View Article : Google Scholar
|
|
11
|
Schönthal AH: Direct non-cyclooxygenase-2
targets of celecoxib and their potential relevance for cancer
therapy. Br J Cancer. 97:1465–1468. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chuang HC, Kardosh A, Gaffney KJ, Petasis
NA and Schönthal AH: COX-2 inhibition is neither necessary nor
sufficient for celecoxib to suppress tumor cell proliferation and
focus formation in vitro. Mol Cancer. 7:382008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshida K, Yamaguchi K, Osada S, Kawaguchi
Y, Takahashi T, Sakashita F and Tanaka Y: Challenge for a better
combination with basic evidence. Int J Clin Oncol. 13:212–219.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mayer A and Vaupel P: Hypoxia, lactate
accumulation, and acidosis: siblings or accomplices driving tumor
progression and resistance to therapy? Adv Exp Med Biol.
789:203–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Covello KL, Kehler J, Yu H, Gordan JD,
Arsham AM, Hu CJ, Labosky PA, Simon MC and Keith B: HIF-2alpha
regulates Oct-4: effects of hypoxia on stem cell function,
embryonic development, and tumor growth. Genes Dev. 20:557–570.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu L, McArthur C and Jaffe RB: Ovarian
cancer stem-like side-population cells are tumourigenic and
chemoresistant. Br J Cancer. 102:1276–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martin CM, Ferdous A, Gallardo T,
Humphries C, Sadek H, Caprioli A, Garcia JA, Szweda LI, Garry MG
and Garry DJ: Hypoxia-inducible factor-2alpha transactivates Abcg2
and promotes cytoprotection in cardiac side population cells. Circ
Res. 102:1075–1081. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nichols J, Zevnik B, Anastassiadis K, Niwa
H, Klewe-Nebenius D, Chambers I, Schöler H and Smith A: Formation
of pluripotent stem cells in the mammalian embryo depends on the
POU transcription factor Oct4. Cell. 95:379–391. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tai MH, Chang CC, Kiupel M, Webster JD,
Olson LK and Trosko JE: Oct4 expression in adult human stem cells:
evidence in support of the stem cell theory of carcinogenesis.
Carcinogenesis. 26:495–502. 2005. View Article : Google Scholar
|
|
22
|
Dallas NA, Xia L, Fan F, et al:
Chemoresistant colorectal cancer cells, the cancer stem cell
phenotype, and increased sensitivity to insulin-like growth
factor-I receptor inhibition. Cancer Res. 69:1951–1957. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang XQ, Feng YG, Wu MY, Bai HX and Wang
XY: The effect of 5-Fu on the ratio of SP cells and the expression
of HIF-2α and ABCG2 in gastric cancer SGC7901 cell line under
hypoxia. World Chinese Journal of Digestology. 20:1813–1818.
2012.
|
|
24
|
Rosas C, Sinning M, Ferreira A, Fuenzalida
M and Lemus D: Celecoxib decreases growth and angiogenesis and
promotes apoptosis in a tumor cell line resistant to chemotherapy.
Biol Res. 47:272014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steinbach G, Lynch PM, Phillips RK, et al:
The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial
adenomatous polyposis. N Engl J Med. 342:1946–1952. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li M, Lotan R, Levin B, Tahara E, Lippman
SM and Xu XC: Aspirin induction of apoptosis in esophageal cancer:
a potential for chemoprevention. Cancer Epidemiol Biomarkers Prev.
9:545–549. 2000.PubMed/NCBI
|
|
27
|
Kuo CH, Hu HM, Tsai PY, et al: Short-term
celecoxib intervention is a safe and effective chemopreventive for
gastric carcinogenesis based on a Mongolian gerbil model. World J
Gastroenterol. 15:4907–4914. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rocha FT, Lourenço LG, Jucá MJ, Costa V
and Leal AT: Chemoprevention by celecoxib in reflux-induced gastric
adenocarcinoma in Wistar rats that underwent gastrojejunostomy.
Acta Cir Bras. 24:189–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang C, Liu C, Zhou X and Wang C: Enhanced
inhibitive effects of combination of rofecoxib and octreotide on
the growth of human gastric cancer. Int J Cancer. 112:470–474.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dandekar DS, Lopez M, Carey RI and
Lokeshwar BL: Cyclooxygenase-2 inhibitor celecoxib augments
chemotherapeutic drug-induced apoptosis by enhancing activation of
caspase-3 and -9 in prostate cancer cells. Int J Cancer.
115:484–492. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding H, Han C, Zhu J, Chen CS and
D’Ambrosio SM: Celecoxib derivatives induce apoptosis via the
disruption of mitochondrial membrane potential and activation of
caspase 9. Int J Cancer. 113:803–810. 2005. View Article : Google Scholar
|
|
32
|
Kim N, Kim CH, Ahn DW, et al: Anti-gastric
cancer effects of celecoxib, a selective COX-2 inhibitor, through
inhibition of Akt signaling. J Gastroenterol Hepatol. 24:480–487.
2009. View Article : Google Scholar
|
|
33
|
Swamy MV, Herzog CR and Rao CV: Inhibition
of COX-2 in colon cancer cell lines by celecoxib increases the
nuclear localization of active p53. Cancer Res. 63:5239–5242.
2003.PubMed/NCBI
|
|
34
|
Erkinheimo TL, Lassus H, Finne P, van Rees
BP, Leminen A, Ylikorkala O, Haglund C, Butzow R and Ristimäki A:
Elevated cyclooxygenase-2 expression is associated with
alteredexpression of p53 and SMAD4, amplification of HER-2/neu, and
poor outcome in serous ovarian carcinoma. Clin Cancer Res.
10:538–545. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Krysan K, Merchant FH, Zhu L, et al:
COX-2-dependent stabilization of survivin in non-small cell lung
cancer. FASEB J. 18:206–208. 2004.
|