Introduction
Myocardial infarction (MI), often followed by
progressive deterioration of left ventricular function, eventually
leads to overt heart failure (1).
In spite of recent therapeutic advances, heart failure following MI
and left ventricular remodeling are still associated with
significant morbidity and mortality rates. Mesenchymal stem cell
(MSC)-based therapy is a promising treatment to prevent functional
deterioration of MI towards heart failure. Following
transplantation, MSCs differentiate into cardiomyocyte-like cells,
which promote cardiac repair in the ischemic myocardium (2,3).
However, the repair effect from cell therapy is modest or
transient. A possible reason for this is the inefficient
cardiomyocyte-like differentiation from transplanted MSCs in
vivo (4,5). Emerging evidence suggests that
differentiation induction of MSCs prior to transplantation improves
the therapeutic effect from grafted cells (6–8). It
has been shown that 5-azacytidine (5-AZA) induces the
differentiation of MSCs into cardiomyocyte-like cells by random
demethylation in vitro. However, the induction effect of
5-AZA alone is weak and limited (9,10).
Therefore, the development of a more effective induction strategy
is necessary for further research.
Insulin-like growth factor-1 (IGF-1), a 70-amino
acid single-chain protein, plays a significant role in
cardiomyocyte differentiation (11). Therefore, IGF-1 gene overexpression
in MSCs may potentially improve the induction effect of 5-AZA for
cardiomyocyte-like differentiation. In the present study, IGF-1
gene manipulation was combined with 5-AZA treatment to investigate
the synergetic induction effect in vitro.
Materials and methods
Porcine MSC isolation and culture
Bone marrow was collected from the femur of five
healthy female Shanghai white pigs (8–12 weeks old; Shanghai Jiagan
Biological Technology Co., Ltd., Shanghai, China) with a 16-gauge
needle containing 200 U/ml heparin (Sigma-Aldrich, St. Louis, MO,
USA). The aspirates were depleted of mature blood lineages and
purified by centrifugation in a 1.073-g/ml Percoll (Gibco, Grand
Island, NY, USA) density gradient. Mononuclear cells were cultured
in tissue culture flasks in low-glucose Dulbecco’s modified Eagle’s
medium (LG-DMEM; Gibco) supplemented with 10% fetal bovine serum
(Hyclone, Logan, UT, USA), 100 U/ml penicillin and 100 μg/ml
streptomycin (Gibco). Non-adherent cells were discarded after 48 h,
and adherent cells were further expanded until confluent. Cells
were passaged up to eight times. The morphology of isolated cells
was observed under a microscope (DM2700M; Leica, Wetzlar, Germany).
This study was approved by the Institutional Review Board and
Institutional Animal Care and Use Committee of Fudan University
(Shanghai, China).
Flow cytometry analysis
Cells were stained with CD90, CD44, CD34 and CD45
(Ebioscience, San Diego, CA, USA), and then with an appropriate
secondary antibody conjugated with FITC and Cy5 (Ebioscience).
After washing with phosphate-buffered saline (PBS), flow cytometry
was performed on a BD FACSCalibur (BD Biosciences, San Jose, CA,
USA).
Cell proliferation assay
Cell proliferation was monitored by 3-(4,
5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
assay. MSCs from passages 2, 5 and 8 were seeded onto 96-well
plates (1×104 cells/well), and cell proliferation was
documented every 24 h for 12 days. The number of viable cells was
assessed by measurement of the absorbance at 490 nm using a Safire
2 microplate reader (Tecan Systems Inc., San Jose, CA, USA).
Cell vitality assay
MSCs from passages 2, 5 and 8 were prepared at a
high concentration of 106 cells/ml, and 10 μl cell
suspension was mixed with 790 μl PBS and 200 μl trypan blue (20
g/l). The number of stained cells and the total number of cells
were counted in a clean hemocytometer slide under a low-power
microscope (DM2770M; Leica).
Differentiation induction with 5-AZA
alone
To demonstrate the single induction effect of 5-AZA
(Sigma-Aldrich), MSCs from passages 2 and 5 were grown to 70–85%
confluence and treated with 5-AZA (10 μmol/l) for 24 h. The
morphological changes in MSCs from day 0 (prior to treatment) to
day 21 were observed under a microscope (DM2700M; Leica).
Construction of lentivirus encoding the
IGF-1 gene or shRNA-IGF-1
cDNA encoding the IGF-1 gene was cloned and inserted
into the pCDH-CMV-MCS-EF1-coGFP vector (GeneChem, Shanghai, China)
at the EcoRI and XbaI sites. shRNA-IGF-1 was
synthesized as follows: shRNA-IGF-1 sense, CCGGTGTTCAGG AAACAAG
AACTACTCGAGTAGTTCTTGTTTCCTGAACTTTTTTG; and shRNA-IGF-1 antisense,
AATTCA AAAAAGTTCAGGA AACAAGAACTACTCGAGTAGTTCTGTTTCCTGAACA. After
annealing, the shRNA-IGF-1 fragment was cloned into the pLKO.1-TRC
vector. The identities of recombinant plasmids pCDH-IGF-1-GFP and
pLKO.1-shRNA-IGF-1 were confirmed by PCR and DNA sequencing.
Following construction, pCDH-IGF-1-GFP and
pLKO.1-shRNA-IGF-1 were transfected into a packaging cell line
(293T; American Type Culture Collection, Manassas, VA, USA) with
package plasmids psPAX2 and pMD2.G (GeneChem) to produce a
high-titer lentivirus. Concentrated viral supernatants were
generated by ultracentrifugation and assessed by
fluorescence-activated cell sorting (FACS) analysis for GFP on
transduced control cells.
Overexpression or knockdown of IGF-1 gene
in MSCs
MSCs from passage 2 were divided into four groups as
follows: i) MSC group: MSCs were untreated; ii) control group: MSCs
were transfected with control lentivirus; iii) oeIGF-1 group: MSCs
were transfected with lentivirus encoding IGF-1; and iv) siIGF-1
group: MSCs were transfected with lentivirus encoding
shRNA-IGF-1.
Follow-up treatment with 5-AZA in
gene-manipulated MSCs
Following gene manipulation, MSCs from all groups
(MSC, control, oeIGF-1 and siIGF-1) were grown to 70–85% confluence
and then treated with 5-AZA (10 μmol/l) for 24 h. The dual-phase
treated MSCs were then cultured for 21 days.
Immunocytochemistry
One day after the combined induction, IGF-1
expression in all groups was verified by immunocytochemistry. Cells
were fixed for 30 min with 4% formalin and rinsed with PBS.
Following permeabilization with 0.4% Triton X-100 (Sigma-Aldrich)
for 10 min, cells were blocked with 10% bovine serum albumin (Life
Technologies, Grand Island, NY, USA) for 30 min prior to being
incubated with goat anti-human polyclonal IGF-1 primary antibody
(R&D, Santa Fe Springs, CA, USA) at 37°C for 2 h, followed by
incubation in a 1:50 dilution of horseradish peroxidase-conjugated
rabbit anti-goat IgG secondary antibody (R&D). Hematoxylin was
used for nuclear counterstaining and the immunostained cells were
visualized under a microscope (DM2700M; Leica).
RNA isolation and quantitative polymerase
chain reaction (qPCR)
On days 7, 14 and 21, IGF-1 expression and
cardiomyocyte-specific markers (GATA-4, Nkx2.5, β-MHC and MEF2c) of
the induced MSCs were identified by qPCR. Total RNA was isolated
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to
the manufacturer’s instructions. cDNA was prepared from 1 mg total
RNA using a cDNA synthesis kit (Promega, Madison, WI, USA). qPCR
was carried out with SYBR supermix (Takara, Shiga, Japan). The
primers used for amplification are listed in Table I. The expression of each target
mRNA relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
was calculated based on the threshold cycle (CT) as
r=2−Δ(ΔCT).
 | Table ITarget primers and product length. |
Table I
Target primers and product length.
| Gene | Primer sequence
(5′-3′) | Product length
(bp) | Tm (°C) |
|---|
| IGF-1 |
TTCTACTTGGCCCTGTGCTTG | 201 | 60.60 |
|
CACACGAACTGAAGAGCGTC | | 59.22 |
| GATA-4 |
CCTCCGGGGCCCTATGA | 108 | 60.80 |
|
GAGCACAGAGGAGGGCAC | | 61.50 |
| MEF2c |
CACCCTGCTGCTTTTACTATCCT | 166 | 59.70 |
|
GCTCAGCCCATTGTGACATTT | | 58.80 |
| NKX2.5 |
GGGAGGAAGCGGCGAAC | 156 | 61.50 |
|
CGGTTGCCTGCTGACACG | | 60.80 |
| β-MHC |
CTGGGGCTCAAATGGTATGG | 128 | 61.50 |
|
GGCTGGTATTCAAAGGACGG | | 61.90 |
| GAPDH |
CTGGGCTACACTGAGCACC | 101 | 62.00 |
|
AAGTGGTCGTTGAGGGCAATG | | 62.90 |
Immunoblotting
Cardiomyocyte-specific markers (GATA-4, Nkx2.5,
β-MHC and MEF2c) of the induced MSCs were also identified with
immunoblotting every week. Total protein was extracted from MSCs
and differentiated cardiomyocyte-like cells, and then quantified
with a BCA protein assay kit (Pierce, Rockford, IL, USA). Cell
lysates were separated by SDS-PAGE (10%) and incubated with goat
anti-pig polyclonal primary antibodies (anti-GAT4, -Nkx2.5, -β-MHC
and -MEF2c; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
and donkey anti-goat IgG (R&D) secondary antibody conjugated
with horseradish peroxidase. GAPDH was used as a loading control.
Complexes were detected by chemiluminescence (Phototope-HRP Western
Blot Detection System; Cell Signaling, Danvers, MA, USA).
Statistical analysis
Each experiment was conducted at least three times.
Data are presented as the means ± standard deviation. One-way
analysis of variance was used to determine statistical significance
between groups. Data were analyzed with SPSS version 17.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Culture and characterizations of isolated
porcine MSCs
The morphology of isolated porcine MSCs (passage 2)
is shown in Fig. 1A. During the
first two days, the culture was heterogeneous, containing round and
non-adherent cells with lipid micelles in the supernatant. After 10
days, the cell population became more homogeneous, presenting an
adherent fibroblast-like shape, and began to form cell
colonies.
To characterize the MSCs, we performed flow
cytometric analysis with antibodies against CD90, CD44, CD34 and
CD45. As shown in Fig. 1B, the
majority of cells expressed CD90 and CD44 at moderate to high
levels, while these cells were negative for CD34 and CD45 (surface
markers for hematopoietic stem cells). MSCs could be passaged up to
eight times and the proliferation rate and cell viability of MSCs
varied among the different passages, as shown in Fig. 1C and D. MSCs in the second passage
grew faster and exhibited higher viability than MSCs in the fifth
or eighth passage (P<0.05). The growth curve for each group
showed a similar ‘S’ shape. The first two days represented the
latent phase, and the logarithmic growth phase was from day 3 to 6.
Days 7 and 8 represented the plateau phase.
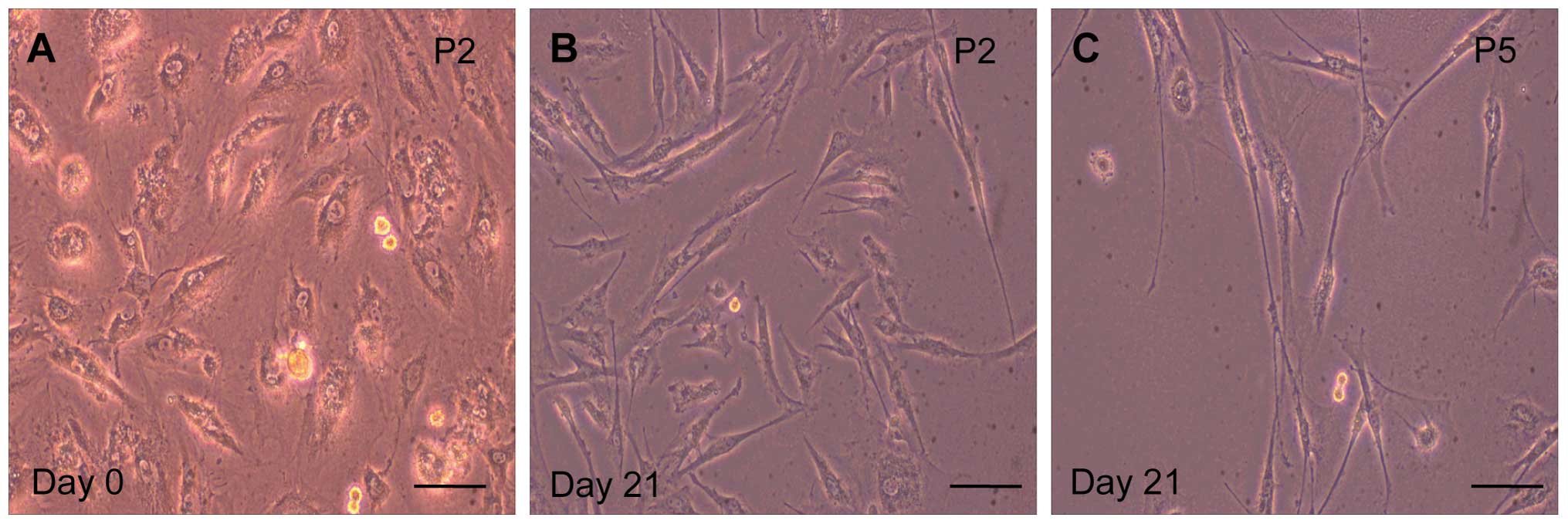
5-AZA promotes the commitment of MSCs to
myocardial differentiation
As shown in Fig.
2A, MSCs demonstrated a fibroblast-like morphology prior to
5-AZA treatment (0 weeks). Following 5-AZA treatment for 48 h, ~60%
of the MSCs detached from the plate, and the morphology of the
remaining cells gradually changed. The remaining 40% of the MSCs
gradually increased in size, formed a ball-like appearance, or
lengthened in one direction and formed a stick-like morphology at
one week. As shown in Fig. 2B and
C, the cells connected with adjoining cells after three weeks.
MSCs in the second passage grew faster and exhibited a
significantly higher cardiomyocyte-like induction ratio than those
in the fifth passage (Table
II).
 | Table IICardiomyocyte induction ratio. |
Table II
Cardiomyocyte induction ratio.
| Passage number | Concentration of
5-AZA (μM) | General average of
total cellular score (n) | General average of
cardiomyocyte score (n) | Inductivity |
|---|
| P2 | 10 | 74.8 | 25.1 | 33.6a |
| P5 | 10 | 60.3 | 10.9 | 18.1b |
Overexpression of IGF-1 gene enhances
myocardial differentiation of MSCs in the presence of 5-AZA
IGF-1 expression in the oeIGF-1 group is shown in
Fig. 3A. The combination of IGF-1
overexpression with 5-AZA treatment induced the cardiomyocyte-like
differentiation of MSCs, which was demonstrated by the expression
of specific cardiomyocyte markers (GATA-4, Nkx2.5, β-MHC and
MEF2c). Compared with the MSC and control groups, the oeIGF-1 group
expressed mRNA and protein of specific markers at a higher level on
day 14 and day 21 following treatment with 5-AZA (P<0.05), but
no significant difference was shown on day 7 (Fig. 3B and C).
 | Figure 3Insulin-like growth factor-1 (IGF-1)
expression and cardiomyocyte-specific marker expression. (A) The
expression of IGF-1 in different mesenchymal stem cell (MSC) groups
was determined by immunocytochemistry. Scale bars, 50 μm. (B)
Cardiomyocyte-like differentiation of MSCs with 5-azacytidine
(5-AZA) treatment was detected by quantitative polymerase chain
reaction, which was demonstrated by upregulation of GATA-4, Nkx2.5,
β-MHC and MEF2c mRNA expression on days 14 and 21. Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) was used as a reference gene. (C)
Cardiomyocyte-like differentiation of MSCs with 5-AZA treatment was
detected by western blot analysis, which was demonstrated by
upregulation of GATA-4, Nkx2.5, β-MHC and MEF2c protein expression
on days 14 and 21. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
was used as a loading control. MSC, untreated MSCs; control, MSCs
transfected with control lentivirus; oeIGF-1 group, MSCs
transfected with lentivirus encoding IGF-1; siIGF-1 group, MSCs
transfected with lentivirus encoding shRNA-IGF-1.
*P<0.05 and **P<0.01, vs. the control
group. |
Knockdown of IGF-1 gene attenuates
myocardial differentiation of MSCs
IGF-1 expression in the siIGF-1 group is shown in
Fig. 3A. The mRNA and protein
expression of the siIGF-1 group was significantly downregulated for
the majority of the specific cardiomyocyte markers (P<0.05)
(Fig. 3B and C).
Discussion
MSCs differentiate into cardiomyocyte-like cells
in vivo and promote cardiac repair in the ischemic
myocardium. Pre-induction of the MSCs prior to transplantation
improves the therapeutic effect significantly. In this study, a
novel combined induction strategy, including IGF-1 gene
manipulation and 5-AZA treatment, was developed for MSC
differentiation into cardiomyocyte-like cells in vitro.
Until now, the mechanisms underlying the
differentiation of MSCs into cardiomyocyte-like cells have been
unclear. Numerous researchers believe that the paracrine effects of
the cells are more likely to play a key role than any direct effect
of the cells. A number of induction agents have been used to mimic
the paracrine effect (12–15). 5-AZA, a DNA-demethylating chemical
compound, induces the demethylation of CpG islands that normally
remain unmethylated in the germ line, leading to an altered
expression of certain genes that may regulate differentiation
(16,17). Our study, consistent with previous
literature, demonstrated that 5-AZA could induce a
cardiomyocyte-like morphological change in MSCs. Furthermore, MSCs
in the second passage exhibited better differentiation than those
in the fifth passage when using a stand-alone treatment of 5-AZA,
which indicated that the number of cell passages may be an
influencing factor in the differentiation induction.
It has been demonstrated that IGF-1 promotes growth,
proliferation and differentiation of numerous cell types, including
cardiomyocytes and vascular smooth muscle cells, in vivo and
in vitro, and inhibits cell apoptosis and necrosis. In
addition, patients or experimental animals with IGF-1 deficiency
show evidence of cardiac atrophy and impaired cardiac function
(18–20). In the present study, the siIGF-1
group exhibited poorer differentiation than the control group,
which indicates that the absence of the IGF-1 gene in MSCs
inhibited their capacity of differentiation. Therefore, we
hypothesize that intrinsic activation of the IGF-1 gene is
essential to initiate the 5-AZA-based cardiomyocyte-like induction
process.
IGF-1 treatment alone is not capable of inducing the
differentiation of MSCs into cardiomyocyte-like cells, while the
combination of multiple factors, including IGF-1, VEGF and bFGF,
induces MSCs to differentiate and express cardiomyocyte markers
under physiological conditions (21,22).
These studies indicate that multiple factors are required during
the differentiation process. The present study demonstrated that
the combination of IGF-1 gene overexpression and 5-AZA treatment
enhanced myocardial differentiation of MSCs for 21 days, which
suggested a synergistic effect of IGF-1 gene manipulation and
5-AZA. It is known that IGF-1 binds to its receptor (IGF-1R), which
possesses intrinsic tyrosine kinase activity and activates a number
of downstream mediators, including phosphatidyl-inositol
3-kinase/Akt and MAP kinase (23,24).
However, the exact molecular mechanism of IGF-1 gene manipulation
involved in the cardiomyocyte-like differentiation of MSCs needs to
be further explored in the future.
In conclusion, our findings demonstrate that the
IGF-1 gene in MSCs is essential for cardiomyocyte-like
differentiation. In addition, the combination of IGF-1 gene
manipulation and 5-AZA treatment is feasible and effective for
differentiation induction in vitro, which could be of
significance for MSC-based cardiac repair in the ischemic
myocardium.
Acknowledgements
This study was supported by the National Science
Foundation of China (grant nos. 81301312 and 81270326).
References
|
1
|
Buckberg G, Athanasuleas C and Conte J:
Surgical ventricular restoration for the treatment of heart
failure. Nat Rev Cardiol. 9:703–716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gnecchi M, Danieli P and Cervio E:
Mesenchymal stem cell therapy for heart disease. Vascul Pharmacol.
57:48–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Williams AR and Hare JM: Mesenchymal stem
cells: biology, pathophysiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dimmeler S and Zeiher AM: Cell therapy of
acute myocardial infarction: open questions. Cardiology.
113:155–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hill JM, Dick AJ, Raman VK, et al: Serial
cardiac magnetic resonance imaging of injected mesenchymal stem
cells. Circulation. 108:1009–1014. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Li T, Wei X, et al: Mesenchymal
stem cell transplantation improves regional cardiac remodeling
following ovine infarction. Stem Cells Transl Med. 1:685–695. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Yu B, Zhang Y, et al: Jagged1
protein enhances the differentiation of mesenchymal stem cells into
cardiomyocytes. Biochem Biophys Res Commun. 341:320–325. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H, Zuo S, Pasha Z, et al: GATA-4
promotes myocardial transdifferentiation of mesenchymal stromal
cells via up-regulating IGFBP-4. Cytotherapy. 13:1057–1065. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haghani K, Bakhtiyari S and Nouri AM: In
vitro study of the differentiation of bone marrow stromal cells
into cardiomyocyte-like cells. Mol Cell Biochem. 361:315–320. 2012.
View Article : Google Scholar
|
|
10
|
Burlacu A: Can 5-Azacytidine convert the
adult stem cells into cardiomyocytes? A brief overview. Arch
Physiol Biochem. 112:260–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lai NC, Tang T, Gao MH, et al: Improved
function of the failing rat heart by regulated expression of
insulin-like growth factor I via intramuscular gene transfer. Hum
Gene Ther. 23:255–261. 2012. View Article : Google Scholar :
|
|
12
|
Cai B, Li J, Wang J, et al: MicroRNA-124
regulates cardiomyocyte differentiation of bone marrow-derived
mesenchymal stem cells via targeting STAT3 signaling. Stem Cells.
30:1746–1755. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xing Y, Lv A, Wang L, et al: The
combination of angiotensin II and 5-azacytidine promotes
cardiomyocyte differentiation of rat bone marrow mesenchymal stem
cells. Mol Cell Biochem. 360:279–287. 2012. View Article : Google Scholar
|
|
14
|
Zhao JW, Zhang MR, Ji QY, et al: The role
of slingshot-1 L (SSH1L) in the differentiation of human bone
marrow mesenchymal stem cells into cardiomyocyte-like cells.
Molecules. 17:14975–14994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Samper E, Diez-Juan A, Montero JA, et al:
Cardiac cell therapy: boosting mesenchymal stem cells effects. Stem
Cell Rev. 9:266–280. 2013. View Article : Google Scholar
|
|
16
|
Tomita S, Li R-K, Weisel RD, et al:
Autologous transplantation of bone marrow cells improves damaged
heart function. Circulation. 100:II247–II256. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Song J, Liu W, et al: Growth and
differentiation of rat bone marrow stromal cells: does 5-AZA
trigger their cardiomyogenic differentiation? Cardiovasc Res.
58:460–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Q, Li B, Wang X, et al: Overexpression
of insulin-like growth factor I in mice protects from myocyte death
after infarction, attenuating ventricular dilation, wall stress,
and cardiac hypertrophy. J Clin Invest. 100:1991–1999. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee WL, Chen JW, Ting CT, et al:
Insulin-like growth factor I improves cardiovascular function and
suppresses apoptosis of cardiomyocytes in dilated cardiomyopathy.
Endocrinology. 140:4831–4840. 1999.PubMed/NCBI
|
|
20
|
Buerke M, Murohara T, Skurk C, et al:
Cardioprotective effect of insulin-like growth factor I in
myocardial ischemia followed by reperfusion. Proc Natl Acad Sci
USA. 92:8031–8035. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Muguruma Y, Reyes M, Nakamura Y, et al: In
vivo and in vitro differentiation of myocytes from human bone
marrow-derived multipotent progenitor cells. Exp Hematol.
31:1323–1330. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bartunek J, Croissant JD, Wijns W, et al:
Pretreatment of adult bone marrow mesenchymal stem cells with
cardiomyogenic growth factors and repair of the chronically
infarcted myocardium. Am J Physiol Heart Circ Physiol.
292:H1095–H1104. 2007. View Article : Google Scholar
|
|
23
|
Rommel C, Bodine SC, Clarke BA, et al:
Mediation of IGF-1-induced skeletal myotube hypertrophy by PI (3)
K/Akt/mTOR and PI (3) K/Akt/GSK3 pathways. Nat Cell Biol.
3:1009–1013. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu W, Liu Y and Lowe WL Jr: The role of
phosphatidylinositol 3-kinase and the mitogen-activated protein
kinases in insulin-like growth factor-I-mediated effects in
vascular endothelial cells. Endocrinology. 142:1710–1719.
2001.PubMed/NCBI
|