Introduction
Adult-onset hypothyroidism causes a wide range of
central nervous system dysfunctions, including
hippocampus-dependent cognitive dysfunction (1–3).
Cognitive impairments observed in hypothyroidism are associated
with several neurotransmitter systems. In particular, a strong
correlation has been demonstrated between the activity of thyroid
hormones (THs) and the cholinergic system (4–5).
Acetylcholine (ACh) is an important neurotransmitter and is crucial
in hippocampal cognitive functioning. Its activity depends upon the
metabolizing enzyme, acetylcholinesterase (AChE), which is
responsible for the hydrolysis of acetylcholine released from
presynaptic nerve terminals. Several presynaptic proteins mediate
the release of neurotransmitters, including synaptotagmin-1 (syt-1)
and synaptosomal-associated protein of 25 kDa (SNAP-25) (6–8).
Syt-1, an abundant integral membrane protein, which is unique to
small synaptic vesicles and large dense-core vesicles in the brain
(9), directly interacts with
SNAP-25 on the pre-synaptic membrane to facilitate neurotransmitter
release. It has been demonstrated that thyroid dysfunction affects
the cholinergic system and synaptic proteins in adult rats
(10–13).
Thyroxine (T4) is at present the most widely
accepted form of replacement therapy in hypothyroidism. However,
previous studies have revealed that brain impairment induced by
adult-onset hypothyroidism may not be fully restored when T4
replacement therapy returned serum THs to control levels. Clinical
observations have suggested that neurocognitive functioning as well
as psychological well-being may not be completely resolved in
patients with hypothyroidism, despite T4 treatment (14–16).
Furthermore, in morphological studies conducted by Madeira et
al, the normalization of thyroid hormone levels in adult rats
did not restore the hypothyroid-induced reduction in the number of
pyramidal cells in the hippocampal CA1 region, and granule cells in
the dentate gyrus (3,17). Our previous biochemical data also
reported that hypothyroidism altered the expression levels of
synaptic proteins in the brains of adult Sprague-Dawley (SD) rats,
which concurrently, did not fully recover as a result of the
standard hormone substitution therapy (13). These findings emphasize the
necessity for alternative therapeutic approaches, for
hypothyroidism patients that do not respond to T4 monotherapy.
Donepezil (DON) is an acetylcholinesterase inhibitor
(AChEIs) that has demonstrated efficacy in improving cognitive
function and providing neuroprotective effects (18), and it may have applications that
extend to the treatment of hypothyroidism. Therefore, in this
study, we have investigated the concentration of ACh and the
activity of AChE, as well as the expression levels of syt-1 and
SNAP-25 in the hippocampus of adult-onset hypothyroidism.
Furthermore, the effects of T4, DON and the co-administration of
both on the biochemical parameters in hypothyroid adult rats, was
examined.
Materials and methods
Experimental animals
Eight-week-old male SD rats (~260–300g) were
obtained from Nanjing Experimental Animal Center (Nanjing, China).
Animals were maintained under standard laboratory conditions with a
natural light-dark cycle and had free access to standard rodent
chow and water. All experimental procedures adhered to the Animal
Care and Use Committee of Anhui Medical University (Anhui, China)
and the study was approved by the ethics committee of Anhui Medical
University (Anhui, China).
Five groups of 10–12 animals were treated as
follows: i) The hypothyroid group (Hypo) consisted of 12
hypothyroid rats, which were induced by including
6-n-propyl-2-thiouracil (PTU; Sigma Chemicals, Perth, WA, MO, USA)
in their drinking water at a concentration of 0.05% weight/volume
(w/v) for six weeks; ii) the DON group (DON) contained 11 rats, who
were treated with PTU for six weeks as described above. However,
from the fifth week, 0.005% (w/v) of DON (Sigma Chemicals) was
added to the tap drinking water, every day for two weeks; iii) the
T4 group (T4) consisted of 11 rats, who were treated with PTU for
six weeks as described in the Hypo group. However, from the fifth
week, they were treated with intraperitoneally injected T4
(dissolved in a saline solution, 6 μg/100 g body weight) for two
weeks to restore hypothyroid animals to euthyroid status; iv) the
T4 plus DON group (T4+DON) included 11 rats treated according to
the same protocols as the T4 group aside from adding 0.005% (w/v)
DON to the drinking water from the fifth week; v) the control group
(C) contained 11 control rats, who were given the same volume of
saline solution for six weeks.
Serum hormone concentrations
Following the last delivered dose, all rats were
anesthetized by chloral hydrate (350 mg/kg BW). The blood (1.5 ml)
was collected from abdominal aorta and immediately centrifuged at
14,000 × g for 15 min (19). The
serum was collected and quickly frozen at −20°C until the time of
assay. The thyroid status of the rats was determined by measuring
serum triiodothyronine (T3), T4 and thyroid stimulating hormone
(TSH) levels utilizing a chemiluminescence method. All sample
measurements were run in duplicate.
Tissue preparation
The rat brains were dissected on ice following blood
collection. The right hemispheres were fixed in 4% paraformaldehyde
at 4°C for 7 days for immunohistochemical analysis. The hippocampus
from the left hemisphere was quickly isolated and stored at −80°C
for the determination of ACh content and AChE activity.
Determination of ACh content
ACh content in hippocampus homogenates was measured
using the modified method of Hestrin (20), to compare the extent of
neurotransmitter levels in the hippocampus between the groups
(n=11–13/group), as previously described. Briefly, 0.2 ml
supernatant was mixed with 0.35 ml distilled water and was followed
by adding 0.05 ml 1.5 mmol/l calabarine sulfate and 0.2 ml 1.84
mol/l trichloroacetic acid. The mixture was centrifuged at 5,000
rpm for 5 min. The ultimate supernatant (0.1 ml) was added to 0.1
ml alkaline hydroxylamine hydrochloride (equal volumes of 2.0 mol/l
hydroxyl-amine hydrochloride and 3.5 mol/l sodium hydroxide) and
incubated at room temperature for 15 min. This was then was reacted
with 0.05 ml 4.0 mol/l HCl and 0.05 ml 0.37 mol/l ferric chloride
(0.37, containing 0.1 mol/l HCl). Finally, 0.2 ml of the medium and
volumes of tissue homogenates were spotted in duplicate onto
96-well microplates. Physostigmine (1.5 mmol/l) was added to the
reaction mixture to inhibit the activity of AChE. Following another
2 min of incubation, the intensity of brown ferric complex was read
at 540 nm on a BioTek plate reader (BioTek Instruments Inc.,
Winooski, VT, USA). Ach levels were expressed as μg/mg of
hippocampus protein (μg/mg Prot).
Determination of AChE activity
AChE activity in the hippocampus homogenates was
determined by following the hydrolysis of acetylcholine using
commercially available kits purchased from Sigma Chemicals (Poole,
UK).
Briefly, the hippocampus was removed and 10% (w/v)
homogenate was prepared in a sodium phosphate buffer (30 mM, pH
7.0), centrifuged at 10,000 × g for 5 min at 4°C and the
supernatant was used for estimation of AChE activity. Sodium
chloride (0.1 ml) was added to 0.1 ml of homogenate in a test tube
and the solution was vortexed. Reference tubes were then placed in
a waterbath at 60°C for 10 min in order to inactivate the
cholinesterase, prior to being cooled back to room temperature. All
tubes then received 1.5 ml water, 1.0 ml nitrophenol solution and
0.1 ml ACh chloride solution. Exactly 30 min from the addition of
the acetylcholine chloride, 0.2 ml of each sample was transferred
to cuvettes for reading in a spectrophotometer at 540 nm.
Protein assay
Protein in hippocampal homogenates was detected
using a BCA protein assay kit (Thermo Fisher Scientific, Waltham,
MA, USA) according to the manufacturer’s instructions.
Immunohistochemistry
The fixed right hemispheres were embedded in
paraffin and sectioned coronally with a microtome into 6-μm-thick
sections. From each rat, five sections (1/20 serial sections) of
the hippocampus were selected to be mounted on polylysine-coated
slides. Following deparaffinization, antigen retrieval was
performed by heating the sections in 10 mM citrate buffer (pH 6.0)
at 100°C for 10 min. Potential non-specific binding sites were
blocked with 5% normal goat serum in phosphate-buffered saline
(PBS). The sections were then incubated with the primary polyclonal
antibody, rabbit anti-syt-1 (1:5,000; Chemicon, Temecula, CA, USA)
or SNAP-25 (1:2,000; Sigma Chemicals, USA) at 37°C for 1 h and
overnight at 4°C, followed by washes in PBS, incubation with the
biotinylated secondary antibody (goat anti-rabbit IgG kit
[Maixin-Bio Ltd., Fuzhou, China]) for 15 min at 37°C and further
washes in PBS. Sections were further incubated with the HRP for 10
min at 37°C, washed in PBS and colored with diaminobenzidine (DAB;
Maixin-Bio Ltd.) at room temperature for 5 min. Finally, sections
were counterstained with hematoxylin for 3 min, dehydrated, rinsed
and coverslipped with glycerin. The sections were subsequently
dehydrated, rinsed and coverslipped with glycerin. With the
exception of the normal goat serum-inhibition step, the sections
were rinsed in PBS (three times, 5 min each) following each
treatment. The negative control was processed with the same steps,
however the primary antibody was replaced with PBS.
An image analysis system was used for quantitative
analysis of the expression levels of yt-1 and SNAP-25. The system
included MetaMorph image acquisition and processing software (JADA
801D; JEDA Science Technology Development, Co., Ltd., Nanjing,
China) and a Nikon 80i microscope (Nikon, Tokyo, Japan) equipped to
a computer. The layers were analyzed from different subfields of
the hippocampus, including the stratum oriens (SO), stratum
radiatum (SR) and stratum lacunosum-moleculare (SLM) in the CA1;
the SO, stratum lucidum (SL) and SR in the CA3, polymorphic layer
(PL) and molecular layer (ML) in the dentate gyrus (DG). An image
of the entire hippocampal structure was obtained initially at a low
magnifcation (x40) and subsequent images at a higher magnification
(x200), in various subfields of the hippocampus, were acquired
according to the size of each subfeld: three images in CA1 for SO
and SR; one image in CA3 and DG-PL and two images in DG-ML and
CA1-SLM. The digital data were then exported into the MetaMorph
software for analysis and processing. The average optical density
(OD) represented the intensity of the immunohistochemical
staining.
Statistical analysis
All statistical analysis was performed using SPSS
13.0 software (SPSS, Inc., Chicago, IL, USA). Values are expressed
as the mean ± standard error (SEM). The data were analyzed by
one-way analysis of variance (ANOVA) using least-significant
difference for post hoc analysis. P<0.05 was considered to
indicate a statistically significant result.
Results
Serum concentrations of the hormones
Serum T3, T4 and TSH concentrations are presented in
Table I. The serum T3 and T4
levels were significantly lower (P<0.001) and TSH levels were
higher (P<0.001) in SD rats of the Hypo and DON groups, compared
with the controls. T4 and T4+DON treatment restored T3, T4 and TSH
levels, which were not significantly different from the control
values (P>0.05).
 | Table ISerum T3, T4 and TSH levels in five
groups. |
Table I
Serum T3, T4 and TSH levels in five
groups.
| Group | Number | T3 (nmol/l) | T4 (nmol/l) | TSH (μIU/ml) |
|---|
| C | 12 | 0.83±0.03 | 49.81±1.08 | 1.02±0.14 |
| Hypo | 11 | 0.60±0.03* | 18.19±1.72* | 19.78±3.01* |
| DON | 11 | 0.57±0.02* | 18.58±0.91* | 19.55±3.29* |
| T4 | 10 | 0.83±0.08 | 52.42±1.92 | 1.21±0.32 |
| T4+DON | 11 | 0.77±0.07 | 52.71±2.04 | 1.07±0.15 |
Content of ACh in the rat
hippocampus
Alkaline hydroxylamine colorimetry was performed to
detect the content of Ach in the hippocampus of the rats among the
groups. ACh content in the hippocampus is illustrated in Fig. 1. Our results demonstrated that the
amount of ACh was significantly decreased by 27% in the hypothyroid
rats (P=0.027) and the content was restored to control values
(P=0.212, 0.860 and 0.255, respectively) by DON, T4 or T4+DON
treatment.
 | Figure 1Concentration of hippocampal ACh from
Hypo, T4, DON, T4+DON and C groups (n=10–12). Homogenates were
extracted from the hippocampus of each rat. Hypothyroidism induced
a significant decrease in ACh content in the hippocampus, and the
DON (0.005%), T4 (6 μg/100 g body weight) or combined treatment
(T4+DON) restored the Ach levels to the control value. Data shown
are the mean ± SEM of three independent experiments. C, control
group; Hypo, hypothyroid group; DON, hypothyroid rats treated with
0.005% (w/v) DON; T4, hypothyroid rats treated with 6 μg T4/100 g
BW; T4+DON, hypothyroid rats treated with 6 μg T4/100 g BW beside
adding 0.005% (w/v) DON to the drinking water.
*P<0.05, vs C. ACh, acetylcholine; T4, thyroxine;
DON, donepezil; Prot, hippocampus protein; SEM, standard error of
the mean; w/v, weight/volume. |
AChE activity in rat hippocampus
AChE activity in the hippocampus is illustrated in
Fig. 2. The results demonstrated
that AChE activity was significantly decreased by 34, 40 and 39%
(P=0.028, 0.014 and 0.016, respectively) in the Hypo, DON and
T4+DON groups, as compared with the rats in the C group, and T4
administration restored the AChE activity to the control value.
 | Figure 2The AChE activity in the hippocampus
of rats from Hypo, T4, DON, T4+DON and C groups (n=10–12),
homogenates were extracted from the hippocampus of each rat. Data
shown are the mean ± SEM of three independent experiments. C,
control group; Hypo, hypothyroid group; DON, hypothyroid rats
treated with 0.005% (w/v) DON; T4, hypothyroid rats treated with 6
μg T4/100 g body weight; T4+DON, hypothyroid rats treated with 6 μg
T4/100 g body weight beside adding 0.005% (w/v) DON to the drinking
water; C, Control group. *P<0.05;
**P<0.01 compared with C. T4, thyroxine; DON,
donepezil; AChE, acetylcholinesterase; SEM, standard error of the
mean; w/v, weight/volume. |
Protein levels of syt-1 and SNAP-25 in
the hippocampus. Immunohistochemistry
Representative photomicrographs of the
immuno-labeled syt-1 and SNAP-25 in subfields (CA1, CA3 and DG) of
the hippocampus from different groups are demonstrated in Fig. 3 and Fig. 4, respectively. The immunoreactivity
distributions of the proteins were similar among these groups.
Punctate spots of reaction product were distributed in every layer
of the CA1, CA3 and DG subregions of the hippocampus.
 | Figure 3Photomicrographs of coronal sections
showing syt-1 immunoreactivity in CA1, CA3 and DG subregions of
hippocampus of rats from Hypo, T4, DON, T4+DON and C groups
(n=10–12). Distinct punctate spots of reaction product were
observed in every layer of CA1, CA3 and DG subregions; note that
slight decrease in overall staining intensity of CA1-SO, CA1-SR,
CA3-SL, CA3-SR and DG-ML in the Hypo, DON and T4 groups was
observed; the overall staining intensity was equal in CA1-SLM,
CA3-SO and DG-PL of five groups; magnification: ×400, scale bar =
50 μm. Hypo, hypothyroid group; DON, hypothyroid rats treated with
0.005% (w/v) DON; T4, hypothyroid rats treated with 6 μg T4/100 g
body weight; T4+DON, hypothyroid rats treated with 6 μg T4/100 g
body weight beside adding 0.005% (w/v) donepezil to the drinking
water; C, Control group. Syt-1, synaptotagmin-1; T4, thyroxine;
DON, donepezil; DG, dentate gyrus; SO, stratum oriens; SR, stratum
radiatum; SLM, stratum lacunosum-moleculare; SL, stratum lucidum;
ML, molecular layer; PL, polymorphic layer; w/v, weight/volume. |
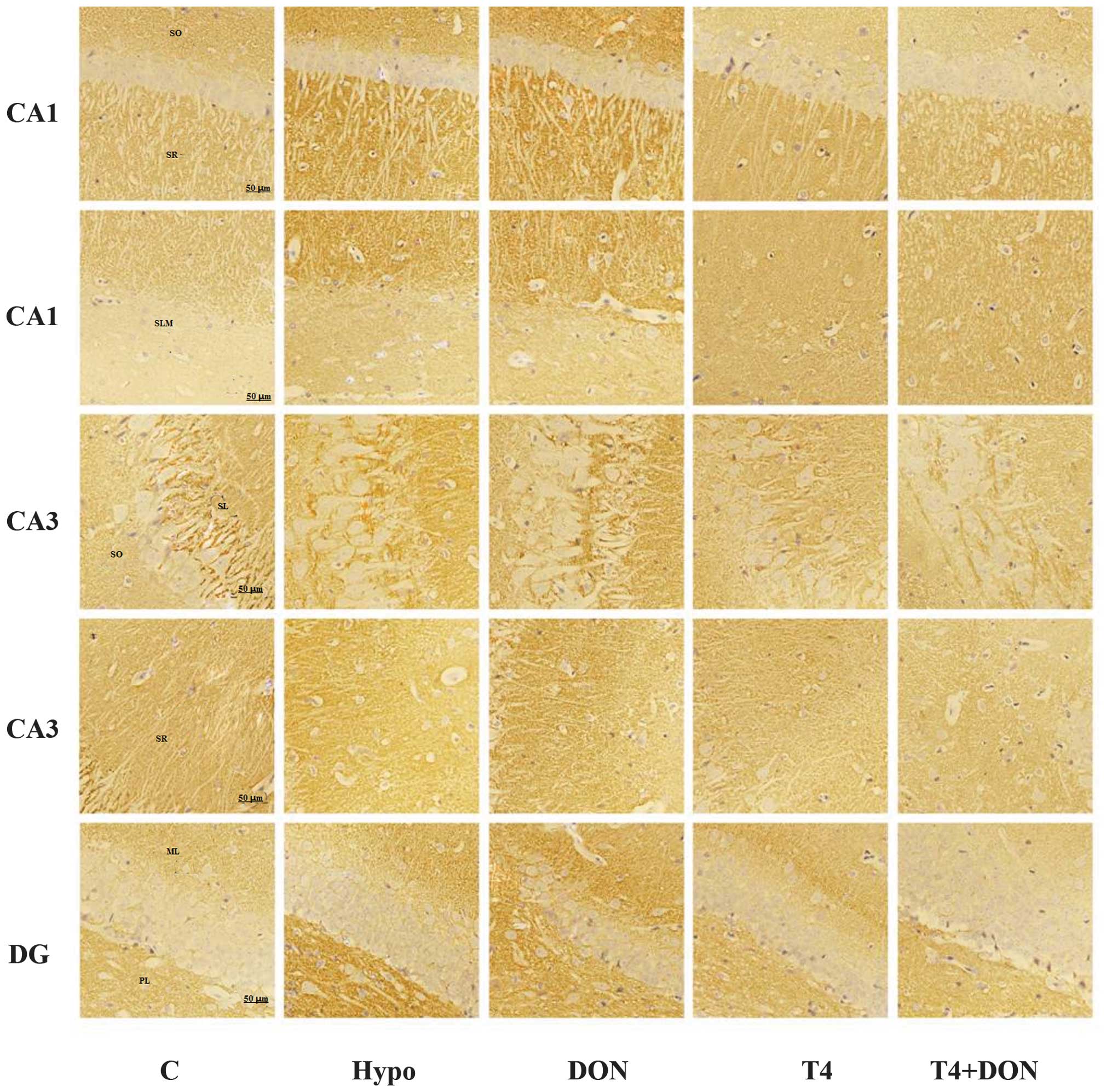 | Figure 4Photomicrographs of coronal sections
showing SNAP-25 immunoreactivity in CA1, CA3 and DG subregions of
hippocampus of rats from Hypo, T4, DON, T4+DON and C groups
(n=10–12). Distinct punctate spots of reaction product were
observed in every layer of CA1, CA3 and DG subregions; note that
the staining for SNAP-25 was more intense in CA3-SO, CA3-SR and in
all layers of CA1 and DG of Hypo and DON groups, and the overall
staining intensity was equal in CA3-SL of five groups;
magnification, ×400; scale bar: 50 μm. C, control group, Hypo,
hypothyroid group; DON, hypothyroid rats treated with 0.005% (w/v)
donepezil; T4, hypothyroid rats treated with 6 μg T4/100 g body
weight; T4+DON, hypothyroid rats treated with 6 μg T4/100 g body
weight beside adding 0.005% (w/v) donepezil to the drinking water.
Syt-1, synaptotagmin-1; T4, thyroxine; SNAP-25,
synaptosomal-associated protein of 25 kDa; DON, donepezil; DG,
dentate gyrus; SO, stratum oriens; SR, stratum radiatum; SLM,
stratum lacunosum-moleculare; SL, stratum lucidum; ML, molecular
layer; PL, polymorphic layer; w/v, weight/volume. |
The OD value corresponding to syt-1 immunoreactivity
in each stratum of hippocampal subfields is summarized in Table II. The OD values of five layers of
CA1, CA3 and DG subfields in the Hypo, DON and T4 groups were
significantly lower compared with the corresponding layers in the C
group (P<0.05), including CA1-SO, CA1-SR, CA3-SL, CA3-SR and
DG-ML. In the T4+DON group, the OD values in all layers were
similar to those in the C group.
 | Table IIsyt-1 in different layers of each
subfield in the hippocampus. |
Table II
syt-1 in different layers of each
subfield in the hippocampus.
| Subfield | Stratum | C | Hypo | DON | T4 | T4+DON |
|---|
| CA1 | SO | 5.06±0.15 | 3.49±0.13** | 3.85±0.14** | 4.09±0.20** | 4.79±0.16 |
| SR | 4.75±0.19 | 3.19±0.14** | 3.59±0.18** | 3.70±0.29* | 4.29±0.28 |
| SLM | 4.31±0.09 | 3.91±0.16 | 3.99±0.11 | 3.99±0.05 | 4.42±0.09 |
| CA3 | SO | 5.01±0.18 | 4.55±0.17 | 4.71±0.08 | 4.87±0.10 | 4.98±0.14 |
| SL | 4.85±0.10 | 3.94±0.04** | 3.95±0.05** | 4.23±0.07** | 4.71±0.08 |
| SR | 4.94±0.05 | 3.90±0.07** | 4.05±0.05** | 4.46±0.09** | 4.81±0.06 |
| DG | ML | 4.75±0.06 | 4.09±0.03** | 4.18±0.09** | 4.37±0.05** | 4.61±0.05 |
| PL | 3.76±0.05 | 3.51±0.03 | 3.57±0.10 | 3.67±0.07 | 3.72±0.06 |
The OD value of SNAP-25 immunoreactivity in each
stratum of hippocampal subfields is also analyzed and summarized in
Table III. It was identified
that the OD values of SNAP-25 in CA3-SO, CA3-SR and in all layers
of CA1 and DG, were significantly higher in Hypo and DON groups
compared with the corresponding layers in the C group (P<0.01).
In the T4 group, the OD values were significantly higher compared
with the C group in all layers of CA1 and CA3-SO (P<0.01). In
the T4+DON group, the OD values in all layers were similar to that
observed in the C group.
 | Table IIISNAP-25 in different layers of each
subfield in the hippocampus. |
Table III
SNAP-25 in different layers of each
subfield in the hippocampus.
| Subfield | Stratum | C | Hypo | DON | T4 | T4+DON |
|---|
| CA1 | SO | 3.55±0.11 | 4.82±0.08** | 4.49±1.13** | 4.39±0.05** | 3.80±0.12 |
| SR | 3.72±0.15 | 4.64±0.14** | 4.43±0.18** | 4.34±0.12* | 4.16±0.13 |
| SLM | 3.52±0.11 | 4.36±0.12** | 4.26±0.09** | 4.00±0.12* | 3.77±0.11 |
| CA3 | SO | 4.07±0.06 | 5.04±0.10** | 5.02±0.10** | 4.83±0.06** | 4.40±0.11 |
| SL | 3.63±0.12 | 4.10±0.12 | 4.09±0.15 | 3.92±0.11 | 4.07±0.09 |
| SR | 3.67±0.14 | 4.36±0.09** | 4.29±0.12** | 3.95±0.09 | 3.80±0.09 |
| DG | ML | 3.61±0.10 | 4.69±0.13** | 4.57±0.06** | 4.00±0.09 | 3.81±0.09 |
| PL | 3.66±0.11 | 4.75±0.04** | 4.46±0.09** | 3.95±0.06 | 3.85±0.05 |
Discussion
In the present study, it has been demonstrated that
there are significant decrements of Ach levels, as well as AChE
activity, in the hippocampus of adult-onset hypothyroidism. A
decrease in AChE activity has previously been identified in various
brain regions, including the cerebellum, frontal cortex, subcortex
and medulla of hypothyroid adult rats (11,21).
The effects induced by hypothyroidism on rat hippocampal Ach and
AChE remain largely unknown. The hippocampus has been reported to
be rich in cholinergic synapses (22) and thus hypothyroidism may result in
hippocampal cholinergic neuronal impairment. This may be because
the hippocampus is vulnerable to the deleterious effects of
hypothyroidism at developmental and adult stages (23), which subsequently leads to an
insufficient synthesis of ACh and its enzyme protein. Indeed, this
hypothesis is consistent with studies that have reported decreased
choline acetyltransferase (ChAT; involved in the synthesis of ACh)
activity and protein concentration in hypothyroidism (11,24–25).
Alternatively, the decrease in AChE activity may also result from
changes in the turnover of the enzyme proteins in hypothyroidism
(26–27).
Using immunohistochemical analysis, the results
indicated that the effects of adult-onset hypothyroidism on syt-1
and SNAP-25 are different, although these proteins are required for
neurotransmitter exocytosis. The relative expression of syt-1 was
decreased in certain layers of hippocampus of hypothyroid rats
compared with the controls, and the simultaneous upregulation of
SNAP-25 expression was also observed. The results obtained are
consistent with our previous study (13). The mechanism underlying this
differential regulation remains elusive. However, it has been
hypothesized that the reduced expression of syt-1 may be due to the
reduced expression of TH receptors as well as a decrease in the
number and/or volume of neurons in the hippocampus associated with
hypothyroidism (3,17,28),
considering it is an abundant constituent of synaptic vesicles
(9). While SNAP-25 overexpression
may be a compensatory mechanism against adult-onset hypothyroidism,
functioning to rescue vesicle exocytosis.
The changes in hippocampal ACh content and AChE
activity observed in the present study, during adulthood, appear to
be reversible, as decreased ACh levels and AChE activity were
restored to control values following T4 treatment. Similar results
were observed in rats studied during the critical stages of
development, in which treatment of 26-day-old neonatally
thyroidectomy rats with thyroid hormone was revealed to virtually
replenish the levels of the brain ACh to control values (29). Therefore, it may be that ACh and
its enzyme protein are under direct thyroid hormone control.
Evidence from tissue culture experiments indicate that as T3 media
concentrations approach the level of total TH in the blood, the
cholinergic enzymatic activities of ChAT and AChE were markedly
enhanced (26). Of note, DON
treatment (via drinking water) for 2 weeks also increased the
levels of ACh in the hippocampus of adult hypothyroid rats to meet
the values recorded from the control rats. This result is
consistent with previous studies demonstrating that AChEIs,
including physostigmine and galantamine, increase ACh levels
(30–31). Therefore it is probable that DON
increases ACh levels through a well-established mechanism of
preventing its enzymatic degradation and thus prolonging the
availability of ACh once it is released into the synapse.
By the administration of T4, it was observed that
hypothyroidism-induced downregulation of the syt-1 protein and
upregulation of the SNAP-25 protein was restored in the
hippocampus. However, the parameters did not reach the control
level. Previous animal studies have also reported that normal
ranges of hormone substitution did not reverse the reduction in
protein kinase C-γ and syt-1 levels in hypothyroid rats (13,32).
However, T4 and DON treatment in combination, did result in the
normalization of the hippocampal syt-1 and SNAP-25 levels in the
adult hypothyroid rats. This suggests that DON treatment resulted
in amelioration of hypothyroidism-induced synaptic protein
impairment, although the exact mechanism underlying this regulation
remains elusive. In recent years, the AChEIs have demonstrated
neuroprotective effects. DON has been reported to ameliorate
synaptic loss and tau pathology in the tauopathy mouse model, which
may be due to ACh-induced anti-inflammatory effects (18). In addition, it has been suggested
that DON slows the progression of hippocampal atrophy in patients
with Alzheimer’s disease (33–34)
and reduce cell death induced by exogenous cytotoxins, including
glutamate, amyloid-β, okadaic acid and carbon monoxide gas
(35–39). The observed normalization of the
synaptic proteins in hypothyroidism may also occur as a result of
DON-induced neuroprotection against hippocampal impairment, leading
to an alteration in the synthesis of these synaptic proteins. To
elucidate the mechanisms underlying the neuroprotective effects of
DON on hypothyroidism, further investigation is required.
In conclusion, this study demonstrated that
hypothyroidism induces alterations of hippocampal Ach level and
AChE activity, as well as syt-1 and SNAP-25 expression, in adult
rats. Ach levels and AChE activity were restored by T4
administration, while expression of syt-1 and SNAP-25 did not reach
the control level. Significantly, co-administration of T4 and DON
led to normalization of the syt-1 and SNAP-25 levels, suggesting
that DON treatment may facilitate the recovery of synaptic protein
impairment induced by hypothyroidism. Nevertheless, the efficacy of
DON treatment and the molecular mechanisms underlying this
regulation require further exploration to be of therapeutic
value.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81272152).
References
|
1
|
Alzoubi KH, Gerges NZ, Aleisa AM and
Alkadhi KA: Levothyroxin restores hypothyroidism-induced impairment
of hippocampus-dependent learning and memory: Behavioral,
electrophysiological, and molecular studies. Hippocampus. 19:66–78.
2009. View Article : Google Scholar
|
|
2
|
Tong H, Chen GH, Liu RY and Zhou JN:
Age-related learning and memory impairments in adult-onset
hypothyroidism in Kunming mice. Physiol Behav. 91:290–298. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Madeira MD, Sousa N, Lima-Andrade MT,
Calheiros F, Cadete-Leite A and Paula-Barbosa MM: Selective
vulnerability of the hippocampal pyramidal neurons to
hypothyroidism in male and female rats. J Comp Neurol. 322:501–518.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith JW, Evans AT, Costall B and Smythe
JW: Thyroid hormones, brain function and cognition: a brief review.
Neurosci Biobehav Rev. 26:45–60. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hefti F, Hartikka J and Bolger MB: Effect
of thyroid hormone analogs on the activity of choline
acetyltransferase in cultures of dissociated septal cells. Brain
Res. 375:413–416. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chapman ER: Synaptotagmin: a
Ca(2+) sensor that triggers exocytosis? Nat Rev Mol Cell
Biol. 3:498–508. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mehta PP, Battenberg E and Wilson MC:
SNAP-25 and synaptotagmin involvement in the final
Ca(2+)-dependent triggering of neurotransmitter
exocytosis. Proc Natl Acad Sci USA. 93:10471–10476. 1996.
View Article : Google Scholar
|
|
8
|
de Wit H, Walter AM, Milosevic I, et al:
Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25
acceptor complexes. Cell. 138:935–946. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perin MS, Brose N, Jahn R and Südhof TC:
Domain structure of synaptotagmin (p65). J Biol Chem. 266:623–629.
1991.PubMed/NCBI
|
|
10
|
Salvati S, Attorri L, Campeggi LM, et al:
Effect of propylthiouracil-induced hypothyroidism on cerebral
cortex of young and aged rats: lipid composition of synaptosomes,
muscarinic receptor sites, and acetylcholinesterase activity.
Neurochem Res. 19:1181–1186. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carageorgiou H, Pantos C, Zarros A, et al:
Changes in antioxidant status, protein concentration,
acetylcholinesterase, (Na+,K+)-, and
Mg2+-ATPase activities in the brain of hyper- and
hypothyroid adult rats. Metab Brain Dis. 20:129–139. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang HY, Sun CP, Jia XM, Gui L, Zhu DF and
Ma WQ: Effect of thyroxine on SNARE complex and synaptotagmin-1
expression in the prefrontal cortex of rats with adult-onset
hypothyroidism. J Endocrinol Invest. 35:312–316. 2012.
|
|
13
|
Liu CL, Xu YX, Zhan Y, et al: Effect of
thyroxine on synaptotagmin 1 and SNAP-25 expression in dorsal
hippocampus of adult-onset hypothyroid rats. J Endocrinol Invest.
34:280–286. 2011. View Article : Google Scholar
|
|
14
|
Wekking EM, Appelhof BC, Fliers E, et al:
Cognitive functioning and well-being in euthyroid patients on
thyroxine replacement therapy for primary hypothyroidism. Eur J
Endocrinol. 153:747–753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Samuels MH, Schuff KG, Carlson NE, Carello
P and Janowsky JS: Health status, psychological symptoms, mood, and
cognition in L-thyroxine-treated hypothyroid subjects. Thyroid.
17:249–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saravanan P, Chau WF, Roberts N, Vedhara
K, Greenwood R and Dayan CM: Psychological well-being in patients
on ‘adequate’ doses of l-thyroxine: results of a large, controlled
community-based questionnaire study. Clin Endocrinol (Oxf).
57:577–585. 2002. View Article : Google Scholar
|
|
17
|
Madeira MD, Cadete-Leite A, Andrade JP and
Paula-Barbosa MM: Effects of hypothyroidism upon the granular layer
of the dentate gyrus in male and female adult rats: a morphometric
study. J Comp Neurol. 314:171–186. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshiyama Y, Kojima A, Ishikawa C and Arai
K: Anti-inflammatory action of donepezil ameliorates tau pathology,
synaptic loss, and neurodegeneration in a tauopathy mouse model. J
Alzheimers Dis. 22:295–306. 2010.PubMed/NCBI
|
|
19
|
Gerges NZ, Stringer JL and Alkadhi KA:
Combination of hypothyroidism and stress abolishes early LTP in the
CA1 but not dentate gyrus of hippocampus of adult rats. Brain Res.
922:250–260. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hestrin S: The reaction of acetylcholine
and other carboxylic acid derivatives with hydroxylamine, and its
analytical application. J Biol Chem. 180:249–261. 1949.PubMed/NCBI
|
|
21
|
Carageorgiou H, Pantos C, Zarros A, et al:
Changes in acetylcholinesterase,
Na+,K+-ATPase, and Mg2+-ATPase
activities in the frontal cortex and the hippocampus of hyper- and
hypothyroid adult rats. Metabolism. 56:1104–1110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Everitt BJ and Robbins TW: Central
cholinergic systems and cognition. Annu Rev Psychol. 48:649–684.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koromilas C, Liapi C, Schulpis KH,
Kalafatakis K, Zarros A and Tsakiris S: Structural and functional
alterations in the hippocampus due to hypothyroidism. Metab Brain
Dis. 25:339–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Z, Yang R and Chen Z: Effects of iodine
and thyroid hormone deficiency during brain development on
activities of cholinergic neurone-related enzymes in central
nervous system of rats. Zhonghua Yu Fang Yi Xue Za Zhi. 30:337–339.
1996.(In Chinese). PubMed/NCBI
|
|
25
|
Kojima M, Kim JS, Uchimura H, Hirano M,
Nakahara T and Matsumoto T: Effect of thyroidectomy on choline
acetyltransferase in rat hypothalamic nuclei. Brain Res.
209:227–230. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Honegger P and Lenoir D: Triodothyronine
enhancement of neuronal differentiation in aggregating fetal rat
brain cells cultured in a chemically defined medium. Brain Res.
199:425–434. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akuzawa K and Wakabayashi K: A serum-free
culture of the neurons in the septal, preoptic, and hypothalamic
region. Effects of triiodothyronine and estradiol. Endocrinol Jpn.
32:163–173. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Constantinou C, Margarity M and Valcana T:
Region-specific effects of hypothyroidism on the relative
expression of thyroid hormone receptors in adult rat brain. Mol
Cell Biochem. 278:93–100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hrdina PD, Ghosh PK, Rastogi RB and
Singhal RL: Ontogenic pattern of dopamine, acetylcholine, and
acetylcholinesterase in the brains of normal and hypothyroid rats.
Can J Physiol Pharmacol. 53:709–715. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saiyed M and Riker WK: Cholinergic and
anticholinergic drug effects on survival during hypoxia:
significant gender differences. J Pharmacol Exp Ther.
264:1146–1153. 1993.PubMed/NCBI
|
|
31
|
Dimitrova DS and Getova-Spassova DP:
Effects of galantamine and donepezil on active and passive
avoidance tests in rats with induced hypoxia. J Pharmacol Sci.
101:199–204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alzoubi KH, Gerges NZ and Alkadhi KA:
Levothyroxin restores hypothyroidism-induced impairment of LTP of
hippocampal CA1: electrophysiological and molecular studies. Exp
Neurol. 195:330–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hashimoto M, Kazui H, Matsumoto K, Nakano
Y, Yasuda M and Mori E: Does donepezil treatment slow the
progression of hippocampal atrophy in patients with Alzheimer’s
disease? Am J Psychiatry. 162:676–682. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Krishnan KR, Charles HC, Doraiswamy PM, et
al: Randomized, placebo-controlled trial of the effects of
donepezil on neuronal markers and hippocampal volumes in
Alzheimer’s disease. Am J Psychiatry. 160:2003–2011. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Akaike A: Preclinical evidence of
neuroprotection by cholinesterase inhibitors. Alzheimer Dis Assoc
Disord. 20(Suppl 1): S8–S11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takada-Takatori Y, Kume T, Sugimoto M,
Katsuki H, Sugimoto H and Akaike A: Acetylcholinesterase inhibitors
used in treatment of Alzheimer’s disease prevent glutamate
neurotoxicity via nicotinic acetylcholine receptors and
phosphatidylinositol 3-kinase cascade. Neuropharmacology.
51:474–486. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Meunier J, Ieni J and Maurice T: The
anti-amnesic and neuroprotective effects of donepezil against
amyloid beta25–35 peptide-induced toxicity in mice involve an
interaction with the sigma1 receptor. Br J Pharmacol. 149:998–1012.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meunier J, Ieni J and Maurice T:
Antiamnesic and neuroprotective effects of donepezil against
learning impairments induced in mice by exposure to carbon monoxide
gas. J Pharmacol Exp Ther. 317:1307–1319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arias E, Gallego-Sandín S, Villarroya M,
García AG and López MG: Unequal neuroprotection afforded by the
acetylcholinesterase inhibitors galantamine, donepezil, and
rivastigmine in SH-SY5Y neuroblastoma cells: role of nicotinic
receptors. J Pharmacol Exp Ther. 315:1346–1353. 2005. View Article : Google Scholar : PubMed/NCBI
|


















