Introduction
Breakdown of the blood-retinal barrier (BRB) is an
early event in the pathogenesis of diabetic retinopathy (DR)
(1). One consequence of BRB
breakdown in diabetics is the accumulation of plasma protein and
osmotically obliged fluid in the neural interstitium. This causes
pathological retinal swelling, neuronal disorganization and vision
loss.
Vascular endothelial growth factor (VEGF), one of
the vascular permeability factors, mediates increased vascular
permeability and colocalizes with extravasated albumin in major
retinal diseases such as DR (2,3).
Increased levels of VEGF have been shown to induce a reduction in
the expression levels of occludin and ZO-1, two important
junctional proteins that are expressed in vascular endothelial
junctional complexes, thereby leading to increased tissue
permeability (4).
Glucocorticoids (GCs) have been shown experimentally
to reduce the breakdown of the BRB by directly affecting the
endothelial cells through regulating the phosphorylation,
organization and content of tight junction proteins (5). It has been reported that
triamcinolone acetonide (TA) can upregulate the expression of the
tight junction transmembrane protein occludin in animals (6). Although GCs have been shown to
ameliorate macular edema, the side effects accompanying treatment
make their frequent use problematic. One of the major side effects
of intravitreal injection of TA is a steroid-induced increase in
intraocular pressure (IOP). One study demonstrated that a rise in
IOP to values higher than 21 mmHg is expected to occur in ~50% of
the eyes treated (7). The
frequency of cataract surgery following intravitreal injection of
high-concentration TA in elderly patients is increasing (8).
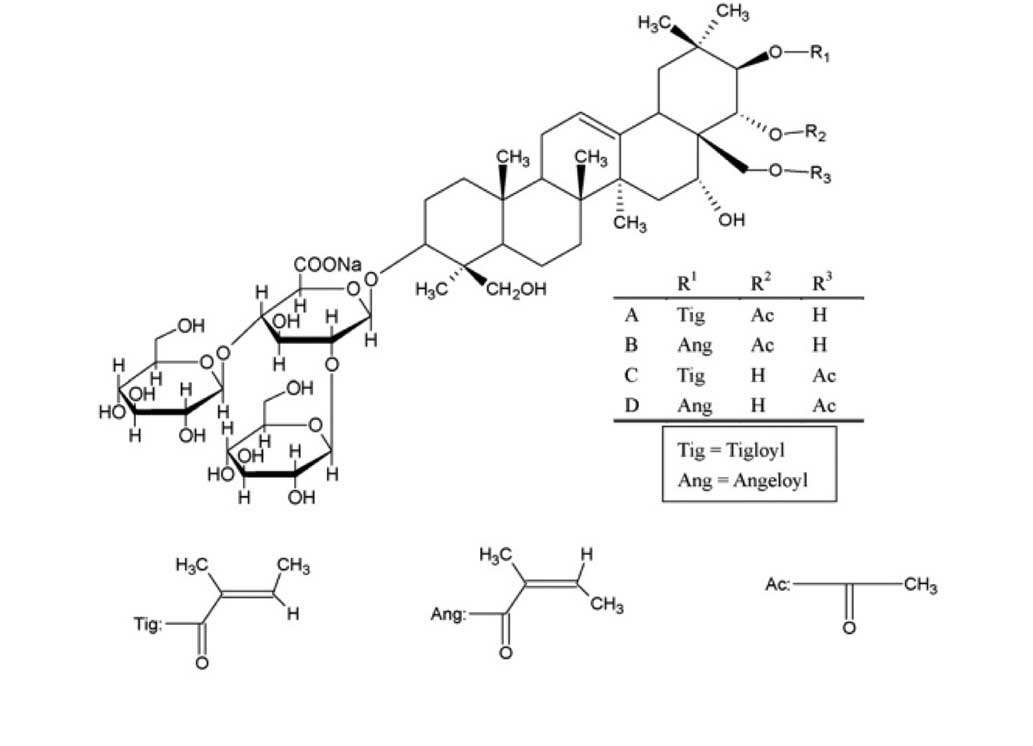
Escin is a natural mixture of triterpene saponins,
which mainly consists of A, B, C and D escin (Fig. 1). Previous studies have indicated
that escin exerts anti-inflammatory and anti-edematous effects
(9,10). Escin has been reported to be a safe
and potent anti-inflammatory agent with a long-lasting, effective
anti-inflammatory action without immunosuppression (11,12).
It also exerts synergistic anti-inflammatory effects with low doses
of GCs in vivo and in vitro (13). In China, escin is widely used
clinically for the treatment of retinal vein occlusion and central
serous chorioretinopathy (14–16).
The current study was designed to investigate
whether a combination of escin and GCs could produce synergistic
protective effects against BRB breakdown in retinal pigment
epithelial (RPE) cells and human umbilical vein endothelial cells
(HUVECs).
Materials and methods
Drugs and cells
Sodium aescinate, for injection with escin, was
obtained from Shandong Luye Pharmaceutical Company Limited (batch
no.: 1212024; Yantai, China). Triamcinolone acetonid (batch no.:
SLBB0079V; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 100%
dimethyl sulfoxide (DMSO; Sigma-Aldrich) and diluted with DMSO and
the appropriate cell culture medium to the desired concentration,
with a final DMSO concentration of 0.1% for in vitro
studies. DMSO was added to cultures at 0.1% (v/v) as a solvent
control. ARPE-19 human RPE cell cultures, were obtained from the
Shandong Eye Institute (Qingdao, China). HUVECs were obtained from
Shandong Luye Pharmaceutical Company Limited (Yantai, China).
Limited Dulbecco’s modified Eagle medium (DMEM; Gibco-BRL,
Carlsbad, CA, USA) supplemented with 10% fetal calf serum (FCS;
Gibco-BRL) and 1% penicillin/streptomycin (Beyotime Institute of
Biotechnology, Haimen, China) was used as the cell culture medium.
Cells were cultured in a humidified atmosphere of 5% CO2
in air at 37°C.
Cell culture
ARPE-19 cells and HUVECs were cultured in 5 ml
complete medium dishes. When the culture reached 90% confluence,
usually after ~4–5 days, cells were washed twice with sterile
D-Hank’s solution (Beyotime Institute of Biotechnology) and
separated by short trypsinization (0.05% trypsin-EDTA; Invitrogen,
Carlsbad, CA, USA). The remaining trypsin-EDTA was removed, the
tubes were gently shaken until cells were completely ablated and
then supplemented with complete DMEM. The pellet was agitated with
a pipette. Cells were transferred to three culture bottles and
incubated for all experiments.
MTT assay
HUVECs (100 μl) and ARPE-19 (100 μl) cells were
seeded into 96-well plates at a density of 4×104 cells
per well. The cells were seeded on the bottom side of the well and
cultured in complete DMEM at 37°C in 5% CO2 for 24 h. To
investigate the effects of different concentrations of escin and TA
on the viability of HUVECs and ARPE-19 cells, following the 24-h
culture, the cells were treated with escin at final concentrations
of 1.25, 2.5, 5, 10, 20 and 40 μg/ml for another 24 h. TA was added
to each well at final concentrations of 0.3125, 0.625, 1.25, 2.5, 5
and 10 μmol/l for another 24 h. The controls were maintained in
complete culture without penicillin/streptomycin for the same time
period. Following treatment with TA the MTT assay was carried out.
In short, the medium was removed, the cells were washed with
phosphate-buffered saline (PBS), and 150 μl MTT solution (5 mg/ml)
was added to each well. ARPE-19 cells and HUVECs were incubated at
37°C for 4 h. The medium was removed and the solution aspirated.
The resulting formazan crystals were dissolved in 150 μl DMSO per
well. Absorption was measured with a scanning multi-well
spectrophotometer (SpectraMax M3; Molecular Devices, Sunnyvale, CA,
USA) at a wavelength of 570 nm. The results are expressed as the
mean of the percentage of proliferation, with the control set as
100% proliferation.
Tight junction building
DMEM was added into a 24-well plate with Transwell
inserts (diameter 6.5 mm, pore size 0.45 μm; Corning Costar,
Canton, MA, USA). HUVECs and ARPE-19 cells (4×104 cells
per transwell insert) in a volume of 100 μl were seeded on the
bottom side of the insert. The medium was changed every two days.
Under these conditions, the formation of tight junctions between
cells was detected using the cell resistance meter (Millicell-ERS;
Millipor, Billerica, MA, USA). The barrier properties were assessed
by daily TEER measurements. TEER was not stable until ~2–3 weeks
and in vitro BRB models were established. Measurements were
performed at 37°C and are expressed as relative values after
subtracting TEER values from the inserts without cells.
Permeability analysis
Once cell tight junctions had been established, 2 μl
recombinant human (rh)VEGF (batch no: F0311; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) was added (final concentration:
ARPE-19 cells 10 ng/ml; HUVECs 100 ng/ml) along with escin ( 0.1, 1
or 10 μg/ml), TA (0.01, 0.1, or 1 μmol/l) or TA combined with
escin. Each concentration was added to three parallel wells. The
blank control was kept in complete culture except for rhVEGF and
antibiotics. At 12 h, the effects of the agents on the cell tight
junctions were detected by the cell resistance meter.
Protein extraction and western
blotting
HUVECs (200 μl; 8×105 cells per transwell
insert) were added to each well. The HUVECs in 30-mm tissue culture
dishes were incubated in a humidified atmosphere of 5%
CO2 in air at 37°C for 7 days. After 7 days, cells were
stimulated with rhVEGF (VEGF). HUVECs were randomly divided into
control, VEGF (100 ng/ml), VEGF + TA (0.01 μmol/L), VEGF + escin
(0.1 μg/ml) and VEGF+ TA (0.01 μmol/L) + escin (0.1 μg/ml) groups.
HUVECs were incubated in air at 37°C for 12 h. Following incubation
the HUVECs were homogenized on ice in cold lysis buffer (Beyotime
Institute of Biotechnology) plus 1:100 volume of phenylmethyl
sulfonylfluoride. The homogenates were centrifuged at 14,000 × g
for 5 min at 4°C. The supernatants were aliquoted and stored at
−80°C following the removal of a small aliquot for protein
estimation. Protein concentrations were determined using a
bicinchoninic acid (BCA) Protein Assay kit (Beyotime). The samples
were thawed on ice, mixed with 4× sample buffer (Invitrogen) and
heated at 100°C for 5 min. Equivalent amounts of proteins (50 μg)
were loaded onto 12% Tris-glycine, SDS-polyacrylamide gels for
fractionation at 160 V. Predetermined molecular weight standards
(Beyotime Institute of Biotechnology) were used as markers.
Proteins on the gel were blotted onto nitrocellulose membranes
(Beyotime Institute of Biotechnology) at 106 V for 70 min at 4°C.
Following transfer, the membranes were incubated with a blocking
buffer (5% skim milk in a washing buffer) for 2 h at room
temperature and washed three times (5 min/wash) with 0.1% Tween 20
in Tris-buffered saline (TBST). Incubation with occludin (cat.no
ab31721), ZO-1 (cat.no. ab59720) or GC receptor (cat.no. ab3578)
antibodies (all supplied by Abcam, Cambridge, MA, USA) in diluent
buffer (5% bovine serum albumin (Beyotime Institute of
Biotechnology) and 0.1% TBST) was performed overnight at 4°C
(1:1000 dilution). The membrane was subsequently washed three times
(5 min per wash) with TBST. The primary antibody was probed with
horseradish peroxidase-conjugated IgG secondary antibody (1:2000)
for 2 h, washed three times in TBST and processed with enhanced
chemiluminescence detection reagents (Beyotime). The processed
membrane was exposed to photographic films for visualization of the
signal. A β-actin western blot was performed for each membrane as
an internal protein loading control.
Evaluation of drug interactions
The interaction between escin and TA was analyzed
using the Berenbaum method to determine whether the combination was
synergistic. The method is performed based on the following
equation: E(da,db)<E(da) + E(db), where E is the observed
effect, da and db are the doses of agents a and b. Synergism is
indicated when the total effect of a combination is greater than
expected from the sum of its effects (17).
Statistical analysis
Quantitative data from the experiments are expressed
as the mean ± standard deviation, significance was determined by
one-way analysis of variance followed by Tukey’s test. In all
cases, P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of escin and TA on the HUVECs and
ARPE-19 cells survival rate
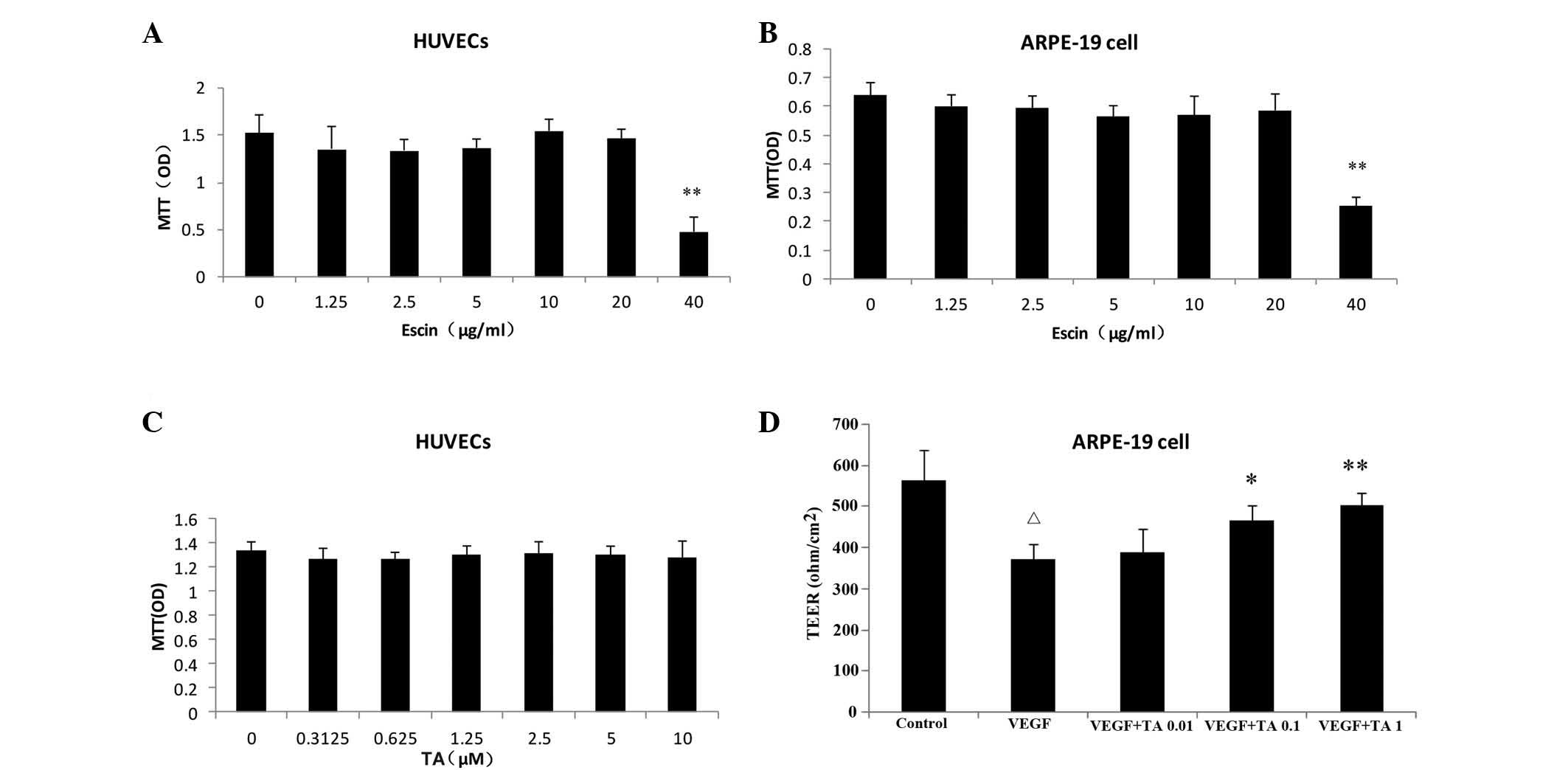
The results of the viability testing showed that
escin at a final concentration of <20 μg/ml had no effect on the
cell viability of either cell line and was chosen for all further
experiments regarding tissue permeability, junctional protein, and
receptor expression. All experimental concentrations of TA had no
effects the cell viability of the two cell lines (Fig. 2).
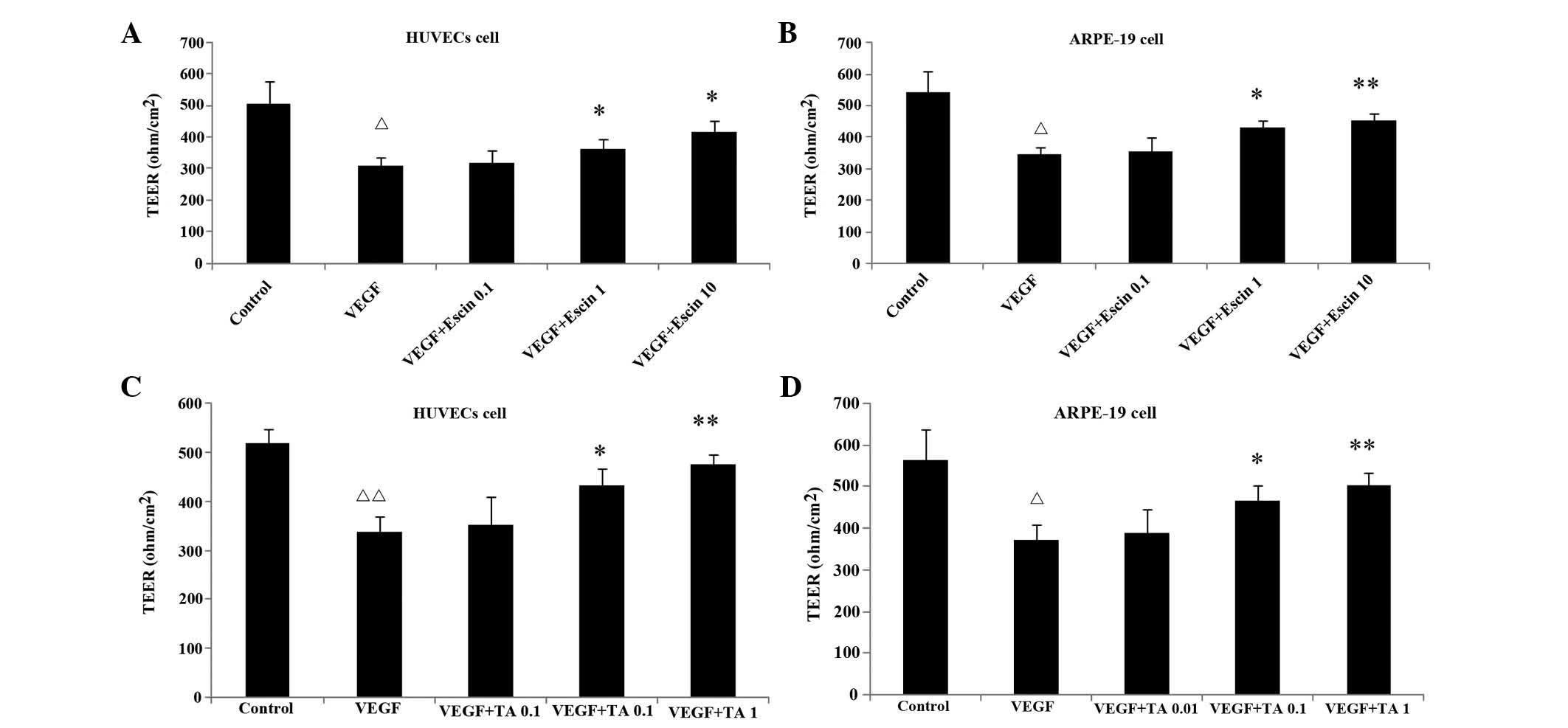
Effects of escin and TA on TEER of the
HUVECs and ARPE-19 cells
The TEER of cellular tight junctions in the VEGF
group was significantly lower than that of control group
(P<0.05). Escin (1, 10 μg/ml) and TA (0.1, 1 μmol/l)
significantly increased the reduced TEER in cells stimulated with
VEGF compared with that in the VEGF-treated control cells
(P<0.05 or P<0.01) (Fig. 3).
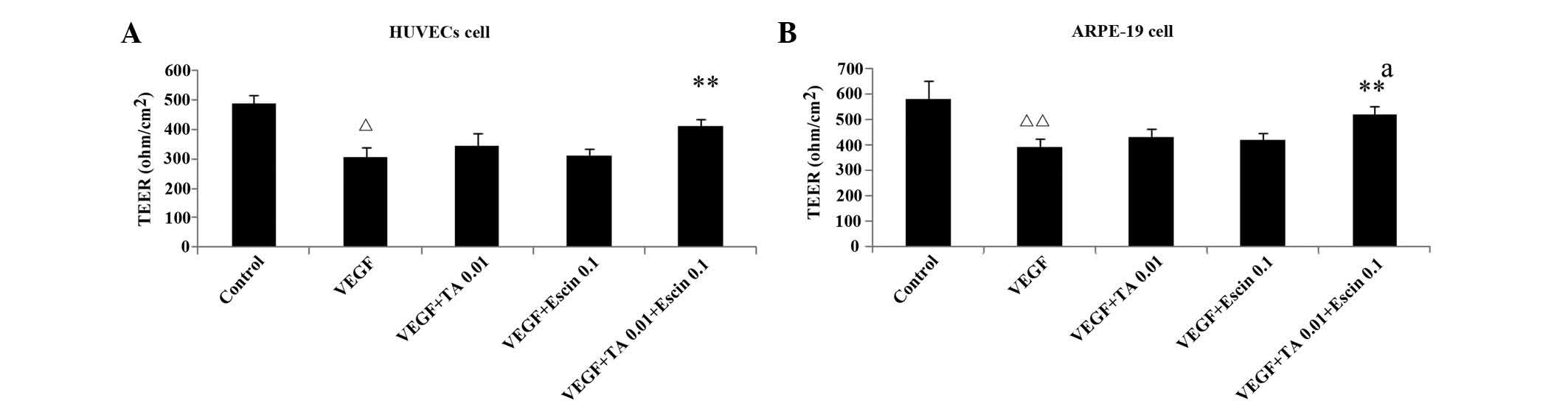
Escin (0.1 μg/ml) and TA (0.01 μmol/L) administered separately had
no significant impact on the TEER of cellular tight junctions,
however, administered together they significantly increased the
TEER of cellular tight junctions in the presence of VEGF
(P<0.05) (Fig. 4).
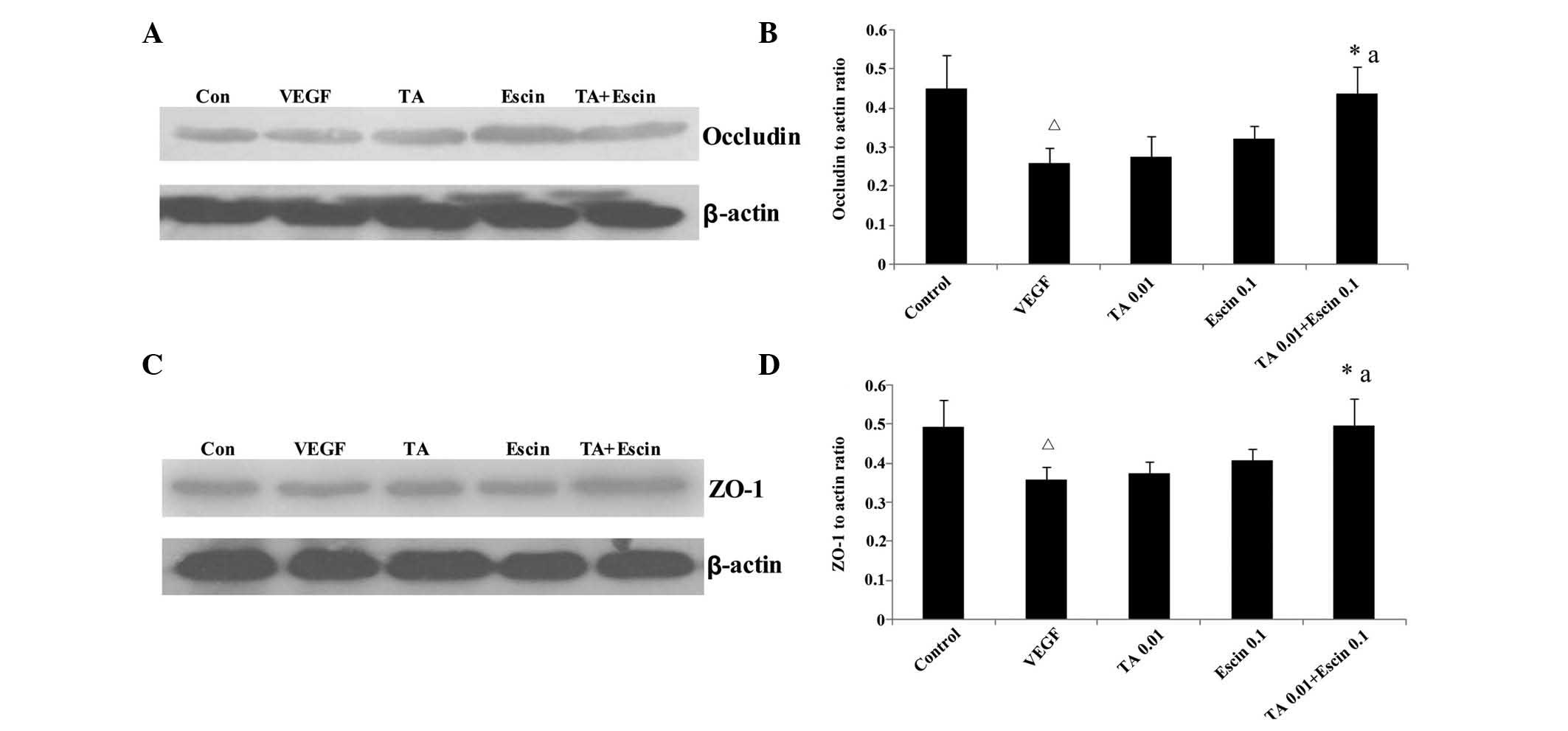
Effects of TA combined with escin on the
expression levels of occludin and ZO-1 in HUVECs
Compared with the control group, the occludin and
ZO-1 protein expression levels in HUVECs treated with VEGF was
significantly reduced (P<0.05). Low concentrations of escin or
TA alone did not enhance the occludin and ZO-1 expression levels
compared with those observed in the VEGF group. However, when escin
and TA were administered together, occludin and ZO-1 expression
levels increased significantly compared with those of the control
group (P<0.05) (Fig. 5).
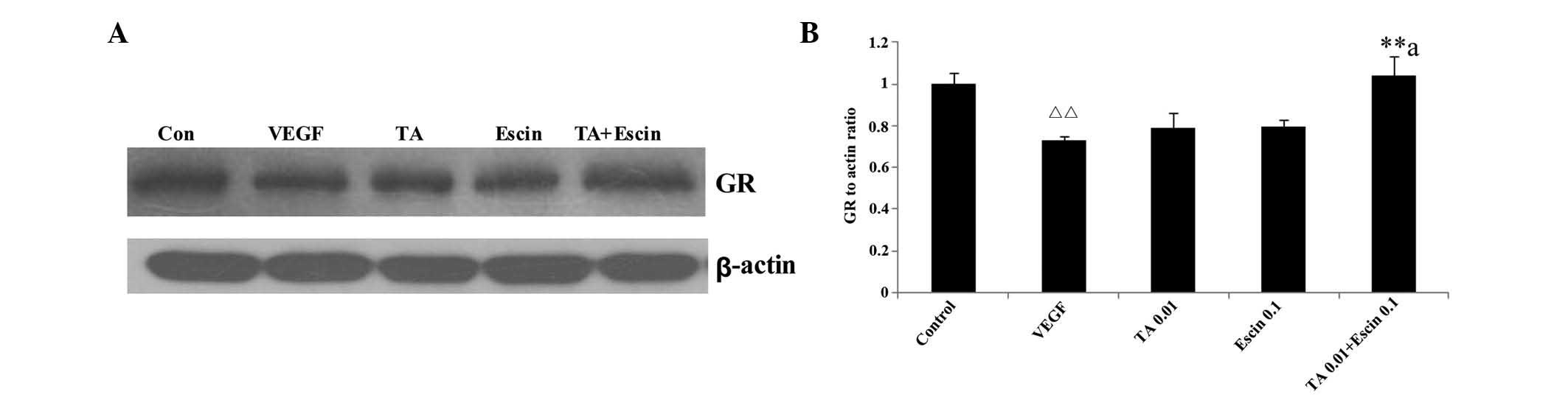
Effects of TA combined with escin on GC
receptor expression of HUVECs
Low dose escin or TA alone did not enhance the GC
receptor expression compared with the VEGF group. However, when
escin and TA were administered together, GC receptor expression
increased significantly (P<0.05) (Fig. 6).
Discussion
The BRB is composed of tight and adherent junction
complexes. Retinal vascular endothelium and pigment epithelium have
well developed tight junctions that confer a high degree of control
on solute and fluid permeability, which maintains the neural
environment of retina. Intact BRB function is essential for proper
vision and its breakdown greatly contributes to the pathology and
vision loss in retinal disorders including DR, age-related macular
degeneration and uveitis.
TEER measurement is a common method for evaluating
the formation of tight junctional complexes. The present study used
an in vitro model of BRB based on ARPE-19 cells and HUVECs
on permeable transwell inserts. The BRB model established the
tightness of the created monolayer as reflected by high TEER and
high expression levels of adherens and tight junctional proteins.
Occludin and ZO-1 are two important transmembrane proteins in tight
junctions that are responsible for forming the permeability barrier
(18).
VEGF has an important role in BRB breakdown
(19,20). Elevated levels of VEGF can alter
the integrity of the BRB and increase the vascular permeability in
a number of pathological conditions, including diabetic macular
edema (21,22). In the present study, following VEGF
treatment, TEER and the expression levels of the tight junctional
proteins occludin and ZO-1 were significantly reduced. The levels
of TEER and tight junctional proteins in cells treated with escin
(1, 10 μg/ml) and TA (0.1, 1 μmol/l) were significantly increased
when compared with those observed in control cells treated VEGF.
Separately, escin (0.1 μg/ml) and TA (0.01 μmol/L) had no
significant impact on the TEER and cellular tight junctions,
however, when administered together they significantly inhibited
the reduction of TEER, indicating that escin and TA have
synergistic effects which reduce BRB breakdown.
Currently, GCs are the most commonly used medicine
for ocular diseases. Several studies have reported on the efficacy
and complications associated with intravitreal GC injection for the
treatment of diabetic macular edema (23,24).
GCs may act by suppressing inflammation and directly affecting the
endothelial cells through regulating the phosphorylation,
organization, and content of tight junction proteins. The effect of
GCs is dependent on the GC receptor as demonstrated by siRNA
(25). In the present study,
administration of escin and TA together increased GC receptor
expression significantly, which may be one of the mechanisms by
which escin protects against the BRB breakdown.
In conclusion, escin and GCs have synergistic
protective effects on BRB breakdown, and the molecular mechanism is
related to the upregulation of occludin and ZO-1. Administration of
escin allows for the reduction of the dose of GCs for treatment of
macular edema. The combination of escin with GCs indicates a
beneficial method for the treatment of BRB breakdown.
Acknowledgements
This study was supported by the Project of Yantai
Science and Technology Development Program (no. 2013WS205).
References
|
1
|
Sander B, Larsen M, Moldow B and
Lund-Andersen H: Diabetic macular edema: passive and active
transport of fluorescein through the blood-retina barrier. Invest
Ophthalmol Vis Sci. 42:433–438. 2001.PubMed/NCBI
|
|
2
|
Qaum T, Xu Q, Joussen AM, et al:
VEGF-initiated blood-retinal barrier breakdown in early diabetes.
Invest Ophthalmol Vis Sci. 42:2408–2413. 2001.PubMed/NCBI
|
|
3
|
Mathews MK, Merges C, McLeod DS and Lutty
GA: Vascular endothelial growth factor and vascular permeability
changes in human diabetic retinopathy. Invest Ophthalmol Vis Sci.
38:2729–2741. 1997.
|
|
4
|
Kernt M, Thiele S, Liegl RG, et al:
Axitinib modulates hypoxia-induced blood-retina barrier
permeability and expression of growth factors. Growth Factors.
30:49–61. 2012. View Article : Google Scholar
|
|
5
|
Wilson CA, Berkowitz BA, Sato Y, Ando N,
Handa JT and de Juan E Jr: Treatment with intravitreal steroid
reduces blood-retinal barrier breakdown due to retinal
photocoagulation. Arch Ophthalmol. 110:1155–1159. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McAllister IL, Vijayasekaran S, Chen SD
and Yu DY: Effect of triamcinolone acetonide on vascular
endothelial growth factor andoccludin levels in branch retinal vein
occlusion. Am J Ophthalmol. 147:838–846. 2009. View Article : Google Scholar
|
|
7
|
Jonas JB, Kreissig I and Degenring R:
Intraocular pressure after intravitreal injection of triamcinolone
acetonide. Br J Ophthalmol. 87:24–27. 2003. View Article : Google Scholar
|
|
8
|
Jonas JB, Degenring R, Vossmerbauemer U
and Kamppeter B: Frequency of cataract surgery after intravitreal
injection of high-dosage triamcinolone acetonide. Eur J Ophthalmol.
15:462–464. 2005.PubMed/NCBI
|
|
9
|
Zhang L, Fu F, Zhang X, Zhu M, Wang T and
Fan H: Escin attenuates cognitive deficits and hippocampal injury
after transient global cerebral ischemia in mice via regulating
certain inflammatory genes. Neurochem Int. 57:119–127. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xin W, Zhang L, Fan H, Jiang N, Wang T and
Fu F: Escin attenuates acute lung injury induced by endotoxin in
mice. Eur J Pharm Sci. 42:73–80. 2011. View Article : Google Scholar
|
|
11
|
Wang T, Fu F, Zhang L, Han B, Zhu M and
Zhang X: Effects of escin on acute inflammation and the immune
system in mice. Pharmacol Rep. 61:697–704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Wang H, Fan H, et al: The potent
anti-inflammatory agent escin does not increase corticosterone
secretion and immune cell apoptosis in mice. Fitoterapia.
82:861–867. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xin W, Zhang L, Sun F, et al: Escin exerts
synergistic anti-inflammatory effects with low doses of
glucocorticoids in vivo and in vitro. Phytomedicine. 18:272–277.
2011. View Article : Google Scholar
|
|
14
|
Gong YY, Yu SQ, Wang H, et al: Clinical
therapy of retinal vein occlusion with aescuven forte. Chin J New
Drugs Clin Rem. 12:965–968. 2005.
|
|
15
|
Wang W, Gong Y, Wang H, et al: Aescuven
forte in treating central serous chorioretinopathy. Chin J New
Drugs Clin Rem. 12:961–964. 2005.
|
|
16
|
Yuan YL and Wu LL: Clinical observation of
the central serous chorioretinopathy treated by aescinate sodium
tablets combined with argon laser. Guiding Journal of TCM and
Pharmacology. 15:58–59. 2009.(In Chinese).
|
|
17
|
Berenbaum MC: What is synergy? Pharmacol
Rev. 41:93–141. 1989.PubMed/NCBI
|
|
18
|
Furuse M, Hirase T, Itoh M, et al:
Occludin: a novel integral membrane protein localizing at tight
junctions. J Cell Biol. 123:1777–1788. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibuya M: Vascular endothelial growth
factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for
angiogenesis. Angiogenesis. 9:225–230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duh EJ, Yang HS, Suzuma I, et al: Pigment
epithelium-derived factor suppresses ischemia-induced retinal
neovascularization and VEGF-induced migration and growth. Invest
Ophthalmol Vis Sci. 43:821–829. 2002.PubMed/NCBI
|
|
21
|
Esser S, Wolburg K, Wolburg H, et al:
Vascular endothelial growth factor induces endothelial
fenestrations in vitro. J Cell Biol. 140:947–959. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Antonetti DA, Barber AJ, Hollinger LA,
Wolpert EB and Gardner TW: Vascular endothelial growth factor
induces rapid phosphorylation of tight junction proteins occludin
and zonula occluden-1. A potential mechanism for vascular
permeability in diabetic retinopathy and tumors. J Biol Chem.
274:23463–23467. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stewart MW: Corticosteroid use for
diabetic macular edema: old fad or new trend? Curr Diab Rep.
12:364–375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu L, Wu X, Geng J, Yuan Z and Chen L:
IVTA as adjunctive treatment to PRP and MPC for PDR and macular
edema: a meta-analysis. PLoS One. 7:e446832012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Felinski EA, Cox AE, Phillips BE and
Antonetti DA: Glucocorticoids induce transactivation of tight
junction genes occludin and claudin-5 in retinal endothelial cells
via a novel cis-element. Exp Eye Res. 86:867–878. 2008. View Article : Google Scholar : PubMed/NCBI
|