Introduction
Acute pancreatitis (AP) is one of the most common
pancreatic diseases. Although ~80% of AP can present as a mild
self-limiting disorder, severe acute pancreatitis (SAP) accounts
for ~20% of the cases and is associated with a high rate of
morbidity and mortality. The mechanisms underlying the disease
remain unclear, however, studies have suggested an association with
oxidative stress. Upon acinar cell injury, activation of the
proinflammatory cascade can lead to local pancreatic necrosis,
systemic inflammatory response syndrome as well as distant organ
dysfunction in human and experimental animal models (1). Proinflammatory cytokines from
activated monocytes and macrophages, such as interleukin (IL)-1β,
tumor necrosis factor (TNF)-α, IL-6 and IL-8, are hypothesized to
have an important function in this cascade, and this provides a
challenge in the treatment of SAP. The close association between
the severity of AP and oxidative stress, suggests that the
antioxidant system is a promising therapeutic target for SAP.
Emodin is an anthraquinone compound consisting of
three cyclic rings. Emodin is a major component of the widely used
Chinese herb, rhubarb, which has various physiological effects,
including immunomodulatory (2),
antitumor (3), chemopreventive
(4) and anti-inflammatory
(5) properties. Despite these
findings, the potential therapeutic mechanism underlying the effect
of emodin in acute pancreatitis has not been addressed.
Nuclear factor (NF)-κB is a sequence-specific
transcription factor known to be involved in the inflammatory and
immune response. It functions in numerous physiological and
pathologic conditions as an inducible nuclear factor. NF-κB
mediates various inflammatory mediators involved in acute
pancreatitis, including cytokines and adhesion molecules, as well
as modulating oxidation. Pyrrolidine dithiocarbamate (PDTC) is a
potent inhibitor of NF-κB, which has been administered to rats to
inhibit NF-κB activation (6). The
present study aimed to evaluate the protective roles of emodin and
its possible molecular mechanisms of action in SAP. The effects of
emodin in a rat model with SAP were investigated, and rats treated
with PDTC were used as a control.
Materials and methods
Animals and treatment groups
All experiments were conducted with the consent of
the University Animal Ethics Committee (Ruijin Hospital, School of
Medicine, Shanghai JiaoTong University, Shanghai, China) for the
use of Sprague Dawley (SD) rats of the Shanghai Experimental
Animals Center of the Chinese Academy of Sciences (Shanghai,
China). Healthy male SD rats weighing 220–230 g were housed in
standard cages, under climate and temperature (22±2°C) control, in
a 12-h light/dark cycle. Rats were fed with standard laboratory
chow and water ad libitum. The rats were fasted overnight
prior to each experiment.
The rats (n=72) were randomly divided into four
groups, with each group containing 18 animals: Control group (SO),
SAP group, emodin treated group and PDTC treated group. SAP models
were established in the SAP, emodin and PDTC treated groups by
injection of 200 μl of 5% sterile sodium taurocholate (NaTc; Sigma,
St. Louis, MO, USA) into the biliary-pancreatic duct (BPD) for 2
min. The control (SO) group was administered with saline.
Pyrrolidine dithiocarbamate (PDTC) was administrated
intraperitoneally 1 h prior to the initiation of pancreatitis at a
dose of 100 mg/kg. Emodin was administered once at a dose of 1
mg/kg, following the initiation of pancreatitis. The rats were
sacrificed by exsanguination at 1, 3, 6, and 12 h, and blood
samples and pancreatic tissues were harvested.
The effects of emodin on the survival of SAP-induced
rats were assessed 72 h after the induction of SAP. Healthy rats
(n=30) were randomly divided into three groups, with 10 animals per
group: SAP group (S), Emodin-treated group and PDTC-treated group.
SAP was induced, and PDTC and emodin were administered, as
described for the previous groups used in this study.
Serum amylase assay
Serum amylase was determined using a commercially
available kit (Randox Laboratories, Crumlin, UK) and by a BeckmanDU
530 Life Science UV/VIS spectrophotometer (Beckman Coulter,
Fullerton, CA, USA).
Cytokine assay
Serum TNF-α, IL-1β and IL-6 levels were detected by
enzyme-linked immunosorbent assays (ELISA) using commercially
available kits (Rat Quantikine ELISA kits; R&D Systems,
Minneapolis, MN, USA) according to the manufacturer’s instructions.
The optical density (OD) was measured using a spectrophotometer at
450 nm with a correction wavelength set at 570 nm. The
sensitivities for TNF-α, IL-1β and IL-6 were 15, 5 and 5 pg/ml
respectively.
Oxidative stress analyses
Pancreatic tissue was homogenized in ice-cold
physiological saline. The superoxide dismutase (SOD) content was
measured using a xanthine oxidase technique, based on the
spectrophotometric monitoring of SOD-mediated reduction of DTNB at
an OD of 550 nm. The concentration of malondialdehyde (MDA) was
quantified by a thiobarbituric acid (TBA) reaction, in which MDA or
MDA-like substances and TBA react with the production of a pink
pigment having an absorption maximum at an OD of 532 nm. SOD and
MDA levels were expressed as nm/mg protein.
NF-κB DNA-binding assay
DNA-binding affinity was measured using the TransAM
NF-κB Chemi kit (Active Motif, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Serial dilutions (1:2) were made of
the nuclear extracts and 20 μl of each dilution and 30 μl binding
buffer were added to a microtiter plate (Active Motif) coated with
an oligonucleotide containing the κB site of the immunoglobulin
(Ig) light chain gene promoter (GGGACTTTCC). The wells were
incubated at room temperature for 1 h and were then washed three
times in 200 μl washing buffer. Subsequently, the wells were
incubated with NF-κB antibody (1:1,000; Active Motif) for 1 h, and
were washed three times in washing buffer. The wells were then
incubated for a further 1 h with horseradish peroxidase-conjugated
goat anti-rabbit IgG secondary antibody (1:10,000; Active Motif).
The wells were then washed four times, prior to the addition of
chemiluminescent substrate (Active Motif). Luminescence was
measured using a spectrophotometer set at an OD of 485 nm with a
correction wavelength set at 535 nm.
Western blot analysis
Cells were lysed with radioimmunoprecipitation lysis
buffer (Beyotime, Jiangsu, China) supplemented with 1 mM
phenylmethanesulfonyl fluoride (Sigma). Anti-NF-κB p65 (phospho
S529) antibody was purchased from Abcam (Cambridge, MA, USA).
Signals were detected using an Odyssey infrared imaging system
(LI-COR, Lincoln, NE, USA) after incubating with IRDye 800 goat
anti-rabbit IgG (H + L) (LI-COR Biosciences, Lincoln, NE, USA)
secondary antibodies. Quantification was performed using Image J
software (National Institutes of Health, Bethesda, MD, USA).
Histological examination
The tail of the pancreas was fixed in 5%
paraformaldehyde, then dehydrated and embedded in paraffin wax.
Sections (3-μm thick) were cut, dewaxed and stained with
hematoxylin and eosin for histological examination. The assessment
was performed by an experienced pathologist blind to the
experimental design. For the pancreas, a scale of 0 to 4 was used
to grade the interstitial edema and hemorrhage, inflammatory cell
infiltration, and acinar cell vacuolization and necrosis,
consistent with the method described by Schmidt et al
(7).
Statistical analysis
The data are presented as the mean ± standard error
of the mean. A one-way analysis of variance was performed by SAS
6.12 (SAS Institute Inc., Cary, NC, USA). Mortality was evaluated
using the log rank method. P<0.05 was considered to indicate a
statistically significant difference.
Results
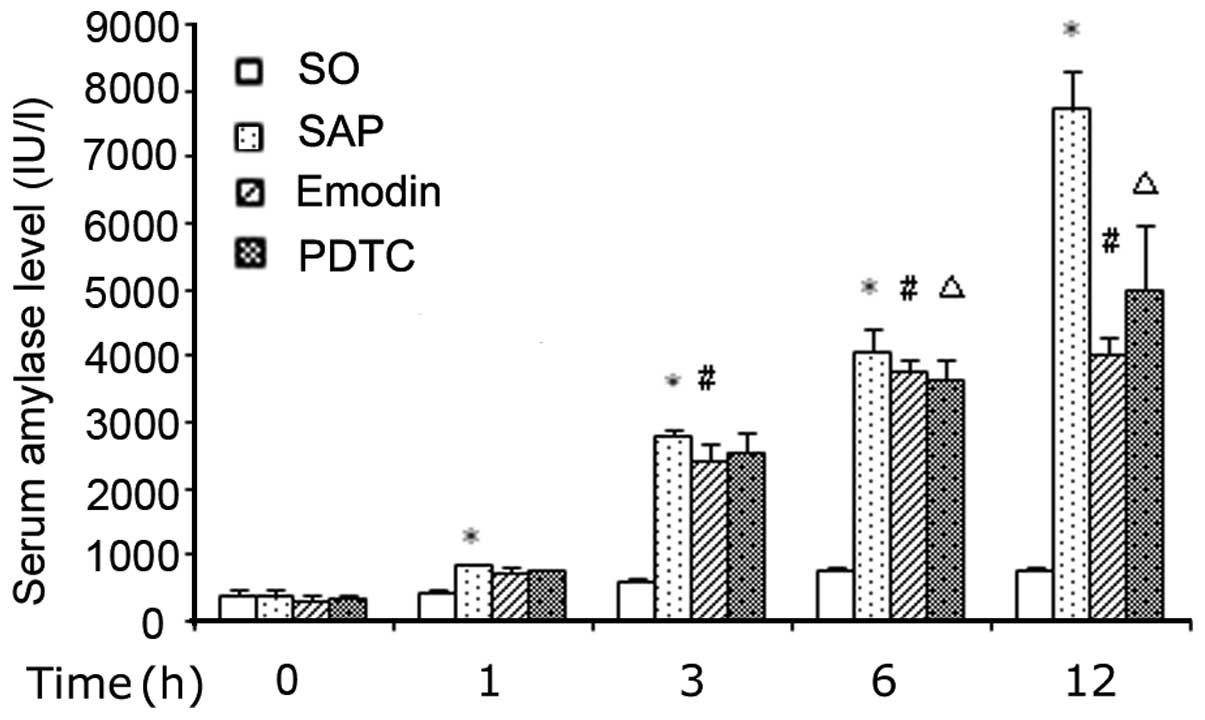
Serum amylase levels
The SAP group showed a marked increase in the level
of serum amylase (P<0.05) after the infusion of 5% NaTc into the
BPD, as compared with the SO group. The application of emodin or
PDTC significantly decreased this elevation at each time point
(Fig. 1).
Serum cytokine levels
The serum levels of TNF-α, IL-1β and IL-6 increased
when SAP was induced, whereas the serum levels decreased when
emodin or PDTC was administered. In comparison with the SAP group,
the emodin and PDTC treated group had a significantly lower level
of TNF-α at 3, 6 and 12 h (P<0.01), a lower level of IL-1β at 1,
3 and 6 h (P<0.01), and a lower level of IL-6 at 1, 3, 6 and 12
h (Fig. 2).
 | Figure 2Serum levels of (A) TNF-α, (B) IL-1β
and (C) IL-6. The induction of SAP increased the serum levels of
TNF-α, IL-1β and IL-6 at 1, 3, 6 and 12 h (*P<0.05,
as compared with the control group). The application of emodin and
PDTC markedly decreased TNF-α expression levels at 3, 6 and 12 h,
IL-1β levels at 1, 3 and 6 h and IL-6 levels at 1, 3, 6 and 12 h
(#P<0.05, as compared with the SAP group). SO,
control group; SAP, severe acute pancreatitis; PDTC, pyrrolidine
dithiocarbamate; IL, interleukin; TNF-α, tumor necrosis
factor-α. |
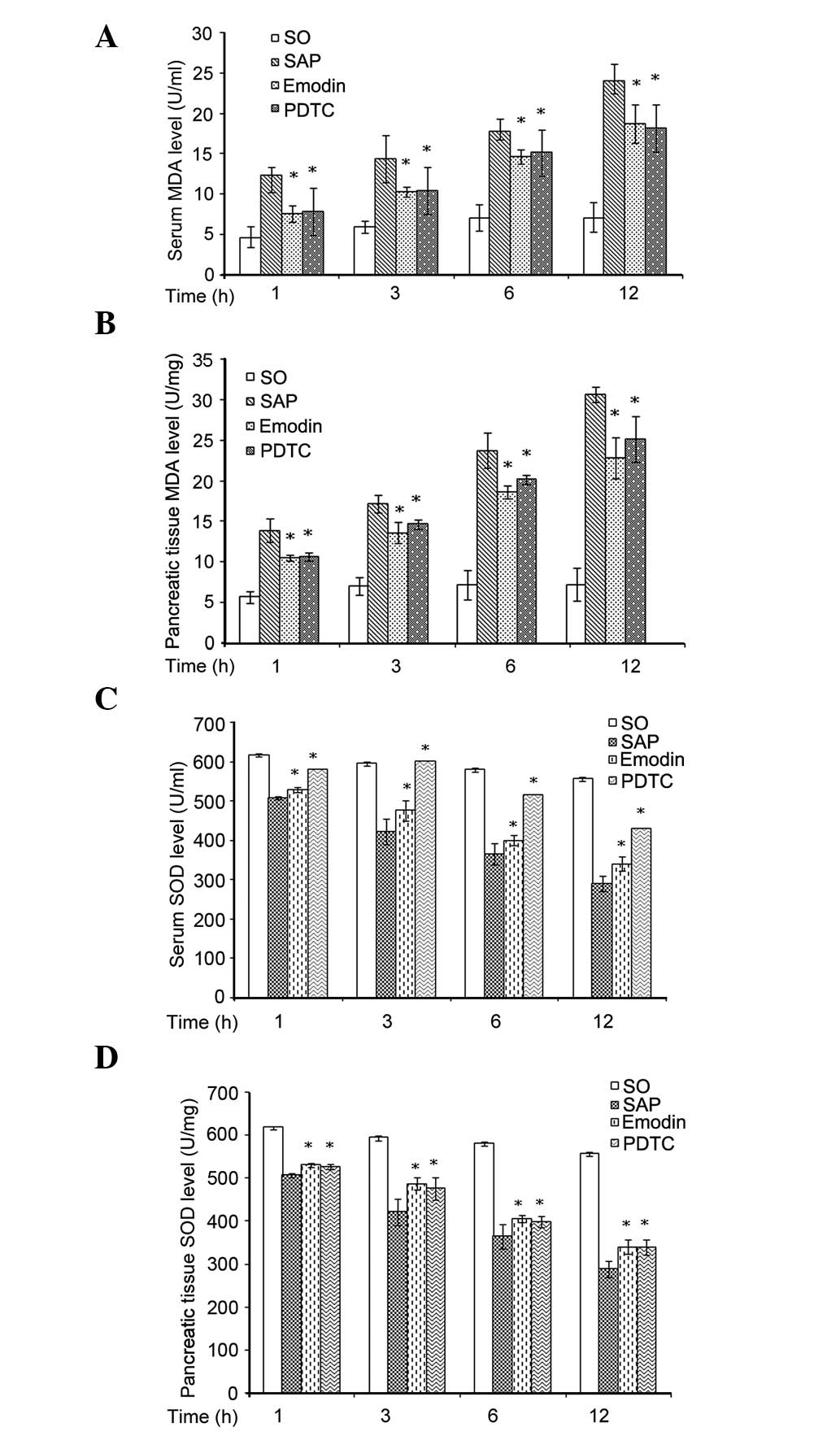
Oxidative stress analyses
There was a significantly higher level of MDA in the
pancreatic tissue (P<0.05), and depleted SOD level (P<0.05)
at each hourly time point investigated in the SAP group. The
application of emodin or PDTC markedly decreased the expression
levels of MDA and increased the expression level of SOD at 1, 3, 6
and 12 h (Fig. 3).
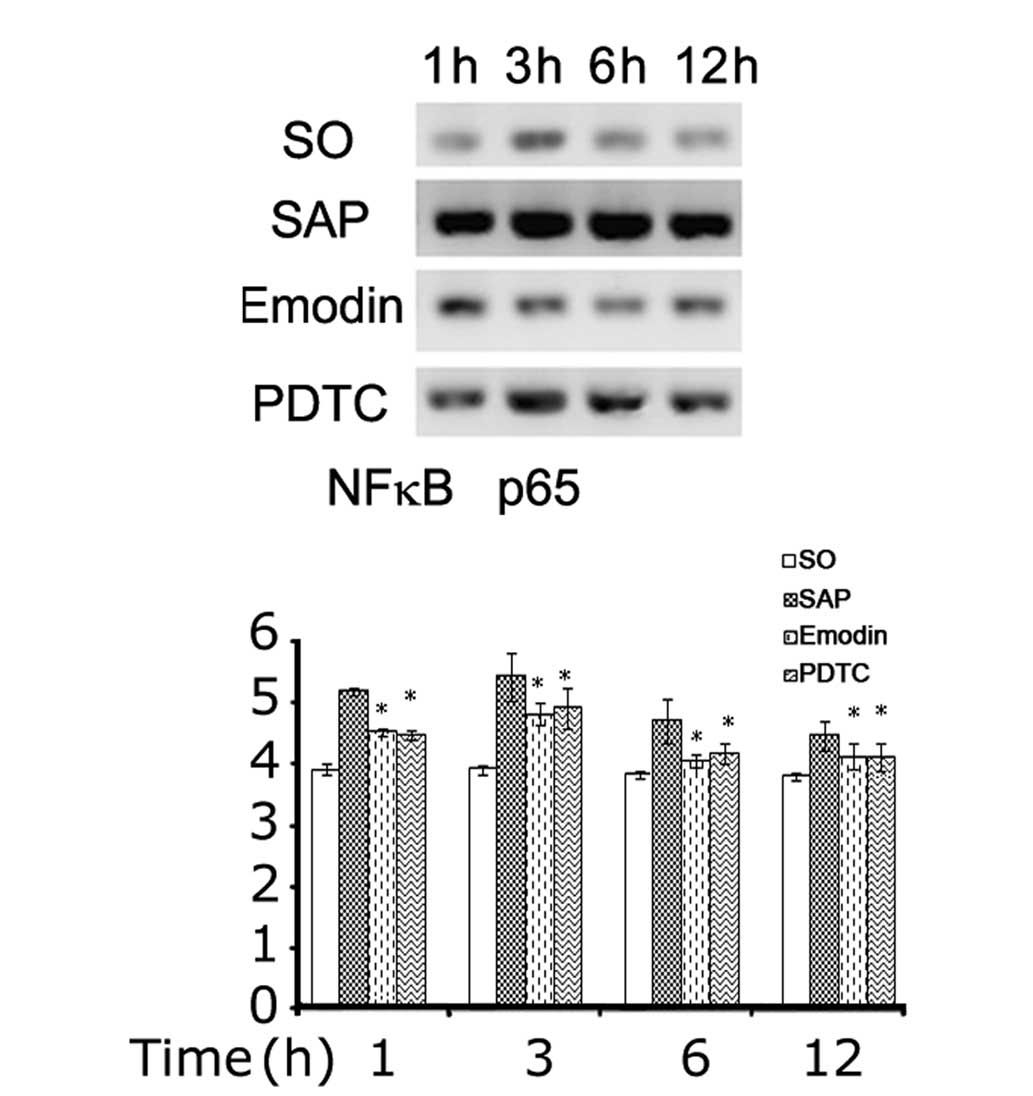
NF-κB DNA-binding assay
NF-κB was shown to be activated in the pancreatic
tissue at 1, 3, 6 and 12 h after the induction of SAP. Emodin and
PDTC were observed to inhibit this activation (Fig. 4).
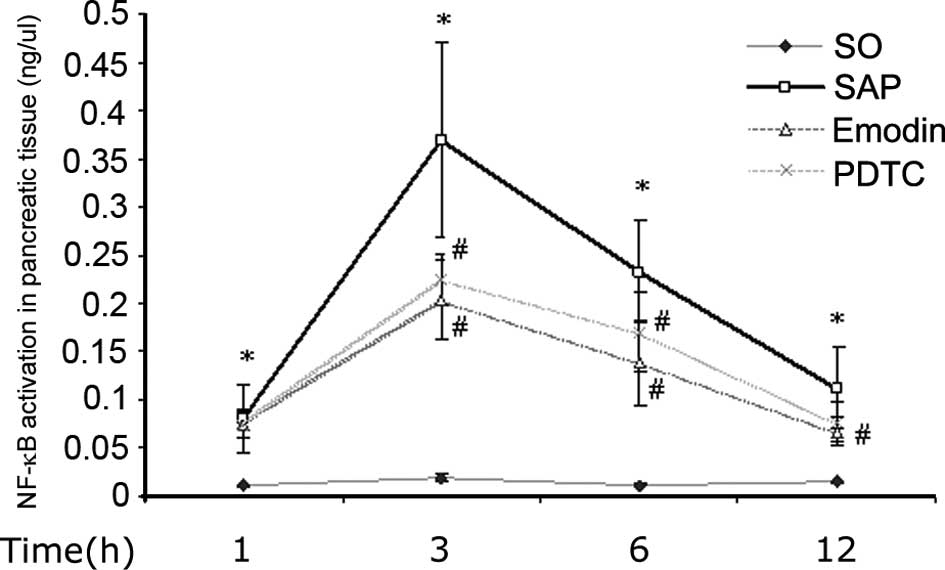 | Figure 4NF-κB activation in pancreatic tissue.
The activation of NF-κB in pancreatic tissue was higher after
induction of SAP at 1, 3, 6 and 12 h (*P<0.01, as
compared with control group, respectively), while application of
emodin or PDTC inhibited this elevation (#P<0.01, as
compared with SAP group, respectively). SO, control group; SAP,
severe acute pancreatitis; PDTC, pyrrolidine dithiocarbamate;
NF-κB, nuclear factor kappa-light-chain-enhancer of activated B
cells. |
NF-κB p65 western blot assay
NF-κB p65 protein was detected by western blotting
in the pancreatic tissue at 1, 3, 6 and 12 h after the induction of
SAP. Emodin and PDTC were observed to inhibit this activation
(Fig. 5).
Histological findings
The grade of pancreatitis was evaluated based on the
Schmidt histological scoring system (5). Large areas of necrosis, together with
infiltration of inflammatory cells and hemorrhage, were identified
in the SAP group, which had a higher histological score as compared
with the control group (Table I).
Following emodin and PDTC treatment, less inflammation and
hemorrhage was observed, and necrosis was detected in <30% of
the total areas.
 | Table IChanges of histological scores. |
Table I
Changes of histological scores.
| Group | 1 h | 3 h | 6 h | 12 h |
|---|
| SO | 0.5±0.55 | 0.67±0.52 | 0.83±0.41 | 1.17±0.41 |
| SAP | 5.67±0.52 | 9.67±0.52 | 12.5±1.05 | 13.83±1.17 |
| Emodin | 4.67±0.52a | 7.67±0.82a,b | 10.33±1.03a,b | 11.17±0.98a,b |
| PDTC | 4.67±0.52a | 8.67±0.82a | 10.67±0.82a,b | 11.83±0.75a,b |
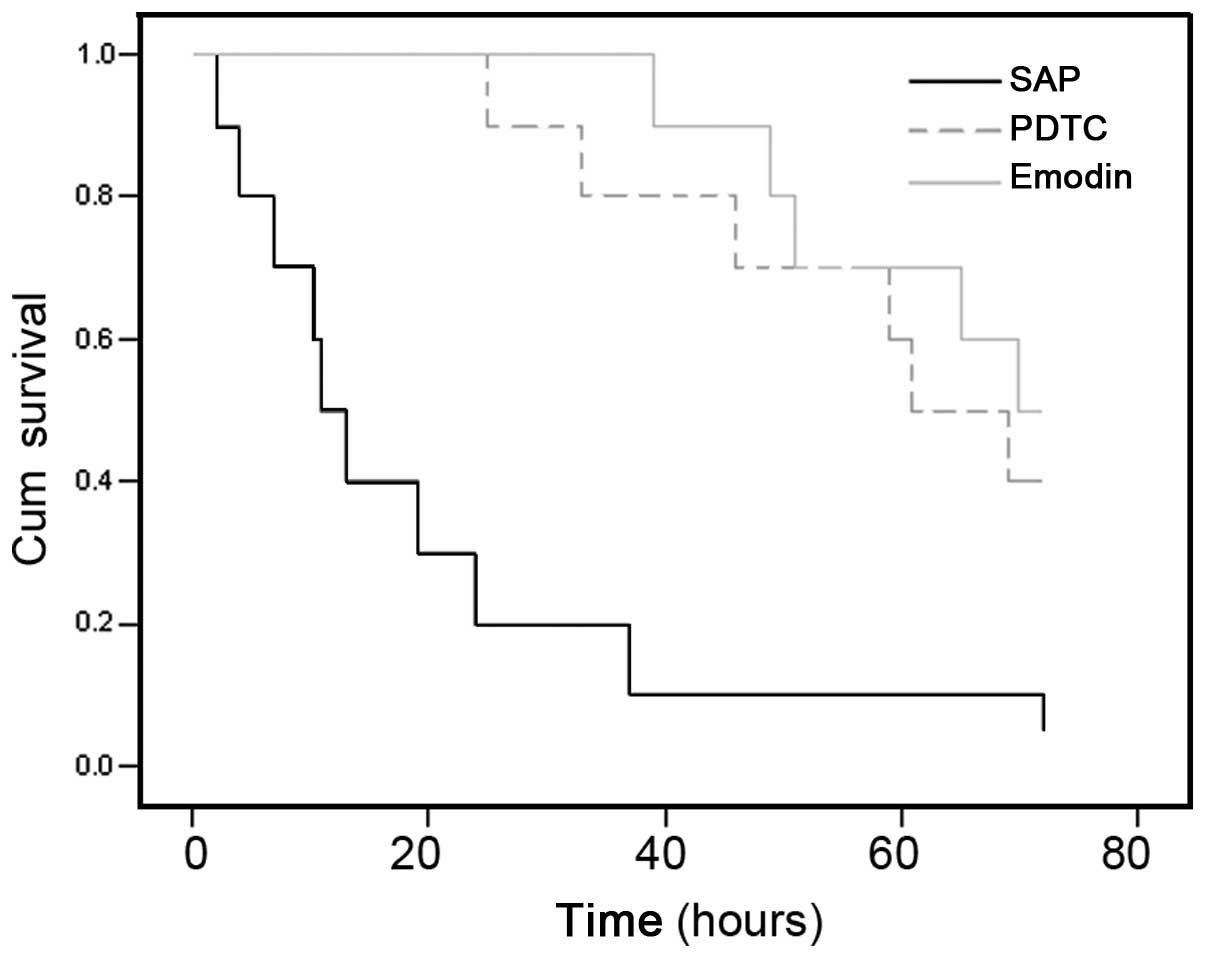
Survival
The majority of the SAP rats died within two days
when no treatment was applied. By contrast, emodin or PDTC could
significantly improve the survival rate, with 40% of the rats still
alive at the end of the study (three days) (Fig. 6).
Discussion
The present study used an experimental SAP rat model
to determine whether emodin could significantly improve the
oxidative stress and morphological injury in pancreatic tissues.
Previous studies have indicated that emodin may influence
antioxidant function, cytokine production and TGF signaling, which
has been demonstrated in an ischemia injury model (8). Furthermore, it has been proposed that
emodin could enhance tissue regeneration through the regulation of
the Smad-TGF-β1 pathway (9). Few
studies have focused on the effects of emodin on SAP. A reliably
induced model with severe, acute onset of hemorrhagic and necrotic
pancreatitis, was established in rats by retrograde infusion of
NaTc in the BPD. It was subsequently shown that emodin could
decrease the serum levels of TNF-α and IL-1β, thus improving the
morphological damage and survival rate. Similar results have been
reported demonstrating the effective treatment of clinical and
experimental acute pancreatitis by emodin (10–12).
It has been shown that significant lipid
peroxidation occurs in AP (13,14).
Reactive oxygen species can activate NF-κB, which can in turn
regulate the expression of inflammatory cytokines, such as TNF-α
and ILs in acinar cells, and lead to the activation of a cascade of
inflammatory cytokines (15). This
is hypothesized to function in the pathogenesis and the progression
of acute pancreatitis and the systemic complications. PDTC is
commonly used as an antioxidant, which can regulate gene expression
by modulating the activity of transcription factors (16). PDTC can block NF-κB from binding to
its target DNA by preventing the degradation of IκB-α from the
ubiquitylation-proteasome proteolytic pathway. NF-κB is known to
regulate numerous proinflammatory molecules, including IL-6, IL-1,
TNF-α, and inducible nitric oxide synthase, cell surface adhesion
molecules (such as E-selectin, intercellular adhesion molecule-1
and vascular cell adhesion molecule-1) and cell surface mediators
(such as major histocompatibility complexes class I and II) through
NF-κB binding sites in their promoter regions. It has been shown
that antioxidant treatment could inactivate NF-κB and inhibit the
activation of neutrophils in acute pancreatitis (17). In the present study, treatment with
PDTC significantly attenuated SAP, as determined by serum amylase
and histological assessment of edema, vacuolization, inflammation
and necrosis (P<0.01). Furthermore, treatment with PDTC markedly
inhibited NF-κB DNA-binding activity (P<0.01, as compared with
the SAP group) and the expression of TNF-α, IL-6 and IL-1 in the
localized tissues and the serum (P<0.05). The observed
attenuation was associated with decreased MDA and increased SOD
levels, in the pancreatic tissues and serum (P<0.05). This is
consistent with previous studies investigating other inflammatory
diseases (18–20). In the present study, treatment with
emodin had a similar effect to treatment with PDTC. Emodin could
inhibit NF-κB activation and regulate cytokine production, thereby
regulating the oxidative stress response and improving the
histological changes and survival rate. In conclusion, this study
has shown that in an experimental rat model of SAP, emodin
treatment significantly improved the oxidative stress and
morphological injury in the pancreatic tissue, suggesting
antioxidant treatment strategies may have clinical benefits.
Understanding the therapeutic effects of emodin in AP and SAP, and
its mechanisms of action is likely to guide future studies in this
field.
Acknowledgements
This study was supported by the National Scientific
Fund (grant no. 81101849).
References
|
1
|
Makhija R and Kingsnorth AN: Cytokine
storm in acute pancreatitis. J Hepatobiliary Pancreat Surg.
9:401–410. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujimoto H, Nakamura E, Okuyama E and
Ishibashi M: Six immunosuppressive features from an ascomycete,
Zopfiella longicaudata, found in a screening study monitored by
immunomodulatory activity. Chem Pharm Bull (Tokyo). 52:1005–1008.
2004. View Article : Google Scholar
|
|
3
|
Srinivas G, Babykutty S, Sathiadevan PP
and Srinivas P: Molecular mechanism of emodin action: transition
from laxative ingredient to an antitumor agent. Med Res Rev.
27:591–608. 2007. View Article : Google Scholar
|
|
4
|
Braumann C, Tangermann J, Jacobi CA,
Muller JM and Dubiel W: Novel anti-angiogenic compounds for
application in tumor therapy - COP9 signalosome-associated kinases
as possible targets. Mini Rev Med Chem. 8:421–428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Y, Tu X, Lin G, et al: Emodin-mediated
protection from acute myocardial infarction via inhibition of
inflammation and apoptosis in local ischemic myocardium. Life Sc.
81:1332–1338. 2007. View Article : Google Scholar
|
|
6
|
Yucel M, Kucuk A, Bayraktar AC, Tosun M,
Yalcinkaya S, Hatipoglu NK, Erkasap N and Kavutcu M: Protective
effects of the nuclear factor kappa B inhibitor pyrrolidine
dithiocarbamate in bladder ischemia-reperfusion injury in rats. Mol
Biol Rep. 40:5733–5740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmidt J, Lewandrowsi K, Warshaw AL,
Compton CC and Rattner DW: Morphometric characteristics and
homogeneity of a new model of acute pancreatitis in the rat. Int J
Pancreatol. 12:41–51. 1992.PubMed/NCBI
|
|
8
|
Lu JS, Liu JX, Zhang WY, et al: Preventive
effects of emodin on cerebral ischemia injury and expression of the
inflammatory factors in rats with cerebral ischemia. Zhongguo Zhong
Yao Za Zhi. 30:1939–1943. 2005.(In Chinese).
|
|
9
|
Tang T, Yin L, Yang J and Shan G: Emodin,
an anthraquinone derivative from Rheum officinale Baill, enhances
cutaneous wound healing in rats. Eur J Pharmacol. 567:177–185.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang G, Sun B, Gao Y, Meng QH and Jiang
HC: An experimental study of emodin assisted early enteral
nutrition for the treatment of severe acute pancreatitis.
Hepatogastroenterology. 55:33–40. 2008.PubMed/NCBI
|
|
11
|
Gong Z, Yuan Y, Lou K, et al: Mechanisms
of Chinese herb emodin and somatostatin analogs on pancreatic
regeneration in acute pancreatitis in rats. Pancreas. 25:154–160.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu JX, Xu JY and Yuan YZ: Effect of emodin
and sandostatin on metabolism of eicosanoids in acute necrotizing
pancreatitis. World J Gastroenterol. 6:293–294. 2000.
|
|
13
|
Gutierrez PT, Folch-Puy E, Bulbena O and
Closa D: Oxidised lipids present in ascitic fluid interfere with
the regulation of the macrophages during acute pancreatitis,
promoting an exacerbation of the inflammatory response. Gut.
57:642–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abu-Hilal M, McPhail MJ, Marchand L and
Johnson CD: Malondialdehyde and superoxide dismutase as potential
markers of severity in acute pancreatitis. JOP. 7:185–192.
2006.PubMed/NCBI
|
|
15
|
Ji LL, Gomez-Cabrera MC and Vina J: Role
of nuclear factor kappaB and mitogen-activated protein kinase
signaling in exercise-induced antioxidant enzyme adaptation. Appl
Physiol Nutr Metab. 32:930–935. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mingyan E, Hongli L, Shufeng L and Bo Y:
Effects of pyrrolidine dithiocarbamate on antioxidant enzymes in
cardiomyopathy induced by adriamycin in rats. Cardiology.
111:119–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Surh YJ: NF-kappa B and Nrf2 as potential
chemopreventive targets of some anti-inflammatory and antioxidative
phytonutrients with anti-inflammatory and antioxidative activities.
Asia Pac J Clin Nutr. 17:269–272. 2008.PubMed/NCBI
|
|
18
|
Jaworek J, Nawrot-Porabka K, Leja-Szpak A,
et al: Melatonin as modulator of pancreatic enzyme secretion and
pancreatoprotector. J Physiol Pharmacol. 58:65–80. 2007.
|
|
19
|
Lawinski M, Sledzinski Z, Kubasik-Juraniec
J, et al: Does resveratrol prevent free radical-induced acute
pancreatitis? Pancreas. 31:43–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wereszczynska-Siemiatkowska U, Mroczko B,
Siemiatkowski A, et al: The importance of interleukin 18,
glutathione peroxidase, and selenium concentration changes in acute
pancreatitis. Dig Dis Sci. 49:642–650. 2004. View Article : Google Scholar : PubMed/NCBI
|