Introduction
Cervical cancer, which develops in the tissues on
the surface of the cervix, is one of the most common types of
cancer affecting females worldwide, with >510,000 new cases and
288,000 mortalities per year (1).
The main risk factor for developing cervical cancer is contraction
of human papillomavirus (HPV), which is the cause of almost all
cases of cervical cancer (2). The
introduction of HPV vaccines has afforded major advances in the
prevention and management of cervical cancer; however, current
vaccines can prevent only ~70% of cases (1). HPV infections are often asymptomatic
and transient, but can promote in situ cervical pre-cancer,
although the majority of cases spontaneously regress, indicating
that HPV alone is not sufficient to cause cervical cancer. In
addition, females in the early stages of cervical cancer may not
exhibit any symptoms (2). Regular
Pap tests are now more common, making cervical cancer more
detectable (3), and the early
detection of cervical cancer tends to lead to more favorable
treatment outcomes compared with the detection of more advanced
stages (4).
There are several treatment options for cervical
cancer, including radiation and chemotherapy, which can be used in
conjunction with various herbal remedies (5,6).
While controversial in the medical field, herbal treatments can
produce positive results when used alongside more standard
therapies (7). Furthermore,
traditional Chinese medicine (TCM) combined with a healthy
lifestyle can often lead to the complete resolution of mild and
moderate diseases (8). Several
doctors have used the TCM ginseng ginsenoside-Rh2 in an attempt to
further improve immune system function, inhibit cancer cell
proliferation and induce the transformation of normal cells in
patients with cervical cancer. Additionally, when used at a dose of
60 g/kg, sophora root has a significant effect on the development
and progression of cervical cancer in mice (http://www.acupuncture.com/herbs/cancerherbc.htm.
Accessed March 4, 2013).
TCM is a holistic medicinal system, which includes
the use of herbal medicines, acupuncture and moxibustion, tuina,
dietary therapy and qigong. TCM has specific methods for diagnosis
and treatment, primarily associated with differentiation of the
syndrome and the prescription of herbal formulas (9). A systematic review of case reports
reveals that there is an abundance of support for the use of TCMs
as therapy for a variety of types of cancer, suggesting the
potential benefits of these therapies (10).
The use of ginseng as a TCM is common in the
treatment of diabetes, cancer, stress and allergies in several
Asian countries (11). In
particular, heat-processed ginseng, which has been used for the
treatment of cancer, inflammation and aging, contains
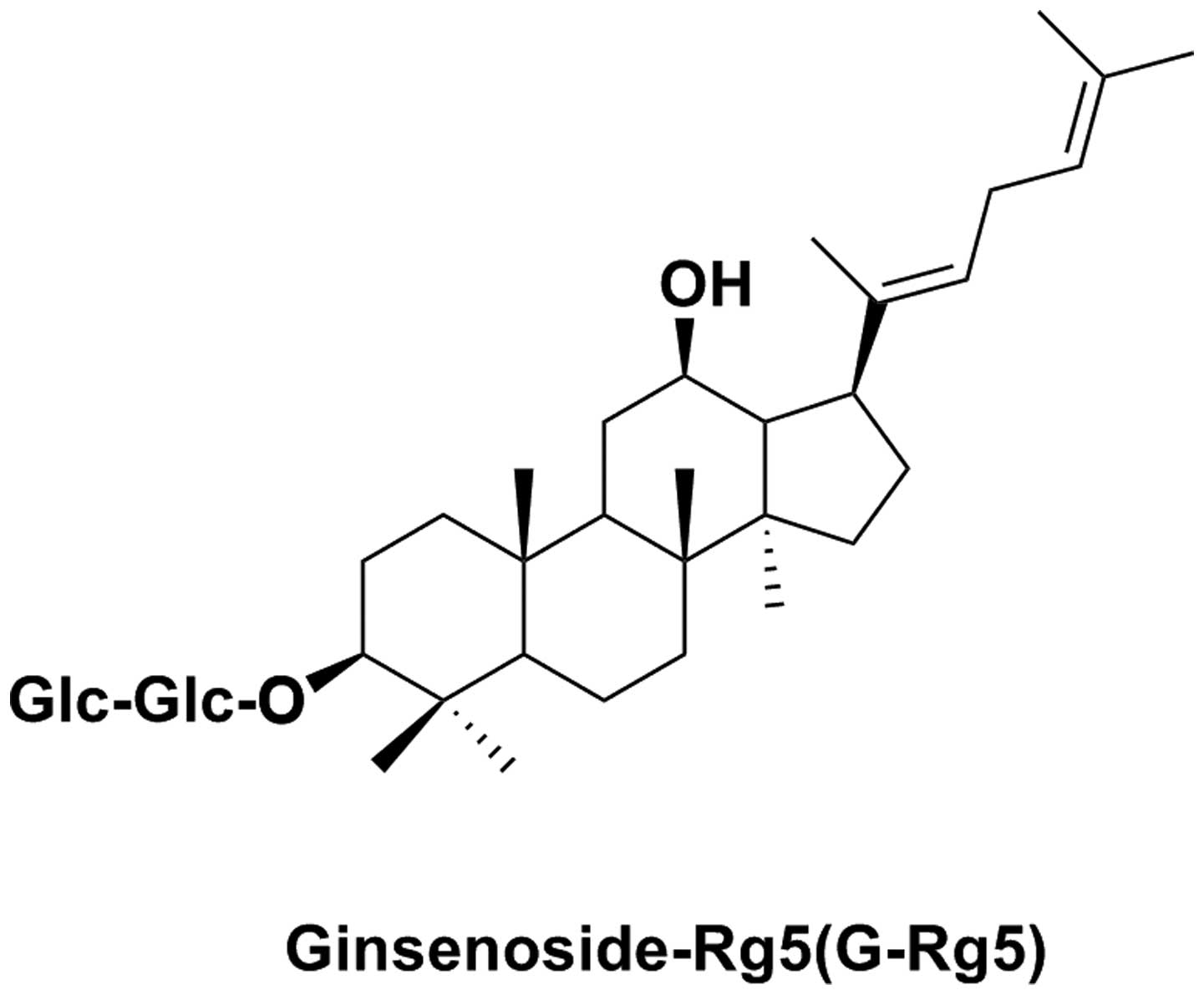
ginsenoside-Rg5 (Fig. 1) as a main
constituent (12–14); ginsenoside-Rg5 belongs to the
family of protopanaxadiol ginsenosides (12,13)
and has been demonstrated to exhibit marked anticancer activity
(15,16), antidermatitic activity (17), anti-inflammatory effects in mouse
lungs (18), neuroprotective
effects (19) and microglial
activation (20). However, the
effects of ginsenoside-Rg5 on cervical cancer remain to be
elucidated. During screening for the identification of TCMs that
inhibit the progression of cervical cancer, heat-processed ginseng
and its main constituent ginsenoside-Rg5 potently induced apoptosis
and DNA damage in human cervical cancer cells in vitro.
Therefore the present study investigated the applicability of
ginsenoside-Rg5 as a potential cytotoxic or genotoxic drug for the
treatment of cervical cancer.
Materials and methods
Cell lines
The human cervical cancer cell lines, C-33A (cat.
no. HTB-31), HT-3 (cat. no. HTB-32), Me180 (cat. no. HTB-33) and
MS751 (cat. no. HTB-34), were obtained from American Type Culture
Collection (Manassas, VA, USA). HeLa cells, derived from human
cervical carcinoma, were purchased from the Cell Bank of the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). The HeLa cells were cultured in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1%
streptomycin and penicillin, followed by incubation at 37°C in a 5%
CO2 incubator. The C33A cells were cultured in DMEM
containing l-glutamine, 10% FBS, 1% non-essential amino acids and
1% sodium pyruvate. The HT-3 and Me180 cells were cultured in
McCoy’s medium (Sigma-Aldrich, St. Louis, MO, USA) with l-glutamine
and 10% FBS. The MS751 cells were cultured in Eagle’s minimum
essential medium (Sigma-Aldrich) with l-glutamine, 10% FBS, 1%
non-essential amino acids and 1% sodium pyruvate.
Chemicals and reagents
Ginsenoside-Rg5 (purity>95%) was provided by Dr
WM Zhao at the Shanghai Institute of Materia Medica of the Chinese
Academy of Sciences (Shanghai, China). Dimethylsulfoxide, Trypan
blue, low melting agarose, 4′,6-diamidino-2-phenylindole (DAPI),
normal melting agarose, 2-amino-2-(hydroxymethyl)-1,3-propanediol,
sodium dodecyl sulfate, ethylenediaminetetraacetic acid,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
N-methyl-N-nitro-N-nitrosoguanidine (MNNG, inducing DNA damage),
Tween 20 and paraformaldehyde were obtained from Sigma-Aldrich.
Propidium iodide (PI) (21) and
DMEM were obtained from Gibco Life Technologies (Carlsbad, CA,
USA). The Apoptosis Detection kit I was obtained from BD Pharmingen
San Diego, CA, USA). Triton X-100, ethidium bromide, FBS, xylene
cyanol and bromphenol blue were obtained from Shanghai Sangon
Biotech Co., Ltd. (Shanghai, China).
Cell viability assessment
The cells were seeded into 96-well plates at a
density of 5×104 cells per well and left overnight for
cell adherence. Cell viability was determined between 12 and 48 h
in the presence or absence of metformin using an MTT assay
(22). Plates were read using an
Automated Microplate Reader (Multiskan EX; Lab Systems, Helskinki,
Finland) at a test wavelength of 570 nm.
DNA extraction and the detection of DNA
fragmentation
The HeLa and MS751 cells were treated using
ginsenoside-Rg5 at concentrations of 0, 2.5 or 5 μM for 12 or 24 h,
respectively. Cells treated with 10 μM MNNG for 24 h were used as a
positive control (CT). The treated cells were then harvested and
washed with phosphate-buffered saline (PBS). DNA was extracted
using a Wizard Genomic DNA Purification kit (Promega Corporation,
Madison, WI, USA) (23). DNA
fragmentation was detected by electrophoresis on a denaturing urea
polyacrylamide gel, which was stained using silver nitrate solution
(24).
Detection of apoptotic incidence using
flow cytometry (25)
Cell apoptosis was determined using an Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit I (BD
Pharmingen). Briefly, the cells were treated with ginsenoside-Rg5
and MNNG at concentrations between 0 and 5 μM and at 10 μM,
respectively, for 24 h. The cells were then washed twice with cold
PBS and resuspended in 500 μl binding buffer at a concentration of
5×105 cells/ml. Subsequently, 5 μl annexin V-FITC
solution and 5 μl PI (1 mg/ml) were added to the cells at 37°C for
30 min. The cells were analyzed using flow cytometry within 1 h.
The number of apoptotic cells were counted and represented as a
percentage of the total cell count.
Alkaline comet assay
The HeLa and MS751 cells were treated with 0, 0.625,
1.25, 2.5 or 5 μM ginsenoside-Rg5 for 24 h and were then
collected, washed and suspended in PBS (pH 7.4). Subsequently, 30
μl cell samples (1×104 cells) were suspended in 110 μl
of 1% molten low-melting-point agarose at 37°C (26). The monosuspension was cast on a
microscopic slide that had been covered with a layer of 0.8%
regular-melting-point agarose. Images were captured using an
Olympus BX53 fluorescent microscope (Olympus Corporation, Tokyo,
Japan) using a filter of 515–560 nm. The extent of DNA migration
was determined using an image analysis system (CASPLab; www.casp.of.pl) and the tail length, indicating DNA
migration from the nucleus, and tail moment (migrated DNA in the
tail multiplied by the tail length) were recorded. All cells
treated with MNNG (10 μM) were included as positive
controls.
γH2AX foci staining
The phosphorylation of histone H2AX as a marker of
DNA double-strand breaks (DSBs) was performed, as previously
described (24). The HeLa and
MS751 cells (5×105) were seeded into six-well culture
plates and treated with 0, 0.625, 1.25, 2.5 or 5 μM ginsenoside-Rg5
and 10 μM MNNG for 24 h. Following treatment, the cells were fixed
in 4% paraformaldehyde for 15 min, washed with PBS (pH 7.4) and
0.1% Tween 20 and permeabilized in 1% Triton-X 100 for 30 min.
Following inhibition with fetal bovine serum (Gibco Life
Technologies, Carlsbad, CA, USA) for 60 min, the cells were
incubated with rabbit monoclonal anti-gH2AX antibodies (1:1,500;
Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C
and conjugated with Alexa594-conjugated anti-rabbit secondary
antibodies (1:360, Cell Signaling Technology, Inc.) for 60 min. For
nuclear staining, 1 mg/ml DAPI was added to the cells and the cells
were incubated for 15 min. The cells were then mounted in antifade
media and images were captured using an Olympus BX53 fluorescent
microscope (Olympus Corporation). The objectives were set at
wavelengths of 594 nm for γH2AX and 350 nm for DAPI.
Statistical analysis
All values are expressed as the mean ± standard
error of the mean. The data were analyzed using one-way analysis of
variance followed by Student-Newman-Keuls test for multiple
comparisons. The Newman-Keuls multiple comparisons test was
applied, and P<0.05 and P<0.01 were considered to indicate
statistically significant differences.
Results
Ginsenoside-Rg5 inhibits the growth of
cervical cancer cells
To determine whether ginsenoside-Rg5 exerted
cytotoxic effects on the cervical cancer cells, the cervical cancer
cell lines were exposed to various concentrations of
ginsenoside-Rg5 and the cell viability was assessed. Although the
sensitivities to the treatment varied depending on the cell line,
ginsenoside-Rg5 treatment at concentrations ranging from 0.625 to
20 μM for 48 h resulted in concentration-dependent cytotoxicity in
the cervical cancer cells (Fig.
2A). The half-maximal inhibitory concentration
(IC50), the concentration at which 50% of the cells
survive, values of the HeLa and MS751 cells were between 2.5 and 10
μM, while the IC50 values of the C-33A, HT-3 and Me180
cells were all higher than 20 μM (Fig.
2A). To further validate the effects of ginsenoside-Rg5, the
cells were exposed to 5 μM ginsenoside-Rg5 for 12, 24 or 48 h.
Ginsenoside-Rg5 reduced the viability of the cervical cancer cells
in a time-dependent manner (Fig.
2B). The HeLa and MS751 cells had a higher sensitivity to the
treatment compared with the C-33A, HT-3 and Me180 cells. Thus,
these results suggested that ginsenoside-Rg5 possessed a cytotoxic
function in certain cervical cancer cell lines, including HeLa and
MS751.
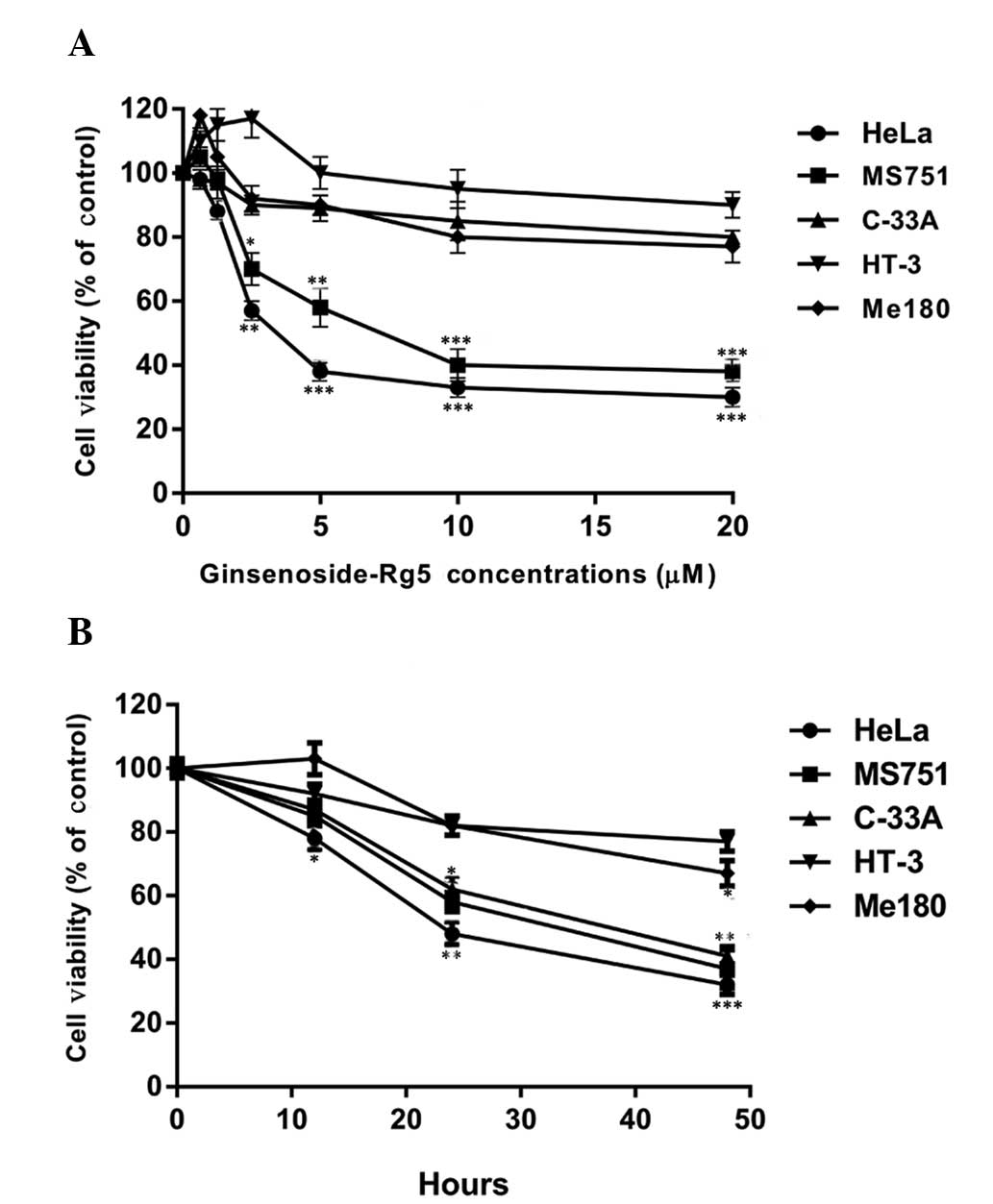 | Figure 2Ginsenoside-Rg5 inhibits the growth of
cervical cancer cells. The human cervical cancer cell lines, HeLa,
MS751, C33A, Me180 and HT-3, were cultured in medium as described
in the Materials and methods. The cells were exposed to (A) 0,
0.625, 1.25, 2.5, 5, 10 or 20 μM ginsenoside-Rg5 for 48 h or (B) 5
μM ginsenoside-Rg5 for 0, 12, 24, or 48 h. Four hours prior to the
final analysis, 100 μg/ml MTT was added to the cell culture medium
and the cell viability was determined using an MTT assay. The
number of cells in the untreated sample was set as 100%. Data are
expressed as the mean ± standard deviation of three independent
experiments.*P<0.05, **P<0.01 and
***P<0.001, compared with the control. MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
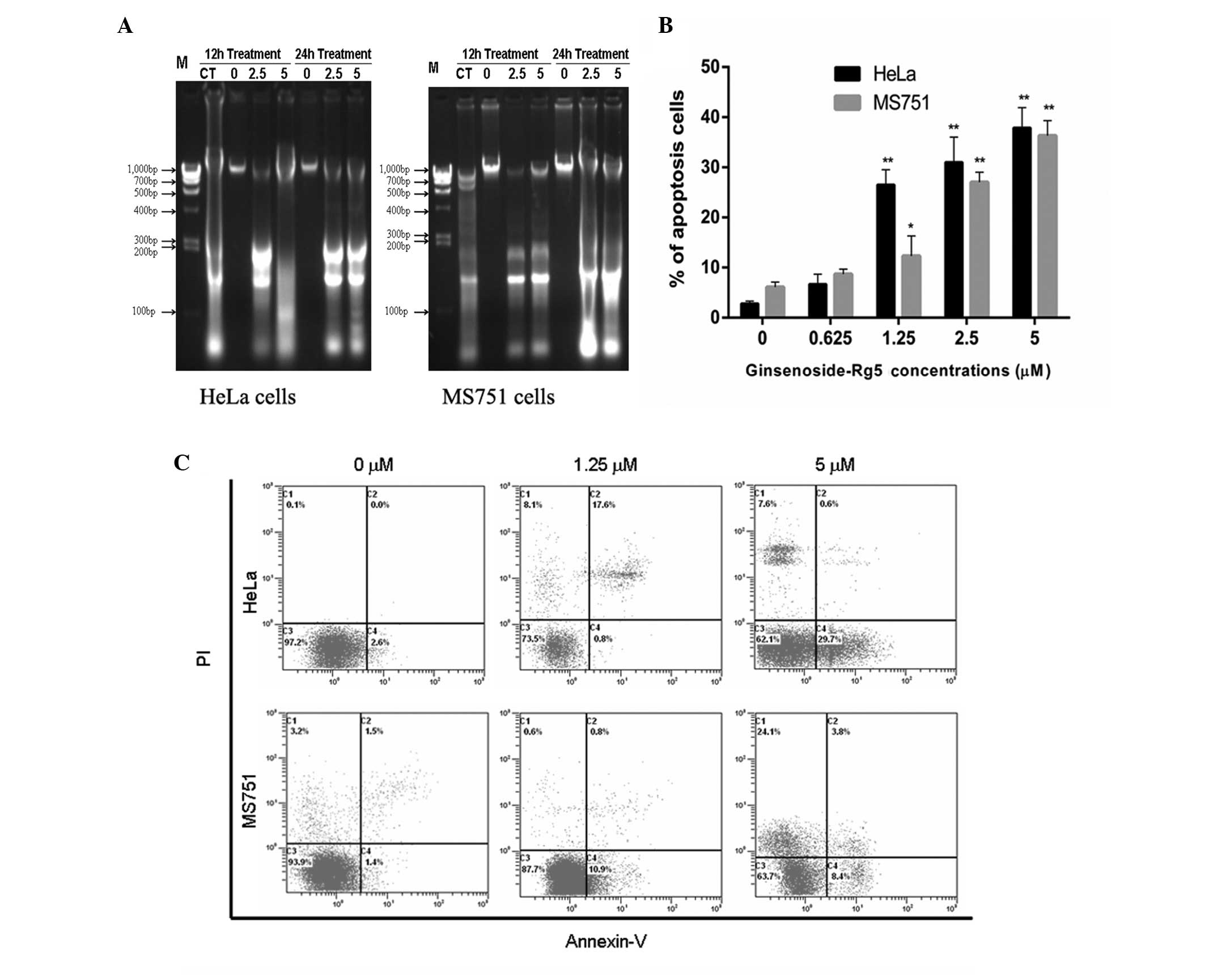
Ginsenoside-Rg5-induces apoptosis in HeLa
and MS751 cells
The HeLa and MS751 cells were significantly more
sensitive to ginsenoside-Rg5. Therefore, to determine whether the
loss in cell viability induced by ginsenoside-Rg5 was associated
with apoptosis, ginsenoside-Rg5-induced apoptosis was examined in
these sensitive cell lines using DNA fragmentation analysis and
flow cytometry. As shown in Fig.
3A, ginsenoside-Rg5 induced a ladder-like pattern on the urea
polyacrylamide gel electrophoresis (PAGE). The DNA in the untreated
cells remained intact. At concentrations of 2.5 and 5 μM,
ginsenoside-Rg5 led to concentration- and time-dependent increases
in DNA fragmentation. In addition, in HeLa and MS751 cells, the
fraction of apoptotic cells increased markedly when the cells were
treated with 1.25–5 μM ginsenoside-Rg5 (Fig. 3B). Taken together, these results
suggested that the anticancer activity of ginsenoside-Rg5 was
mediated, in part, by the induction of apoptosis.
Ginsenoside-Rg5 induces DNA damage in
HeLa and MS751 cells
DNA strand breaks were induced by ginsenoside-Rg5,
as demonstrated by the comet assays. The alkaline comet assay
revealed that DNA fragments migrated to form comet-like images
indicative of DNA damage (27). In
the control cells, high-density DNA was observed in the comet heads
with smooth margins and intact nuclei. The comet frequencies in the
HeLa and MS751 cells were 6.1 and 4.5%, respectively. In the cells
treated with ginsenoside-Rg5, the DNA comets exhibited broom-shaped
tails. The percentages of comet-positive HeLa and MS751 cells
significantly increased at concentrations between 0 and 5 μM
compared with the negative control (Fig. 4).
The mean ± SEM of the tail length and tail moment in
the HeLa and MS751 cells are shown in Table I. These results indicated that the
cells, which were exposed to different concentrations of
ginsenoside-Rg5, exhibited significantly higher DNA damage
(P<0.01) compared with the control samples. In these cell lines,
ginsenoside-Rg5 significantly increased the tail length (P<0.01)
and tail moment (P<0.01) when used at concentrations of 1.25 to
5 μM.
 | Table IParameters of DNA damage from the
comet assays of HeLa and MS751 cells exposed to different
concentrations of ginsenoside-Rg5. |
Table I
Parameters of DNA damage from the
comet assays of HeLa and MS751 cells exposed to different
concentrations of ginsenoside-Rg5.
| Comet assay
parameters |
|---|
|
|
|---|
| Treatment
group | Tail length
(μM) | Tail moment |
|---|
|
|
|
|---|
| Cell
line/compound | Concentration | HeLa | MS751 | HeLa | MS751 |
|---|
| Negative
control | 0 μM | 8.15±2.58 | 5.45±3.42 | 1.54±0.42 | 5.12±1.71 |
|
Ginsenoside-Rg5 | 0.625 μM | 16.46±4.41 | 21.07±3.51 | 8.31±1.34 | 15.09±3.22 |
| 1.25 μM | 30.01±5.21a | 48.42±9.52a | 11.42±3.52a | 25.66±3.16a |
| 2.5 μM | 39.70±7.44a | 57.48±8.87a | 19.33±1.72a | 33.76±4.21a |
| 5 μM | 56.24±10.47a | 72.06±4.70a | 28.25±2.46a | 36.31±2.99a |
| Positive
control | 10 μM | 87.50±4.23a | 80.08±2.82a | 35.27±3.34a | 43.08±6.36a |
γH2AX foci reveal the induction of DNA
DSBs by ginsenoside-Rg5
The phosphorylation of histone H2AX foci formation
has been suggested as a sensitive way to detect DNA DSBs (27). A threshold of four or more γH2AX
foci/cell is optimal for determining the extent of DNA damage
(28). Immunofluorescent images of
histone H2AX phosphorylation in the γH2AX-stained HeLa and MS751
cells are shown in Fig. 5A.
Ginsenoside-Rg5 caused a concentration-dependent induction of γH2AX
foci. In the control, the HeLa and MS751 cells had few
γH2AX-positive foci in the nuclei and there were ~5.5% cells
containing over four foci (Fig.
5B). All treatments with ginsenoside-Rg5 and MNNG induced the
formation of foci and increased the percentages of γH2AX-positive
cells. In addition, ginsenoside-Rg5 and MNNG exhibited distinct
concentration-dependent effects (P<0.01) on the formation of
γH2AX foci in the HeLa and MS751 cells (Fig. 5B).
Discussion
Conventional chemotherapeutic agents are often toxic
to tumor cells and normal cells, which limits their therapeutic
use. The identification of anticancer compounds from natural
products offers a promising alternative to the use of synthetic
compounds due to their favored safety and efficacy (29). Several preclinical and clinical
studies have demonstrated the anticancer potential of Panax
ginseng, which has been used clinically in China for thousands
of years (19). In addition,
ginseng is used to combat stress, fatigue, oxidizing agents, cancer
and diabetes mellitus (30). The
antitumor efficacy of ginseng is attributed mainly to the presence
of saponins, also termed ginsenosides, which induce cell death and
metastasis. Over 30 ginsenosides, which are triterpene derivatives,
have been isolated from the ginseng saponin fraction and the
chemical structures of the individual ginsenosides have been
identified (15). Several types of
eukaryotic cell death, including apoptosis, autophagy, paraptosis,
mitotic catastrophe and necrosis have been recognized (31–33),
of which apoptosis is the most common mechanism of cell death.
Different types of ginsenoside induce apoptosis through a variety
of signaling cascades. Ginsenoside-Rh2 and ginsenoside-Rg3 induce
apoptosis in A549 lung adenocarcinoma cells, prostate cancer cells
(34), hepatoma cells (35) and colorectal cancer cells (36). The induction of apoptosis by other
ginsenosides, including Rb2, Rc and RS4, in different types of
human tumor cell lines has also been observed (37,38).
These findings suggest that the induction of tumor cell apoptosis
by ginsenosides may be one of the mechanisms through which these
compounds exert their antitumor effects. However, only a few
studies have investigated the effects of ginsenosides and, in
particular, ginsenoside-Rg5 in human cervical cancer.
Apoptosis, or programmed cell death, is the result
of a highly complex cascade of cellular events that result in
chromatin condensation and DNA fragmentation (39). It is a fundamental cellular event
during development and is essential for radiation or drug-induced
cytotoxicity, which is characterized by the cleavage of chromatin
DNA into internucleosomal fragments. In the present study,
denaturing urea PAGE was used to detect DNA fragmentation and the
rate of apoptosis was measured using flow cytometry by
double-labeling with annexin V and PI. The data demonstrated that
ginsenoside-Rg5 caused concentration- and time-dependent increases
in DNA fragmentation.
In addition, the majority of evidence has indicated
that ginsenosides induce cell cycle arrest and apoptosis in
mammalian tumor cells (35,40).
However, few studies have described the genotoxicity of
ginsenosides. Thus, the present study investigated the induction of
DNA damage by ginsenoside-Rg5 in human cervical cancer cell lines
in vitro by using alkaline comet assays and measuring γH2AX
foci formation. The alkaline comet assay, a single-cell gel
electrophoresis, is a method used to detect DNA strand breaks
(41). Additionally, the formation
of DNA DSBs induces γH2AX aggregation in the nucleus, thus, the
measurement of γH2AX foci formation may be a sensitive method for
the detection of DNA DSBs (42).
In the present study, these assays revealed concentration-dependent
increases in DNA damage, as evidenced by an increase in comet tail
sizes with a concomitant reduction in head sizes in the comet
assay, as well as an increased number of γH2AX-positive cells in
response to ginsenoside-Rg5 treatment. Thus, these results revealed
that the presence of γH2AX foci may be indicative of DSBs,
confirmed by the comet assay. These results were consistent with
those observed in previous studies (16,17),
indicating that ginsenoside-Rg5 has antiproliferative and apoptotic
activities in cancer cells.
In conclusion, the present study demonstrated that
ginsenoside-Rg5 was anticarcinogenic, inducing cell DNA damage and
apoptosis in vitro, and the DNA damage induced by
ginsenoside-Rg5 may be associated with apoptosis. The results of
the present study confirmed the antitumor effects of
ginsenoside-Rg5 and the potential of ginsenoside-Rg5 as an agent of
chemotherapeutic activity in human cervical cancer cells.
References
|
1
|
Saslow D, Castle PE, Cox JT, et al:
American Cancer Society. Guideline for human papillomavirus (HPV)
vaccine use to prevent cervical cancer and its precursors. CA
Cancer J Clin. 57:7–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steben M and Duarte-Franco E: Human
papillomavirus infection: epidemiology and pathophysiology. Gynecol
Oncol. 107:S2–S5. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Long HJ 3rd: Management of metastatic
cervical cancer: review of the literature. J Clin Oncol.
25:2966–2974. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brinkman JA, Caffrey AS, Muderspach LI,
Roman LD and Kast WM: Inhibitory effect of ginsenoside Rg5 and its
metabolite ginsenoside Rh3 in an oxazolone induced mouse chronic
dermatitis model. Eur J Gynaecol Oncol. 26:129–142. 2005.
|
|
5
|
Leitao MM Jr and Chi DS: Recurrent
cervical cancer. Curr Treat Options Oncol. 3:105–111. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang J, Li J, Sun M and Chen K: Studies of
traditional Chinese medicine monomer on HeLa cell of cervical
cancer. Pak J Pharm Sci. 27(4 Suppl): 1063–1068. 2014.PubMed/NCBI
|
|
7
|
Cohen I, Tagliaferri M and Tripathy D:
Traditional Chinese medicine in the treatment of breast cancer.
Semin Oncol. 29:563–574. 2002. View Article : Google Scholar
|
|
8
|
Li X, Yang G, Li X, et al: Traditional
Chinese medicine in cancer care: a review of controlled clinical
studies published in Chinese. PloS One. 8:e603382013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang G, Li X, Li X, et al: Traditional
chinese medicine in cancer care: a review of case series published
in the chinese literature. Evid Based Complement Alternat Med.
2012.7510–7546. 2012.
|
|
10
|
Liu J, Li X, Liu J, Ma L, Li X and Fønnebø
V: Traditional Chinese medicine in cancer care: a review of case
reports published in Chinese literature. Forsch Komplementarmed.
18:257–263. 2011. View Article : Google Scholar
|
|
11
|
Kwon SW, Han SB, Park IH, Kim JM, Park MK
and Park JH: Liquid chromatographic determination of less polar
ginsenosides in processed ginseng. J Chromatogr A. 921:335–339.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SN, Ha YW, Shin H, Son SH, Wu SJ and
Kim YS: Simultaneous quantification of 14 ginsenosides in Panax
ginseng C.A. Meyer (Korean red ginseng) by HPLC-ELSD and its
application to quality control. J Pharm Biomed Anal. 45:164–170.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yun TK, Lee YS, Lee YH, Kim SI and Yun HY:
Anticarcino genic effect of Panax ginseng C.A. Meyer and
identification of active compounds. J Korean Med Sci. 16:S6–S18.
2001. View Article : Google Scholar
|
|
14
|
Kang KS, Kim HY, Baek SH, et al: Study on
the hydroxyl radical scavenging activity changes of ginseng and
ginsenoside-Rb2 by heat processing. Biol Pharm Bull. 30:724–728.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nag SA, Qin JJ, Wang W, et al:
Ginsenosides as anticancer agents: In vitro and in vivo activities,
structure-activity relationships, and molecular mechanisms of
action. Front Pharmacol. 3:252012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee KY, Lee YH, Kim SI, Park JH and Lee
SK: Ginsenoside-Rg5 suppresses cyclin E-dependent protein kinase
activity via up-regulating p21Cip/WAF1 and down-regulating cyclin E
in SK-HEP-1 cells. Anticancer Res. 17:1067–1072. 1997.PubMed/NCBI
|
|
17
|
Shin YW, Bae EA and Kim DH: Inhibitory
effect of ginsenoside Rg5 and its metabolite ginsenoside Rh3 in an
oxazolone-induced mouse chronic dermatitis model. Arch Pharm Res.
29:685–690. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim TW, Joh EH, Kim B and Kim DH:
Ginsenoside Rg5 ameliorates lung inflammation in mice by inhibiting
the binding of LPS to toll-like receptor-4 on macrophages. Int
Immunopharmacol. 12:110–116. 2012. View Article : Google Scholar
|
|
19
|
Kim EJ, Jung IH, Van Le TK, Jeong JJ, Kim
NJ and Kim DH: Ginsenosides Rg5 and Rh3 protect scopolamine-induced
memory deficits in mice. J Ethnopharmacol. 146:294–299. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee YY, Park JS, Jung JS, Kim DH and Kim
HS: Anti-inflammatory effect of ginsenoside Rg5 in
lipopolysaccharide-stimulated BV2 microglial cells. Int J Mol Sci.
14:9820–9833. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawai K, Liu SX, Tyurin VA, et al:
Antioxidant and antiapoptotic function of metallothioneins in HL-60
cells challenged with copper nitrilotriacetate. Chem Res Toxicol.
13:1275–1286. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu SB, Su JJ, Sun LH, et al: Triterpenoids
and steroids from the fruits of Melia toosendan and their cytotoxic
effects on two human cancer cell lines. J Natl Prod. 73:1898–1906.
2010. View Article : Google Scholar
|
|
23
|
Nakadai A, Li Q and Kawada T: Chlorpyrifos
induces apoptosis in human monocyte cell line U937. Toxicology.
224:202–209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guanggang X, Diqiu L, Jianzhong Y, et al:
Carbamate insecticide methomyl confers cytotoxicity through DNA
damage induction. Food Chem Toxicol. 53:352–358. 2013. View Article : Google Scholar
|
|
25
|
Zhang YH, Li HD, Li B, Jiang SD and Jiang
LS: Ginsenoside Rg3 induces DNA damage in human osteosarcoma cells
and reduces MNNG-induced DNA damage and apoptosis in normal human
cells. Oncol Rep. 31:919–925. 2014.
|
|
26
|
Wu GS, Lu JJ, Guo JJ, et al: Ganoderic
acid DM, a natural triterpenoid, induces DNA damage, G1 cell cycle
arrest and apoptosis in human breast cancer cells. Fitoterapia.
83:408–414. 2012. View Article : Google Scholar
|
|
27
|
Sokolov MV, Smilenov LB, Hall EJ, Panyutin
IG, Bonner WM and Sedelnikova OA: Ionizing radiation induces DNA
double-strand breaks in bystander primary human fibroblasts.
Oncogene. 24:7257–7265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parry MC, Bhabra G, Sood A, et al:
Thresholds for indirect DNA damage across cellular barriers for
orthopaedic biomaterials. Biomaterials. 31:4477–4483. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toh DF, Patel DN, Chan EC, Teo A, Neo SY
and Koh HL: Anti-proliferative effects of raw and steamed extracts
of Panax notoginseng and its ginsenoside constituents on human
liver cancer cells. Chin Med. 6:42011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yue PY, Mak NK, Cheng YK, et al:
Pharmacogenomics and the Yin/Yang actions of ginseng: anti-tumor,
angiomodulating and steroid-like activities of ginsenosides. Chin
Med. 2:62007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bras M, Queenan B and Susin SA: Programmed
cell death via mitochondria: different modes of dying. Biochemistry
(Mosc). 70:231–239. 2005. View Article : Google Scholar
|
|
32
|
Broker LE, Kruyt FA and Giaccone G: Cell
death independent of caspases: a review. Clin Cancer Res.
11:3155–3162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Edinger AL and Thompson CB: Death by
design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol.
16:663–669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim HS, Lee EH, Ko SR, Choi KJ, Park JH
and Im DS: Effects of ginsenosides Rg3 and Rh2 on the proliferation
of prostate cancer cells. Arch Pharm Res. 27:429–435. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park HM, Kim SJ, Kim JS and Kang HS:
Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced
apoptosis in hepatoma cells through mitochondrial signaling
pathways. Food Chem Toxicol. 50:2736–2741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li B, Zhao J, Wang CZ, et al: Ginsenoside
Rh2 induces apoptosis and paraptosis-like cell death in colorectal
cancer cells through activation of p53. Cancer Lett. 301:185–192.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim SE, Lee YH, Park JH and Lee SK:
Ginsenoside-Rs4, a new type of ginseng saponin concurrently induces
apoptosis and selectively elevates protein levels of p53 and
p21WAF1 in human hepatoma SK-HEP-1 cells. Eur J Cancer. 35:507–511.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oh SH and Lee BH: A ginseng saponin
metabolite-induced apoptosis in HepG2 cells involves a
mitochondria-mediated pathway and its downstream caspase-8
activation and Bid cleavage. Toxicol Appl Pharmacol. 194:221–229.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Robertson JD and Orrenius S: Role of
mitochondria in toxic cell death. Toxicology. 181–182:491–496.
2002. View Article : Google Scholar
|
|
40
|
Poon PY, Kwok HH, Yue PY, et al:
Cytoprotective effect of 20S-Rg3 on benzo[a]pyrene-induced DNA
damage. Drug Metab Dispos. 40:120–129. 2012. View Article : Google Scholar
|
|
41
|
Cotelle S and Férard JF: Comet assay in
genetic ecotoxicology: a review. Environ Mol Mutagen. 34:246–255.
1999. View Article : Google Scholar
|
|
42
|
Yu Y, Zhu W, Diao H, Zhou C, Chen FF and
Yang J: A comparative study of using comet assay and gammaH2AX foci
formation in the detection of
N-methyl-N′-nitro-N-nitrosoguanidine-induced DNA damage. Toxicology
In Vitro. 20:959–965. 2006. View Article : Google Scholar
|