Introduction
Morphine is used to relieve pain in patients with
cancer in terminal phases, in order to improve their quality of
life (1). Xenograft mouse models
were established in previous studies in order to analyze the role
of morphine in cancer cells (2,3).
Tegeder et al (2)
discovered that morphine significantly reduced tumor growth through
a p53-dependent mechanism in a mouse model of breast cancer.
Harimaya et al (3)
demonstrated that morphine inhibited tumor metastasis formation in
a mouse model of colon cancer. By contrast, other studies indicated
that morphine increased tumor growth (4,5). A
further study revealed that morphine enhanced tumor
neovascularization by activating vascular endothelial growth factor
receptor (6).
The secreted protein Dickkopf-1 (DKK1) is a member
of the Dickkopf gene family, which comprises Dickkopf-1, -2, -3 and
-4, as well as Dickkopf-3-associated protein, Soggy (7). DKK1 is known to be a negative
regulator of the Wnt/β-catenin signaling pathway (8). Numerous cellular processes are
mediated by Wnt/β-catenin signaling, including proliferation,
differentiation, survival, apoptosis and cell motility (9). Abnormal DKK1 expression is found in
numerous malignant tumors including breast cancer, lung cancer,
esophageal carcinoma and hepatocellular carcinoma (10–13).
DKK1 is typically silenced in colon cancer cells by DNA
hypermethylation, which correlates with the advanced stages of
colorectal tumorigenesis (14).
Other studies have shown that DKK-1 serves as a diagnostic and
prognostic biomarker of hepatocellular carcinomas and osteosarcoma
(15,16).
Morphine and DKK1 are associated with tumorigenesis.
However, to the best of our knowledge, there has been no study
investigating the effects of these two factors simultaneously. The
present study therefore aimed to elucidate the effects and
interactions of morphine and DKK1 in SH-SY5Y human neuroblastoma
cells. Neuroblastoma is a highly malignant pediatric cancer derived
from precursor or immature cells of the sympathetic nervous system
(17). It is characterized by
unexpected clinical behaviors like spontaneous regression or
maturation, but also by aggressive progression and poor treatment
response (18). In this study,
spatial learning and memory were evaluated in mouse models of
neuroblastoma using the water maze and T-maze tests. This is the
first study to our knowledge to investigate the association between
DKK-1 and morphine in human neuroblastoma. Morphine appears to be
of crucial biological importance in protecting neuroblastoma from
the toxicity of DKK1. DKK1 may be used as a novel therapeutic
strategy for neuroblastoma.
Materials and methods
Cell culture
Human neuroblastoma (SH-SY5Y) cells were purchased
from the American Type Culture Collection (Manassas, VA, USA).
Cells were grown at 37°C in medium containing a 1:1 ratio of
Eagle’s minimum essential medium and Ham’s F-12 nutrient mixture
(Hyclone, Logan, UT, USA) as well as 10% fetal bovine serum (FBS;
Hyclone) in a humidified atmosphere containing 5%
CO2.
Plasmid transfection and stable cell
establishment
The plasmid, phosphorylated enhanced green
fluorescent protein (pEGFP)-C1-DKK1 was provided by Dr Wang Rong
(China Medical University, Shenyang, China)(19). pEGFP-C1-DKK1 transfections were
performed using Lipofectamine® 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions. Twenty-four hours post-transfection with
pEGFP-C1-DKK1, cells were selected with G418 (400 μg/ml; Invitrogen
Life Technologies) for 10–12 days. Drug-resistant clones were
isolated and expanded. All gene expression studies were conducted
using pools of colonies (n=50) to avoid clonal bias. The three
resulting DKK1-positive cell lines were named D1, D2 and D3.
Immunofluorescence
Transfected cells were washed with
phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde,
permeabilized in 1% Triton X-100 (Beyotime Institute of
Biotechnology, Shanghai, China) for 5 min and blocked with 5%
bovine serum albumin (BSA) in PBS containing 0.5% Triton X-100 for
1 h. DKK1 expression was detected using goat polyclonal
immunoglobulin G anti-DKK1 antibody (sc-30782; 1:200; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature.
Cells were subsequently washed with PBS and incubated with
appropriate secondary fluorophore-conjugated antibodies (1:1000)
for 1 h at room temperature. Secondary antibody used for detection
of DKK1 was Alexa Fluor® 594 donkey anti-goat
immunoglobulin G, heavy and light chains (1:200; Invitrogen Life
Technologies).
Morphine treatment and cell growth
inhibition assays (MTT assay)
D1, D2 and D3 cells were treated with a single dose
of morphine (1, 5, 10 or 15 μM). Cells were plated in 96-well
plates (1,500 cells/well) and allowed to attach overnight. MTT
solution (Sigma-Aldrich, St. Louis, MO, USA) was added (final
concentration, 0.5 mg/ml) and cells were incubated for 4 h. Next,
the cells were lysed using with dimethyl sulfoxide (Sigma-Aldrich).
Absorbance was measured at 550–560 nm using a 505 microplate reader
(Bio-Rad, Hercules, CA, USA).
Annexin V-fluorescein isothiocyanate
(FITC) and propidium iodide (PI) double staining
D1, D2 and D3 cells were left untreated, or were
treated with morphine. According to the manufacturer’s instructions
(Apoptosis Detection kit, KeyGEN, Nanjing, China), cells were
washed and resuspended in binding buffer prior to the addition of
FITC-labeled Annexin-V and PI for 10 min. Suspensions were
immediately analyzed by a FACSCalibur machine (BD Biosciences,
Baltimore, MD, USA).
Reverse transcription (RT)-polymerase
chain reaction (PCR)
mRNA levels of DKK1 were determined by RT-PCR. GAPDH
served as the negative control. Total RNA (20 μg) was extracted
from transfected cells using TRIzol reagent (Invitrogen Life
Technologies). Reverse transcription was performed using the RT
reaction mix (Promega Corporation, Madison, WI, USA) and the cDNA
obtained was used for PCR. The following primers were used for DKK1
and GAPDH were used: Forward, 5′-CTGCATGCGTCACGCTATGT-3′ and
reverse, 5′-TCCTCGGAAATGATTTTGATCA-3′ for DKK1; and forward,
5′-CAGTCAGCCGCATCTTCTTTT-3′ and reverse, 5′-GTGACCAGGCGCCCAATAC-3′
for GAPDH.
Transwell assay
The migration assay was performed using Boyden
chambers (8-μM pore size polycarbonate membrane; Cell Biolabs, San
Diego, CA, USA). Cells were resuspended in FBS-free Eagle’s minimum
essential medium (EMEM; Hyclone) to a concentration of
3×105 cells/ml. The upper chamber was loaded with 100 μl
cell suspension and the lower chamber was loaded with 600 μl EMEM
containing 10% FBS. Following incubation for 12 h under normal
culture conditions, no cells were observed floating in the upper
chamber, indicating that the cells had not undergone apoptosis at
this time-point. The filter was fixed in 4% paraformaldehyde
(Sigma) and stained with crystal violet (Beyotime, Shanghai,
China). The cells on the upper side of the filter were wiped off
using a cotton swab. Cells that had migrated to the lower side of
the membrane were counted using a light microscope (CX31; Olympus
Corporation, Tokyo, Japan). Ten microscopic fields (x400) were
randomly selected to count the cells. For the cell invasion assay,
the procedure was identical to that outlined above, excluding the
Matrigel-coated insert (BD Biosciences).
Stereotaxic surgery and intracerebral
administration in the mouse
All animal experiments with were performed according
to the guidelines of the China Medical University Ethics Committee
(Shenyang, China). Six to eight week-old NU/NU nude mice (1.4–1.8
g) were purchased from Vital River (Beijing, China). The mice were
kept at 22°C and exposed to a 12 h light/dark cycle (6.30 am–6.30
pm) with free access to food and water. All mice received
intrastriatal injections of SH-SY5Y cells (1×106 in 200
μl), which were administered 5 mm below the dura over a 10-min
duration with a microsyringe (Hamilton, Reno, NV, USA). The needle
was left in place for a further three minutes. The burr hole was
sealed with bone wax and the skin incision was closed with 4-0 silk
sutures. When the tumor diameters had reached 3–5 mm, the mice were
divided randomly into three groups (tumor, DKK1 and DKK1 + morphine
groups). The groups were treated with phosphate-buffered saline, a
100 μl intratumoral injection of DKK1 (20 μg) and 30 μl
Lipofectamine 2000 (Invitrogen Life Technologies), or DKK1 (20 μg)
with Lipofectamine 2000 (30 μl) and morphine. Two injections were
administered at 9 am and 4 pm every four days.
Water maze test
Behavioral observations for spatial learning and
memory were made in the water maze, a modification of the standard
version of the Morris test (20).
Animals were trained to find a hidden platform in a swimming pool.
The circular white pool was filled to a depth of 30 cm with 23°C
water. The pool was located in a testing room which contained
numerous objects that could be used by the mice for spatial
orientation. The position of the cues remained unchanged throughout
the period of testing. Each trial was started by placing a mouse
facing towards the wall of the pool at the start point. The
sequence of the starting positions was randomly selected and
changed each day. The trial was terminated when the animal mounted
the hidden platform. Ten mice were used in each experiment and each
experiment was performed in triplicate.
T-maze test
Two identical four-arm radial mazes arranged in a
single large wagon-wheel structure (120 cm outer diameter; width of
arms, 6 cm) were used for the T-maze test. The center platform was
common to the two mazes. One movable transparent wall on an outer
arm and eight transparent doors around the center allowed the
selection of a specific configuration for each maze. Each mouse was
initially placed at one end of the trajectory and required to
navigate through the correct arms in order to reach the opposite
end and retrieve chocolate sprinkles. The mouse subsequently had to
return to the starting position, where it received chocolate
sprinkles again, to initiate a novel trial. Each recording session
began with a one-hour resting period in a small, separate box. The
mouse was then trained for 15 min on the first maze, allowed to
rest for one hour, then trained for 15 min on the second maze and
allowed to rest for another hour. One maze was selected as the test
maze and the other as the control maze; the temporal order and
physical location of the test maze on the first day was randomized
across mice. For each mouse, the test and control mazes remained
unchanged for the duration of training. The injection of DKK1
and/or morphine was always performed during the resting period
following maze T, and the order of the mazes was alternated each
day for 8–10 days. On the last planned experimental day, no
stimulation was applied. Recordings had to be prematurely halted
following three days of testing for one mouse due to technical
reasons.
Animal magnetic resonance imaging
(MRI)
For the MRI (Aspect M2; Winsun China Ltd., Hong
Kong, China), anesthesia was induced with 4% isoflurane and
maintained with 2.7% isoflurane in 69% N2O and 30%
O2 using a vaporizer. MRI sessions were performed 10 and
11 days following cell inoculation when tumors were 2–3 mm in
diameter.
Terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate nick-end labeling
(TUNEL)
Tumors were resected and the tumor volume was
determined. Tumor biopsies were immediately fixed in 10%
neutral-buffered formalin containing phosphatase inhibitors (NaF
and Na3VO4; Beyotime Institute of
Biotechnology) prior to the preparation of paraffin-embedded
sections. Apoptosis was measured using immunohistochemistry in
paired tumor samples by TUNEL.
Preparation of nuclear and cytoplasmic
protein extracts
Nuclear and cytoplasmic protein fractions were
isolated at the indicated time-points using a CelLytic™ NuCLEAR™
Extraction kit (Sigma), according to the manufacturer’s
instructions. Protein concentrations were determined using a
bicinchoninic acid protein assay with BSA used as a standard
(Pierce Biotechnology, Inc., Rockford, IL, USA).
Western blot analysis
Western blot analysis was performed using cell
extracts from SH-SY5Y cells transfected with pEGFP-C1-DKK1. Cell
extracts were resolved using SDS-PAGE and subsequently transferred
onto nitrocellulose membranes. Membranes were developed and
visualized using enhanced chemiluminescence (Pierce Biotechnology,
Inc., Rockford, IL, USA). Primary antibodies used included: Rabbit
polyclonal IgG anti-low-density lipoprotein receptor-related
protein 6 (LRP6) (sc-15399; Santa Cruz Biotechnology, Inc.), mouse
polyclonal IgG anti-phosphorylated-LRP6 (Ser1490) (2568; Cell
Signaling Technology, Beverly, MA, USA), rabbit monoclonal IgG
anti-Axin2 (5863; Cell Signaling Technology), mouse monoclonal IgG
anti-glycogen synthase kinase 3β (GSK-3β; sc-81462; Santa Cruz
Biotechnology, Inc.), mouse monoclonal IgG anti-β-catenin (610154;
BD Biosciences), and mouse monoclonal IgG anti-β-tubulin (T5201;
Sigma-Aldrich).
Statistical analysis
All values are presented as the mean ± standard
error of the mean. Student’s paired t-test was used to identify
statistical significances. Kaplan-Meier survival plots were
generated and comparisons were made with log-rank statistics.
P<0.05 was considered to indicate a statistically significant
difference between values. Statistical analyses were performed
using GraphPad PRISM 4 software (GraphPad Software Inc., San Diego,
CA, USA).
Results
DKK1-expressing SH-SY5Y cell lines
SH-SY5Y cells were transfected with pEGFP-C1-DKK1,
and EGFP-expressing SH-SY5Y cells were selected for further study.
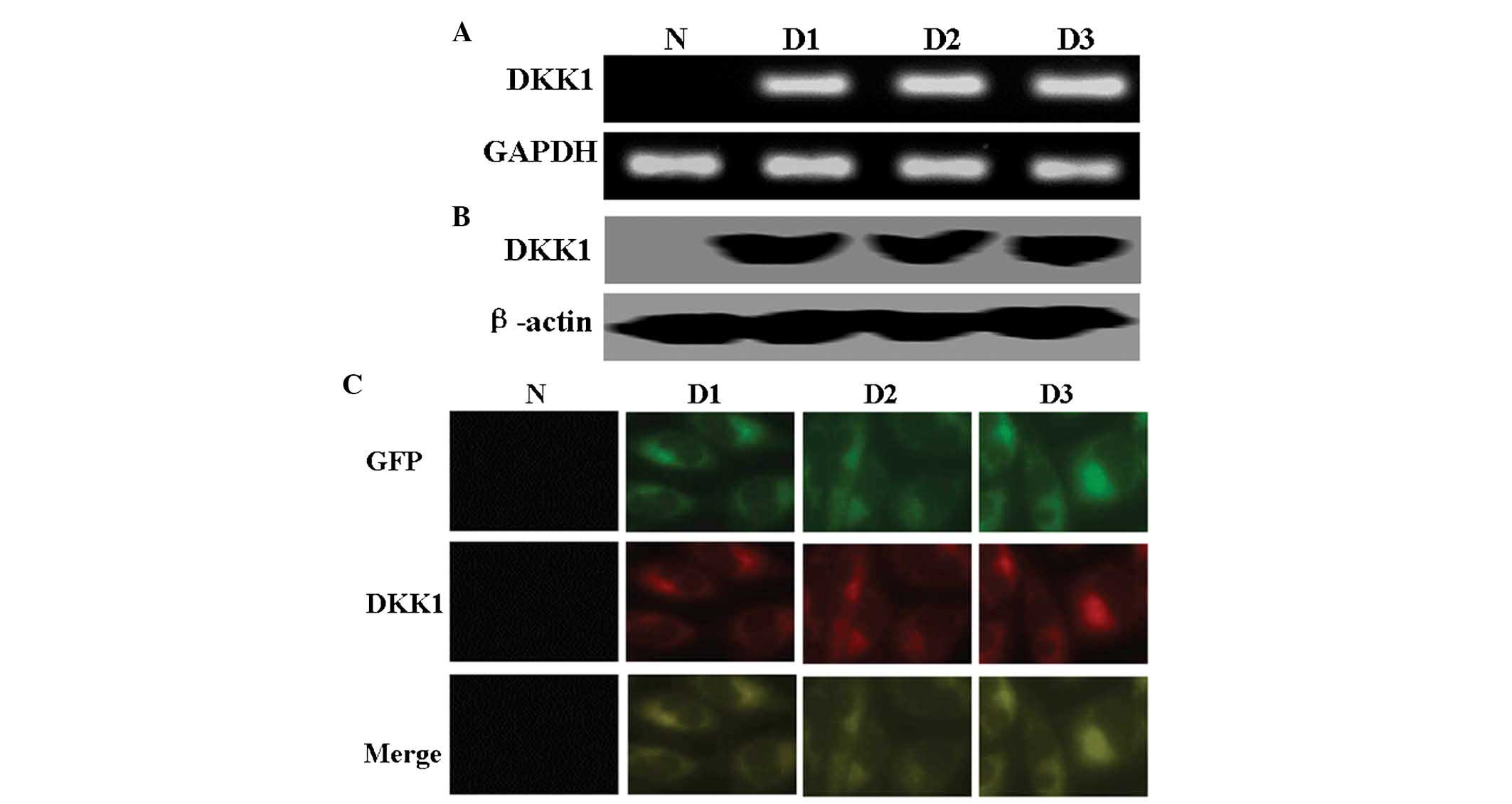
As shown in Fig. 1A and B, the
results of RT-PCR and western blot analysis confirmed exogenous
expression of DKK1 in SH-SY5Y cells following transfection.
Immunofluorescence analysis revealed the localization of DKK1 and
EGFP in DKK1-expressing SH-SY5Y cells (Fig. 1C).
Effects of morphine on DKK1-expressing
SH-SY5Y cells
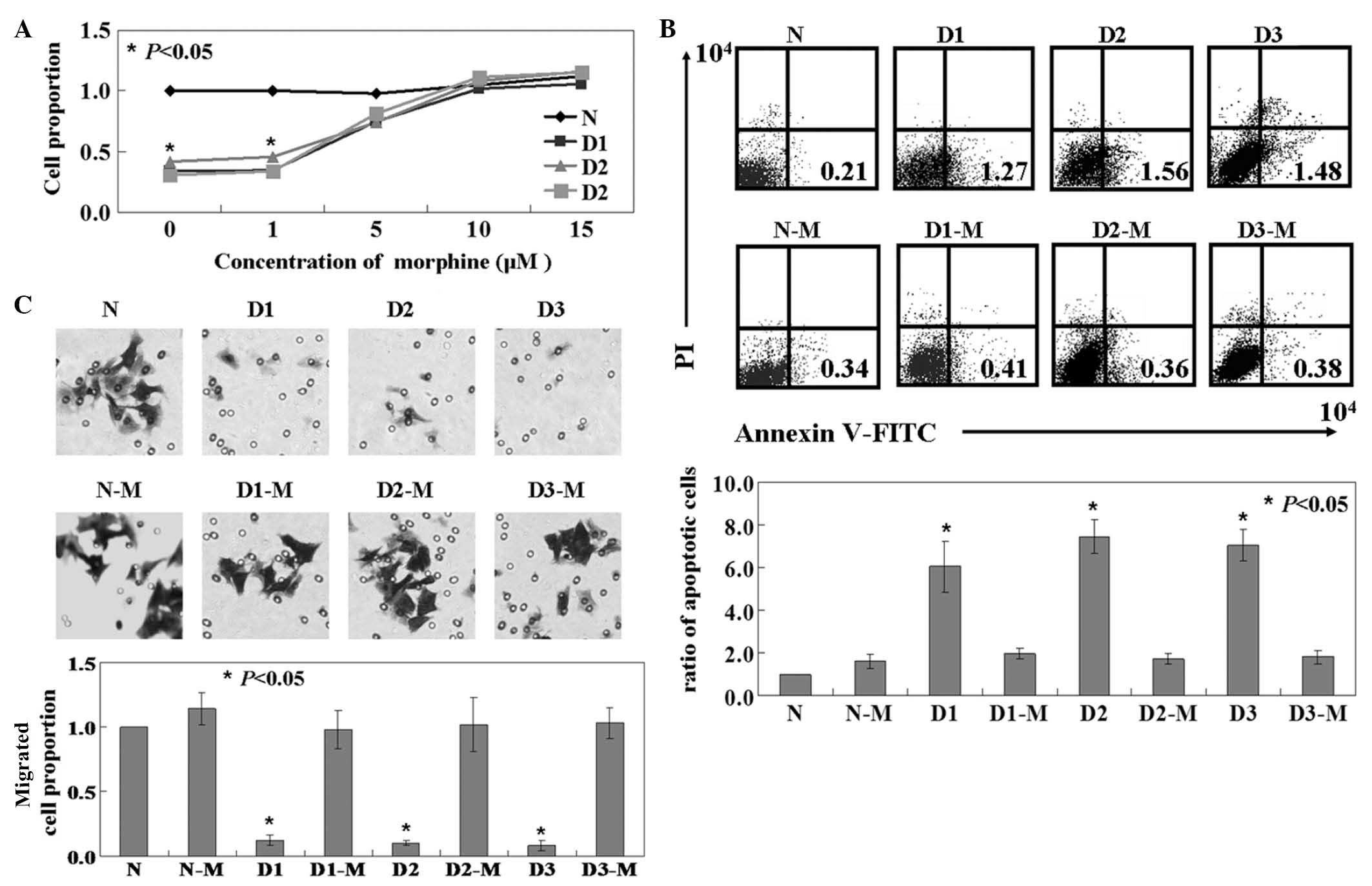
The function of DKK1 on SH-SY5Y cells was
established. The results of the MTT assay indicated that the
proliferation rate of cells in the D1, D2 and D3 groups was
significantly lower than that of untreated cells (P<0.05;
Fig. 2A). The ratio of apoptotic
cells in the D1, D2 and D3 groups was higher than that of the
untreated cells, established by Annexin-V and PI double-staining
(P<0.05; Fig. 2B). The effect
of DKK1-expression on the migration of SH-SY5Y cells was determined
by Transwell assay. Migration was significantly inhibited in D1, D2
and D3 cells compared to that of untreated cells (P<0.05;
Fig. 2C). Subsequently, the effect
of morphine on DKK1-expressing SH-SY5Y cells was determined.
Following morphine treatment, D1, D2 and D3 groups exhibited a
higher proliferation rate, lower apoptotic rate and greater
mobility than untreated DKK1-expressing cells (P<0.05; Fig. 2).
Anti-tumor activity of morphine and DKK1
in a mouse model
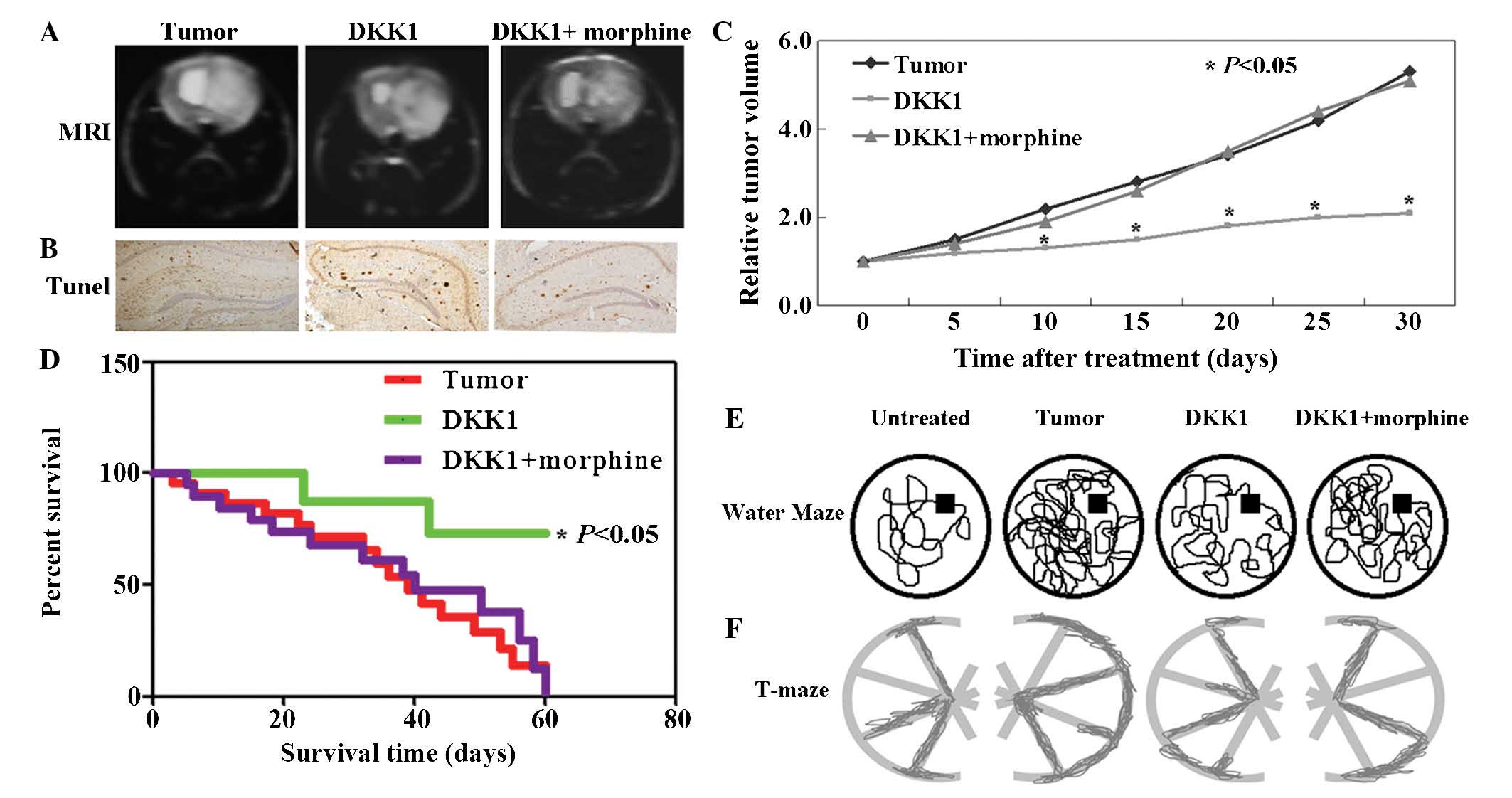
Neuroblastoma mouse models were used in order to
assess the in vivo tumorigenic or anti-tumor effects of DKK1
and morphine. Fig. 3A exhibits
representative T2-weighted MRIs of the mouse tumor model using the
spin-echo pulse sequence method, prior to and following injection
of DKK1 or DKK1+morphine. The results of the TUNEL assay indicated
a higher apoptotic rate of tumor cells in mice following DKK1
treatment and a lower apoptotic rate of cells in mice following
DKK1+morphine treatment (Fig. 3B).
Correspondingly, the volumes of DKK1-treated tumors were lower than
those of untreated and those of DKK1+morphine-treated tumors
(P<0.05; Fig. 3C). In addition,
the survival rate of mice with tumors treated with DKK1 was
significantly improved (P<0.05). Mice in the untreated and
DKK1+morphine-treated groups died at the end of the experiment
(Fig. 3D). Furthermore, the
acquired and retained memory of the mice with DKK1 or morphine
treatment was examined using the water maze. It was confirmed that
the DKK1-treatment group demonstrated a superior memory in
comparison to the other groups (Fig.
3E). The number of Start-to-Finish trajectories completed by
each mouse on the T-maze test as a function of time was evaluated.
Significantly shorter trajectories were observed in the mice with
DKK1 treatment than in those in the untreated and
DKK1+morphine-treated groups (Fig.
3F).
Morphine mitigates DKK1-induced apoptosis
in SH-SY5Y cells
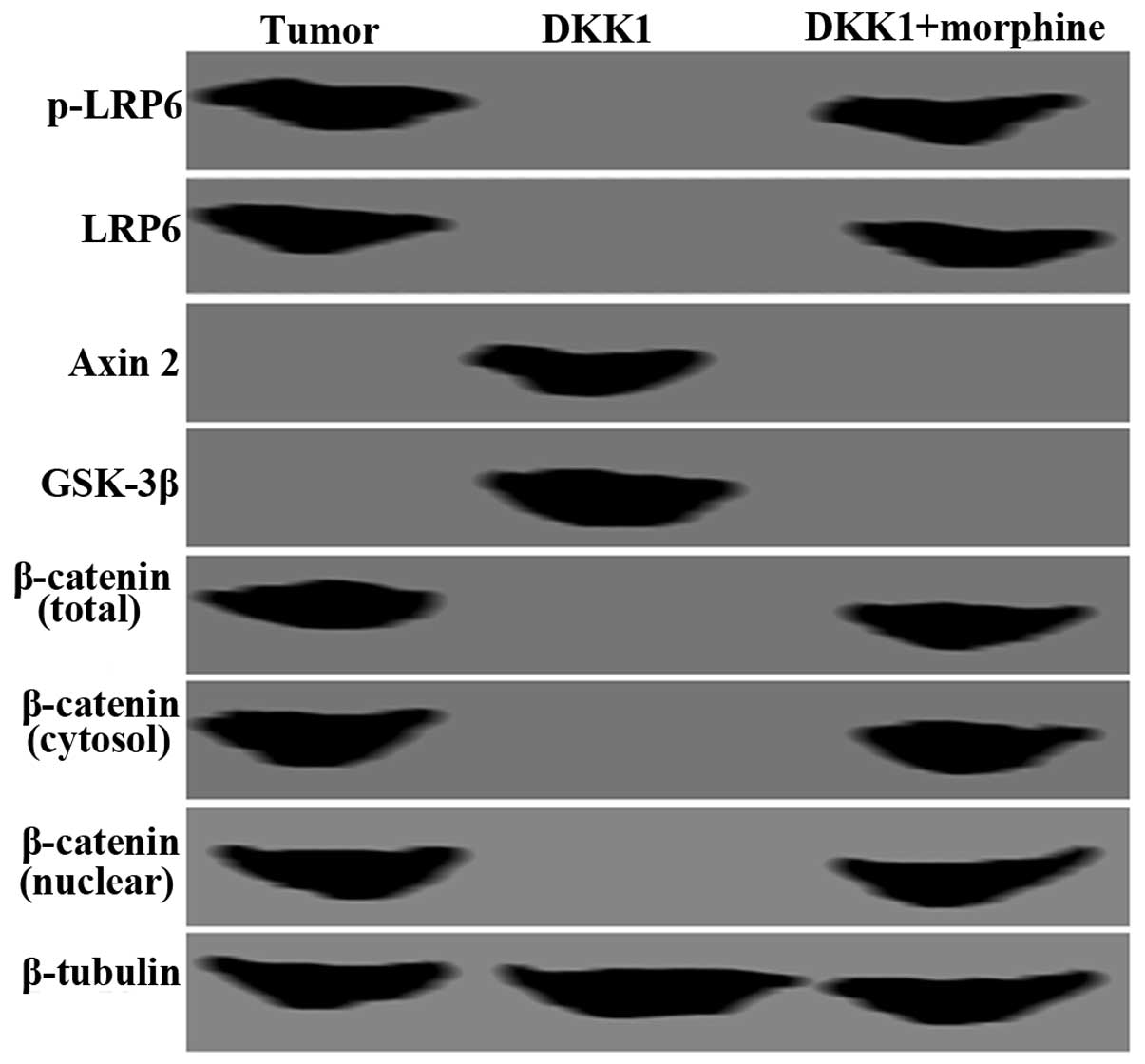
As indicated in Fig.
4, a decrease in p-LRP6 and LRP6 expression and an increase in
Axin2 and GSK-3β expression in SH-SY5Y cells with DKK1 expression
was observed. By contrast, the altered expression levels of these
proteins were reversed following DKK1+morphine treatment (Fig. 4). Total, cytosolic and nuclear
β-catenin protein expression was lost in SH-SY5Y cells following
DKK1 treatment. However, the expression of these proteins was
restored following DKK1+morphine treatment (Fig. 4). β-tubulin was used as an internal
control.
Discussion
Morphine, a potent natural opiate, is commonly used
for the treatment of patients with severe tumor-associated pain.
Despite extensive research, it remains to be elucidated whether
morphine directly modifies tumor cell growth (21). Tegeder et al (2) reported that high concentrations of
morphine inhibited tumor cell proliferation. However, other studies
have drawn opposite conclusions. Lazarczyk et al (22) found that morphine triggered human
glioblastoma T98G cell proliferation. Gupta et al (4) demonstrated that morphine promoted
tumor neovascularization in a human breast tumor xenograft mouse
model, thereby enhancing tumor progression. In the present study,
it was demonstrated that morphine protected neuroblastoma cells
against DKK1-induced apoptosis. The expression of DKK1 in
xenografted tumors also inhibited tumor growth. However, morphine
was found to significantly offset the anti-tumor effects of DKK1
in vivo. In vitro and in vivo results revealed
that morphine protected neuroblastoma cells from DKK1-induced
apoptosis. Furthermore, morphine had neither positive nor negative
effects on the proliferation capacity of DKK1-negative
neuroblastoma cells.
DKK1, a secreted protein, is known to be a negative
regulator of the Wnt/β-catenin signaling pathway (23). It was previously demonstrated that
DKK1 was downregulated in human tumors, indicating that it may act
as a tumor suppressor (24). In
the present study, it was confirmed that DKK1 induced apoptosis in
neuroblastoma cells through the Wnt/β-catenin signaling pathway.
This signaling pathway is commonly dysregulated in various cancers
(25–27). LRP6 is an essential Wnt co-receptor
in the Wnt/β-catenin signaling pathway and its phosphorylation is
essential for Wnt/β-catenin signaling activation (28). The results of the present study
additionally demonstrated a significant inhibition of LRP6
expression and phosphorylation following DKK1 treatment of SH-SY5Y
cells. Moreover, in previous studies, GSK-3 was shown to be active
in the absence of Wnt signaling (29), leading to the degradation of
cytoplasmic β-catenin and inhibition of nuclear translocation
(30). The results additionally
confirmed that cytosolic and nuclear β-catenin protein expression
was lost in SH-SY5Y cells following DKK1 treatment. However,
morphine activated Wnt/β-catenin signaling in DKK1-expressing
SH-SY5Y cells. Neuorblastoma most commonly affects cognitive
functions, including attention, psychomotor speed and memory
(31). The Morris water maze
(MWM), which consists of a circular pool in which mice are trained
to escape from water by swimming to a hidden platform, has been
described previously (32). The
MWM and T-maze tests are widely used to evaluate spatial learning
and memory (33). In this study,
the use of the MWM and T-maze tests revealed that neuroblastoma
impaired the spatial learning and memory of mice. Notably, DKK1
repaired the memory of mice by inducing the apoptosis of
neuroblastoma cells. Following the administration of morphine, the
effect of DKK1 was offset in the mice.
In conclusion, the results of the present study
demonstrated that DKK1 inhibited SH-SY5Y cell proliferation and
migration at least in part by promoting Wnt/β-catenin signaling.
Morphine was also able to protect SH-SY5Y cells against
DKK1-induced apoptosis. The results of this study indicate that
morphine may protect neuroblastoma cells and thus, it may be used
in neuroblastoma patients.
Acknowledgements
The authors would like to thank Dr Wang Rong (Cancer
Research Institute, China Medical University, Shenyang, China) for
providing the pEGFP-C1-DKK1 plasmid.
References
|
1
|
Lickiss JN: Approaching cancer pain
relief. Eur J Pain. 5(Suppl A): 5–14. 2001. View Article : Google Scholar
|
|
2
|
Tegeder I, Grösch S, Schmidtko A, Häussler
A, Schmidt H, Niederberger E, Scholich K and Geisslinger G: G
protein-independent G1 cell cycle block and apoptosis with morphine
in adenocarcinoma cells: involvement of p53 phosphorylation. Cancer
Res. 63:1846–1852. 2003.PubMed/NCBI
|
|
3
|
Harimaya Y, Koizumi K, Andoh T, Nojima H,
Kuraishi Y and Saiki I: Potential ability of morphine to inhibit
the adhesion, invasion and metastasis of metastatic colon 26-L5
carcinoma cells. Cancer Lett. 187:121–127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta K, Kshirsagar S, Chang L, Schwartz
R, Law PY, Yee D and Hebbel RP: Morphine stimulates angiogenesis by
activating proangiogenic and survival-promoting signaling and
promotes breast tumor growth. Cancer Res. 62:4491–4498.
2002.PubMed/NCBI
|
|
5
|
Farooqui M, Li Y, Rogers T, Poonawala T,
Griffin RJ, Song CW and Gupta K: COX-2 inhibitor celecoxib prevents
chronic morphine-induced promotion of angiogenesis, tumour growth,
metastasis and mortality, without compromising analgesia. Br J
Cancer. 97:1523–1531. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen C, Farooqui M and Gupta K: Morphine
stimulates vascular endothelial growth factor-like signaling in
mouse retinal endothelial cells. Curr Neurovasc Res. 3:171–180.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katoh Y and Katoh M: Comparative genomics
on DKK2 and DKK4 orthologs. Int J Mol Med. 16:477–481.
2005.PubMed/NCBI
|
|
8
|
Scott EL and Brann DW: Estrogen regulation
of Dkk1 and Wnt/β-Catenin signaling in neurodegenerative disease.
Brain Res. 1514:63–74. 2013. View Article : Google Scholar :
|
|
9
|
Willert K and Jones KA: Wnt signaling: is
the party in the nucleus? Genes Dev. 20:1394–1404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Forget MA, Turcotte S, Beauseigle D, et
al: The Wnt pathway regulator DKK1 is preferentially expressed in
hormone-resistant breast tumours and in some common cancer types.
Br J Cancer. 96:646–653. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamabuki T, Takano A, Hayama S, et al:
Dikkopf-1 as a novel serologic and prognostic biomarker for lung
and esophageal carcinomas. Cancer Res. 67:2517–2525. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Makino T, Yamasaki M, Takemasa I, et al:
Dickkopf-1 expression as a marker for predicting clinical outcome
in esophageal squamous cell carcinoma. Ann Surg Oncol.
16:2058–2064. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patil MA, Chua MS, Pan KH, et al: An
integrated data analysis approach to characterize genes highly
expressed in hepatocellular carcinoma. Oncogene. 24:3737–3747.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aguilera O, Fraga MF, Ballestar E, et al:
Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1)
gene in human colorectal cancer. Oncogene. 25:4116–4121. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu B, Yang X, Xu Y, et al: Elevated
expression of DKK1 is associated with cytoplasmic/nuclear
beta-catenin accumulation and poor prognosis in hepatocellular
carcinomas. J Hepato. 50:948–957. 2009. View Article : Google Scholar
|
|
16
|
Lee N, Smolarz AJ, Olson S, et al: A
potential role for Dkk-1 in the pathogenesis of osteosarcoma
predicts novel diagnostic and treatment strategies. Br J Cancer.
97:1552–1559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weinstein JL, Katzenstein HM and Cohn SL:
Advances in the diagnosis and treatment of neuroblastoma.
Oncologist. 8:278–292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ko YB, Kim BR, Yoon K, Choi EK, Seo SH,
Lee Y, Lee MA, Yang JB, Park MS and Rho SB: WIF1 can effectively
co-regulate pro-apoptotic activity through the combination with
DKK1. Cell Signal. 26:2562–2572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Widy-Tyszkiewicz E, Scheel-Krüger J and
Christensen AV: Spatial navigation learning in spontaneously
hypertensive, renal hypertensive and normotensive Wistar rats.
Behav Brain Res. 54:179–185. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kissin I: Scientometric assessment of
drugs for chronic pain, 1979–2013: rapid growth of publications,
paucity of successful drugs. J Pain Res. 7:505–514. 2014.
View Article : Google Scholar
|
|
22
|
Lazarczyk M, Matyja E and Lipkowski AW: A
comparative study of morphine stimulation and biphalin inhibition
of human glioblastoma T98G cell proliferation in vitro. Peptides.
31:1606–1612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Z, Li S, Song W, Li X, Li Q, Zhang
Z, Han Y, Zhang X, Miao S, Du R and Wang L: Lysine-specific
demethylase 1 (LSD1/KDM1A) contributes to colorectal tumorigenesis
via activation of the Wnt/β-catenin pathway by down-regulating
Dickkopf-1 (DKK1) [corrected]. PLoS One. 8:e700772013. View Article : Google Scholar
|
|
24
|
Li S, Qin X, Guo X, Cui A, He Y, Wei S,
Wang X and Shan B: Dickkopf-1 is oncogenic and involved in invasive
growth in non small cell lung cancer. PLoS One. 8:e849442013.
View Article : Google Scholar
|
|
25
|
Sawada G, Ueo H, Matsumura T, Uchi R,
Ishibashi M, Mima K, Kurashige J, Takahashi Y, Akiyoshi S, Sudo T,
et al: CHD8 is an independent prognostic indicator that regulates
Wnt/β-catenin signaling and the cell cycle in gastric cancer. Oncol
Rep. 30:1137–1142. 2013.PubMed/NCBI
|
|
26
|
Hidaka Y, Mitomi H, Saito T, Takahashi M,
Lee SY, Matsumoto K, Yao T and Watanabe S: Alteration in the
Wnt/β-catenin signaling pathway in gastric neoplasias of fundic
gland (chief cell predominant) type. Hum Pathol. 44:2438–2448.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mo ML, Li MR, Chen Z, Liu XW, Sheng Q and
Zhou HM: Inhibition of the Wnt palmitoyltransferase porcupine
suppresses cell growth and downregulates the Wnt/β-catenin pathway
in gastric cancer. Oncol Lett. 5:1719–1723. 2013.PubMed/NCBI
|
|
28
|
Zeng X, Huang H, Tamai K, Zhang X, Harada
Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, et al:
Initiation of Wnt signaling: control of Wnt coreceptor Lrp6
phosphorylation/activation via frizzled, dishevelled and axin
functions. Development. 135:367–375. 2008. View Article : Google Scholar
|
|
29
|
Ikeda S, Kishida S, Yamamoto H, Murai H,
Koyama S and Kikuchi A: Axin, a negative regulator of the Wnt
signaling pathway, forms a complex with GSK-3beta and beta-catenin
and promotes GSK-3beta-dependent phosphorylation of beta-catenin.
EMBO J. 17:1371–1384. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aberle H, Bauer A, Stappert J, Kispert A
and Kemler R: beta-catenin is a target for the ubiquitin-proteosome
pathway. EMBO J. 16:3797–3804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schleiermacher G, Janoueix-Lerosey I and
Delattre O: Recent insights into the biology of neuroblastoma. Int
J Cancer. 135:2249–2261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Péczely L, Ollmann T, László K, et al:
Effects of ventral pallidal D1 dopamine receptor activation on
memory consolidation in morris water maze test. Behav Brain Res.
274:211–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oliveira L, Graeff FG, Pereira SR,
Oliveira-Silva IF, Franco GC and Ribeiro AM: Correlations among
central serotonergic parameters and age-related emotional and
cognitive changes assessed through the elevated T-maze and the
Morris water maze. Age (Dordr). 32:187–196. 2010. View Article : Google Scholar
|