Introduction
Worldwide, an estimated two billion people have been
infected with hepatitis B virus (HBV), and >240 million suffer
from chronic HBV (CHB) infection. CHB can result in a wide spectrum
of liver diseases, including CHB, cirrhosis and hepatocellular
carcinoma (1). Although HBV is
noncytopathic, it is generally accepted that the outcome of HBV
infection is mediated by the host immune response to HBV rather
than the virus itself (2).
In CHB, cellular immune responses, particularly
HBV-specific T-cell responses, are weak, oligoclonal and may be
exhausted. These suppressed immune responses result in persistent
HBV infection, and the fluctuating balance between virus
replication and immune reactivity results in chronic liver
inflammation, in which HBV replication is the driving force behind
disease progression. The aim of CHB antiviral treatment is to
inhibit HBV replication before irreversible damage occurs. During
the past ten years, major advances have been made in CHB treatment.
Current antiviral therapy by nucleos(t)ide analogues (NUC) or
interferon (IFN)-α can alleviate liver inflammation, normalize
serum alanine aminotransferase (ALT) levels, effectively suppress
HBV replication and increase the rate of HBV envelope antigen
(HBeAg) loss. It may even result in the HBV surface antigen (HBsAg)
seroconversion in CHB patients (3,4).
However, these treatments do not permanently eradicate the
infection and there is a risk of HBV reactivation at withdrawal.
The permanent suppression of HBV replication following treatment
requires a robust acquired immune response against the HBV core and
envelope antigens (5). There is
evidence that antiviral therapy alters the balance between host
immunity and viral replication, enabling weakened virus-specific
immune responses to strengthen, broaden, and possibly control the
infection, probably due to the decreased HBV antigen levels
allowing the recovery of the T-cell response (6–9).
This mechanism may be important in contributing to complete
recovery from CHB.
To date, the majority of published studies (7,8,10,11)
have focused on the immune profile of HBsAg-negative patients who
are defined as having complete control of HBV infection. These
studies indicate that a substantial restoration of the exhausted
HBV-specific T-cell response results from long-term effective
therapy. Little is known regarding the cellular immune responses of
the patients with persistent suppression of HBV replication
(defined here as undetectable HBV DNA, a low level of HBeAg and
HBsAg positive status). These patients require shorter antiviral
treatment times compared with the HBsAg-negative patients, and it
remains unclear whether different immunological characteristics
exist between these groups. Finding answers to these questions is
vital to the future development of novel therapeutic strategies and
immunomonitoring strategies to enable the earlier withdrawal of
NUCs.
The present study examined changes in HBV-specific
cytotoxic T lymphocytes (CTLs) and HBV-specific T-cell
proliferation (against HBsAg and HBcAg) in the CHB patients with
persistent suppression of HBV replication following antiviral
therapy. The effects of antiviral therapy on the cellular immune
responses and the association between the immune responses and the
HBV virus load during antiviral therapy were investigated.
Patients and methods
Study population
A total of 83 patients with HBV infection, recruited
between May 2012 and January 2013 from the Infection and Immunity
Center at Beijing You’an Hospital at the Capital Medical University
(Beijing, China), were enrolled in the studies. The following
patient categories were used: NUC-treated CHB patients who
presented with persistent suppression of HBV replication and
undetectable HBV DNA, were HBeAg negative and who continued to use
antiviral therapy (n=46); untreated CHB patients who were HBV DNA
positive and HBeAg positive (n=22); and patients who were in
convalescence from acute HBV infection (n=15). Ten healthy adults
who were HBsAb-HBcAb-positive were enrolled as a healthy control
(HC) group. A summary of the demographic, clinical and laboratory
data from each group is shown in Table
I. The study was approved by the Ethics Committee of the
Beijing You’an Hospital in Capital Medical University, and all
subjects provided written informed consent.
 | Table IDemographic and clinical features of
each group. |
Table I
Demographic and clinical features of
each group.
| Variable | Treated patients | Untreated
patients | AHB patients | Control group |
|---|
| Cases (n) | 46 | 22 | 15 | 10 |
| Gender
(male/female) | 29/17 | 18/4 | 11/4 | 6/4 |
| Age (years)a | 38.9±10.5 | 33.5±12.1 | 37.7±9.7 | 39.9±10.7 |
| Serum ALT
(U/L)b,d | 22.9 (8–54) | 116 (34–1373) | 231 (23–1042) | 23.5 (12–34) |
| HBsAg (IU/ml)b,e | 2574 (2–9588) | 4189 (10–52000) | 433 (9.8–12239) | Negative |
| HBeAg (COI)b,f | Negative | 922 (0.08–1481) | 2.03 (0.1–657) | Negative |
| Genotype
(B/C/B+C) | 10/31/5 | 5/15/2 | 3/11/1 | Not available |
| HBV DNA
(IU/ml)a,c,f | Negative | 6.6±1.8 | 3.2±1.2 | Negative |
| Persistent HBV
DNA | 15 (4–40) | Not available | Not available | Not available |
| <12 IU/ml
(months)b | | | | |
Synthetic HBV peptides
To investigate the HBV-specific T-cell responses, 16
to 20-mer peptides overlapping by 10 residues were used, which
correspond to genotype C HBV (the most prevalent genotype in
northern China). A panel of 55 overlapping peptides covering the
full S open reading frame (ORF) and 28 overlapping peptides
covering the full C ORF of HBV were obtained from Sigma-Aldrich
(St. Louis, MO, USA). The two panels of overlapping peptides were
dissolved in dimethyl sulfoxide and were placed into two mixtures,
the S peptide pool (S-pool) and the C peptide pool (C-pool),
respectively. The purity of these peptides exceeded 95%.
Separation of peripheral blood
mononuclear cells (PBMCs)
PBMCs were isolated from fresh blood using
Ficoll-Hypaque density gradient centrifugation with Lymphoprep™
(Axis-Shield, Oslo, Norway) as previously described (8). Peripheral venous blood samples (20
ml) were collected in heparinized test tubes and diluted to a final
volume of 40 ml with phosphate-buffered saline (PBS). This
suspension was poured into a conical tube with 6 ml Lymphoprep. The
PBMCs were isolated using density gradient centrifugation (800 × g,
20 min, without braking). Subsequently, the cells were washed twice
with PBS and resuspended in R10 medium, which consisted of 90%
complete RPMI-1640 medium (HyClone, Logan, UT, USA) and 10%
heat-inactivated fetal bovine serum (FBS; HyClone). After
isolation, the PBMCs were used for the in vitro experiment,
and the rest were cryopreserved in 90% FBS and 10% dimethyl
sulfoxide at −80°C for future use.
Enzyme-linked immunospot (ELISPOT) assay
for HBV-specific interferon-γ (IFN-γ) secretion
To evaluate the HBV-specific reactivity, an IFN-γ
ELISPOT assay was used to analyze PBMC IFN-γ secretion as
previously described (7,8). The antigens for the IFN-γ ELISPOT
assays were the two pools of overlapping peptides (S-pool and
C-pool). The positive controls were stimulated with 2 μg/ml
phytohemagglutinin (PHA; Sigma-Aldrich). The MultiscreenHTS 96-well
filtration plates (Millipore, Billerica, MA, USA) were coated with
15 μg/ml anti-IFN-γ mouse monoclonal antibody 1-D1K (Mabtech, Nacka
Strand, Sweden) overnight at 4°C, according to the manufacturer’s
instructions. The plates were washed six times with PBS and blocked
with R10 for 2 h at room temperature (RT). The freshly isolated
PBMCs (2×105 cells/well) were seeded and cultured in
duplicate in R10 supplemented with CD28 monoclonal antibodies
(Clone, CD28.2; eBioscience, San Diego, CA, USA) for 48 h at 37°C
with 5% CO2, and each specimen was respectively
stimulated with the S-pool, C-pool and PHA at a final concentration
of 2 μg/ml. Following washing, 50 μl of 1 μg/ml biotinylated mouse
monoclonal antibody 7-B6-1 (Mabtech) was added and incubated for 2
h at RT. Then, 50 μl of 1,000-fold dilution streptavidin-alkaline
phosphatase was added and incubated for 1 h at RT. Diluted BCIP/NBT
(100 μl; Invitrogen, Carlsbad, CA, USA) was then added to each well
and quenched with distilled water until distinct spots emerged.
Following air-drying, the spot-forming cells (SFCs) were counted
using an ELISpot reader (Sagecreation, Hangzhou, China). The
quantity of SFCs was taken as the mean number of spots stimulated
with antigen minus the spots in the absence of antigen per
1×106 PBMCs.
Cell proliferation analysis
To assess the cell proliferation, CellTrace™
carboxyl fluorescein diacetate succinimidyl ester (CFSE) was used
as a cell proliferation-tracing reagent (Invitrogen). Freshly
isolated PBMCs were diluted to ~20 million/ml in PBS and labeled
with a final concentration of 2 μmol/ml CFSE in the dark for 10 min
at 37°C. Free CFSE was inactivated with FBS and washed away. The
stained PBMCs were moved into a flat-bottom 96-well plate with a
concentration of 200,000 cells per well. The PBMCs were cultured in
R10 medium at 37°C and 5% CO2. The HBV-specific T-cell
proliferation was evaluated after seven days of incubation in the
presence of HBV antigens (S-pool and C-pool) at a final
concentration of 2 μg/ml. The positive control was in the presence
of 1 mg/ml purified anti-CD3 and anti-CD28 mouse monoclonal
antibodies (eBioscience), whilst the negative control was incubated
in the R10 medium only. Cell proliferation was assessed by
examining the dilution of CFSE by flow cytometry (FCM).
Flow cytometric analysis
Following incubation, PBMCs were harvested, washed
with PBS and cell surface markers were stained with mouse
monoclonal phycoerythrin-anti-CD3, mouse monoclonal
allophycocyanin-anti-CD4 and mouse monoclonal
peridinin-chlorophyll-protein-anti-CD8 antibodies (eBioscience,
Inc.) for 20 min at RT. The stained cells were washed with PBS,
fixed with 4% phosphate-buffered paraformaldehyde, and analyzed by
FCM. All results were collected using a BD FACS CantoTM II with
corresponding antibodies (BD Biosciences, Franklin Lakes, NJ, USA),
and at least 20,000 gated lymphocytes were collected for each
sample. The proportion of proliferating cells was calculated using
FlowJo software (Tree Star Inc., Ashland, OR, USA), and the results
were expressed as the percentage of divided cells among gated
lymphocytes.
HBV DNA assay and HBV marker assays
Serum HBV DNA was extracted from 850 μl plasma by
the Cobas AmpliPrep® automated extractor and quantified
using Roche COBAS® AmpliPrep/COBAS®
TaqMan® HBV Test system and Roche original reagent
(Roche China, Ltd., Shanghai, China) for the automated quantitative
polymerase chain reaction amplification according to the
manufacturer’s instructions. The HBV DNA load data were analyzed
with AMPLILINK® software (Roche China, Ltd.) and
expressed in IU/ml. The detection limit of the assay was 12 IU/ml.
The quantification of the serum HBV markers (HBsAg, HBsAb, HBeAg,
HBeAb, and HBcAb) was determined by the Abbott
Architect® i2000 system and the corresponding ARCHITECT
assay (Abbott Laboratories, Chicago, IL, USA).
Statistical analysis
Continuous data was presented as the mean ± standard
deviation or median (range). The cell proliferation data from the
patients were compared using one-way analysis of variance following
the post hoc analysis of least significant difference. Data from
the ELISPOT assays were evaluated using the Kruskal-Wallis and
Mann-Whitney U-tests. The rates of positive response were compared
using the χ2 test. Correlations were examined using
Pearson’s correlation. P<0.05 was considered to indicate a
statistically significant difference.
Results
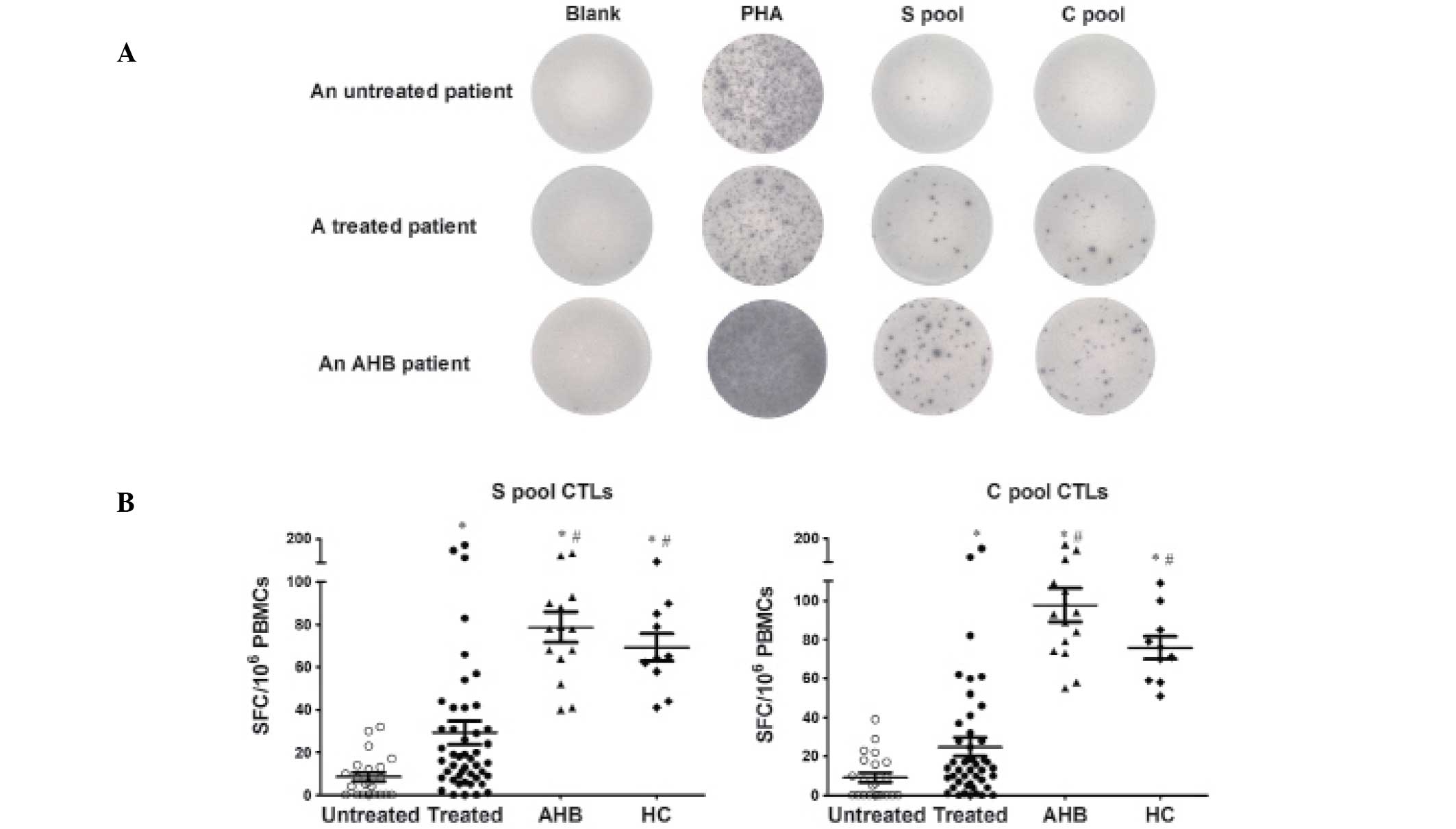
HBV-specific CTLs are suppressed in
untreated patients and are restored by antiviral therapy
To confirm the effects of antiviral therapy on the
frequency and functional changes of HBV-specific CTLs, an IFN-γ
ELISPOT assay was performed on 22 untreated CHB patients, 46
NUC-treated CHB patients, 15 AHB patients and 10 healthy adults.
There were no significant differences in age and gender between the
four groups. The HBsAg and alanine transaminase (ALT) levels in the
treated group were significantly lower than those in the untreated
group (P<0.01). The antigens for the human IFN-γ ELISPOT assays
were the S-pool peptides and the C-pool peptides.
The untreated CHB patients mounted weak HBV-specific
CTL responses following stimulation with the S-pool and C-pool
peptides. S-specific CTL responses were detectable in 63.6% of the
untreated patients with a magnitude of 5.5 (range, 0–32) SFCs per
106 PBMCs. C-specific CTL responses were detectable in
54.5% of the untreated patients with a magnitude of 6.0 (range,
0–39) SFCs per 106 PBMCs. In the treated CHB patients
who were undetectable for HBV-DNA following antiviral therapy, the
positive rates of the S-specific and C-specific CTL responses
increased to 93.5% and 91.3%, respectively. The magnitudes of the
S-pool and C-pool specific CTLs increased to 16.5 (range, 0–175)
and 13.5 (range, 0–163) SFCs per 106 PBMCs,
respectively. The S-pool and C-pool specific CTL responses were
consistently and significantly higher than those in the untreated
CHB patients. P<0.01 for the positive response to the S-pool,
and P<0.001 for the positive response to the C-pool, as assessed
by a χ2 test. The P-values for the difference in the
magnitude of response between the two groups for the S-pool and
C-pool were P<0.01 and P<0.05, respectively, as assessed by
the Mann-Whitney test. The HBV-specific CTL responses of the
treated CHB patients were significantly weaker than those of the
AHB patients, who exhibited a 100% positive CTL response and a
higher frequency of SFCs. The S-pool and C-pool specific CTL
responses were consistently higher in the HC group compared with
the two groups of CHB patients, and were comparable to those in AHB
patients. These results are shown in Fig. 1 and Table II.
 | Table IIHBV-specific T-cell responses in
different patient categories. |
Table II
HBV-specific T-cell responses in
different patient categories.
| S pool | C pool |
|---|
|
|
|
|---|
| Patients | Number of SFCs | Positive rate
(%) | Number of SFCs | Positive rate
(%) |
|---|
| Untreated
(n=22) | 5.5 (0–32) | 63.60 | 6.0 (0–39) | 54.50 |
| Treated (n=46) | 16.5
(0–175)a | 93.50a | 13.5
(0–163)a | 91.30a |
| AHB (n=15) | 78.0
(40–140)b | 100.00 | 79.5
(41–177)b | 100.00 |
| HC (n=10) | 64.5
(41–105)b | 100.00 | 59.5
(45–116)b | 100.00 |
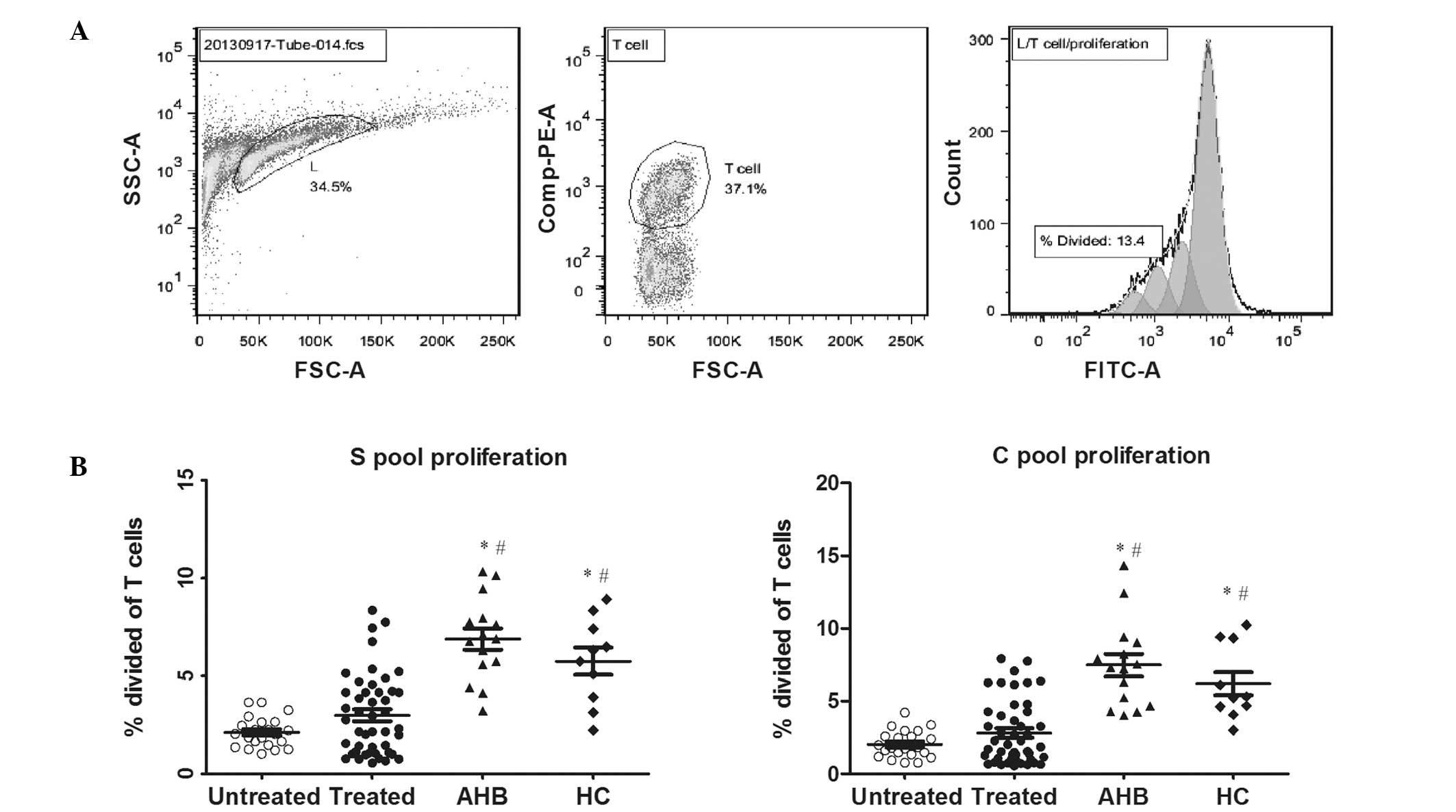
HBV-specific T-cell proliferation, as
determined by a proliferation-tracing reagent, CFSE
The HBV-specific T-cell proliferation was also
determined by CFSE staining in the presence of the HBV peptides
(S-pool and C-pool). The untreated CHB patients showed
significantly lower total (CD4+ and CD8+)
HBV-specific T-cell proliferation (S-pool, 2.1±0.8, C-pool,
2.0±0.9). The treated CHB patients showed moderate proliferative
responses (S-pool, 3.0±2.0; C-pool, 2.8±2.1). The AHB patients
showed vigorous total HBV-specific T-cell proliferation (S-pool,
6.9±2.1; C-pool, 7.5±2.9), which were significantly higher than
that of the two CHB patients groups (P<0.05). The HBV-specific
T-cell proliferation of the HC group (S-pool, 5.7±2.1; C-pool,
6.2±2.5) was comparable to that of the AHB patients (P>0.05),
and was significantly higher than those of the two CHB patient
groups (P<0.001). These results are shown in Fig. 2.
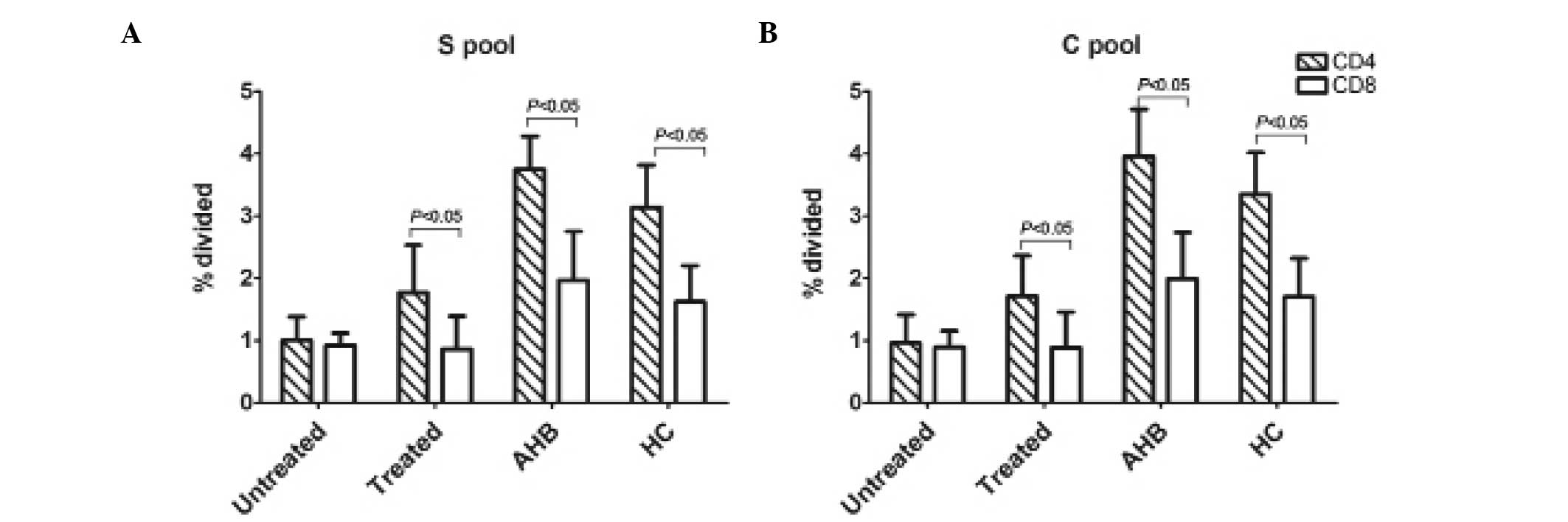
The total T-cell numbers were further divided into
subpopulations of CD8+ and CD4+ T-cells. The
proliferative responses of the CD4+ and CD8+
T-cells among the three groups were in accordance with the data
pertaining to the total HBV-specific T-cell proliferation.
CD4+ and CD8+ T-cells contributed to the
overall HBV-specific T-cell response observed in the four groups,
although CD4+ responses were significantly greater in
treated CHB patients, AHB patients and healthy adults than in
untreated patients (P<0.05), as shown in Fig. 3.
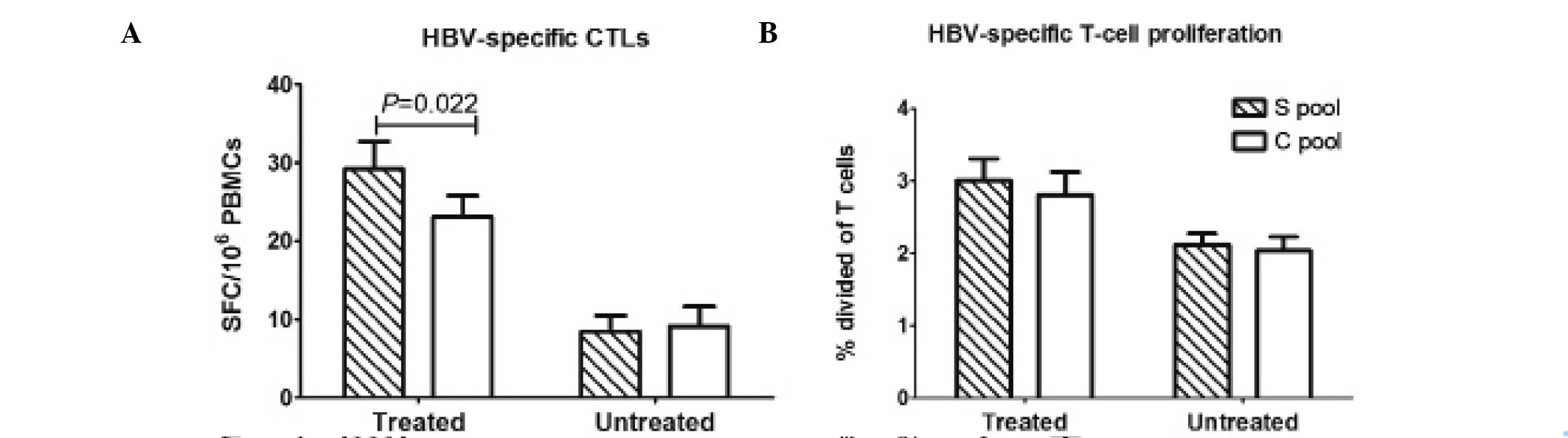
Antiviral therapy primarily improves the
S-peptide-specific CTL response
The S-specific and C-specific CTL responses were
observed in a proportion of the untreated CHB patients (S pool: 14
patients, 63.6%; C pool: 12 patients, 54.5%)., but were weak in
these individuals. There was no significant difference between the
magnitude of the S-specific CTLs and that of the C-specific CTLs in
this group. The treated CHB patients showed vigorous HBV-specific
CTL responses. The HBV-specific CTL responses in the treated CHB
patients mainly reacted with the S-pool peptides, and the number of
the S-specific CTLs (median, 16.5 SFCs per 106 PBMCs;
range, 0–175) was significantly higher than that of the C-specific
CTLs (median, 13.5 SFCs per 106 PBMCs; range, 0–163;
P<0.05; Fig. 4A). No
significant difference was identified between the S-specific T-cell
proliferation and C-specific T-cell proliferation in treated and
untreated CHB patients.
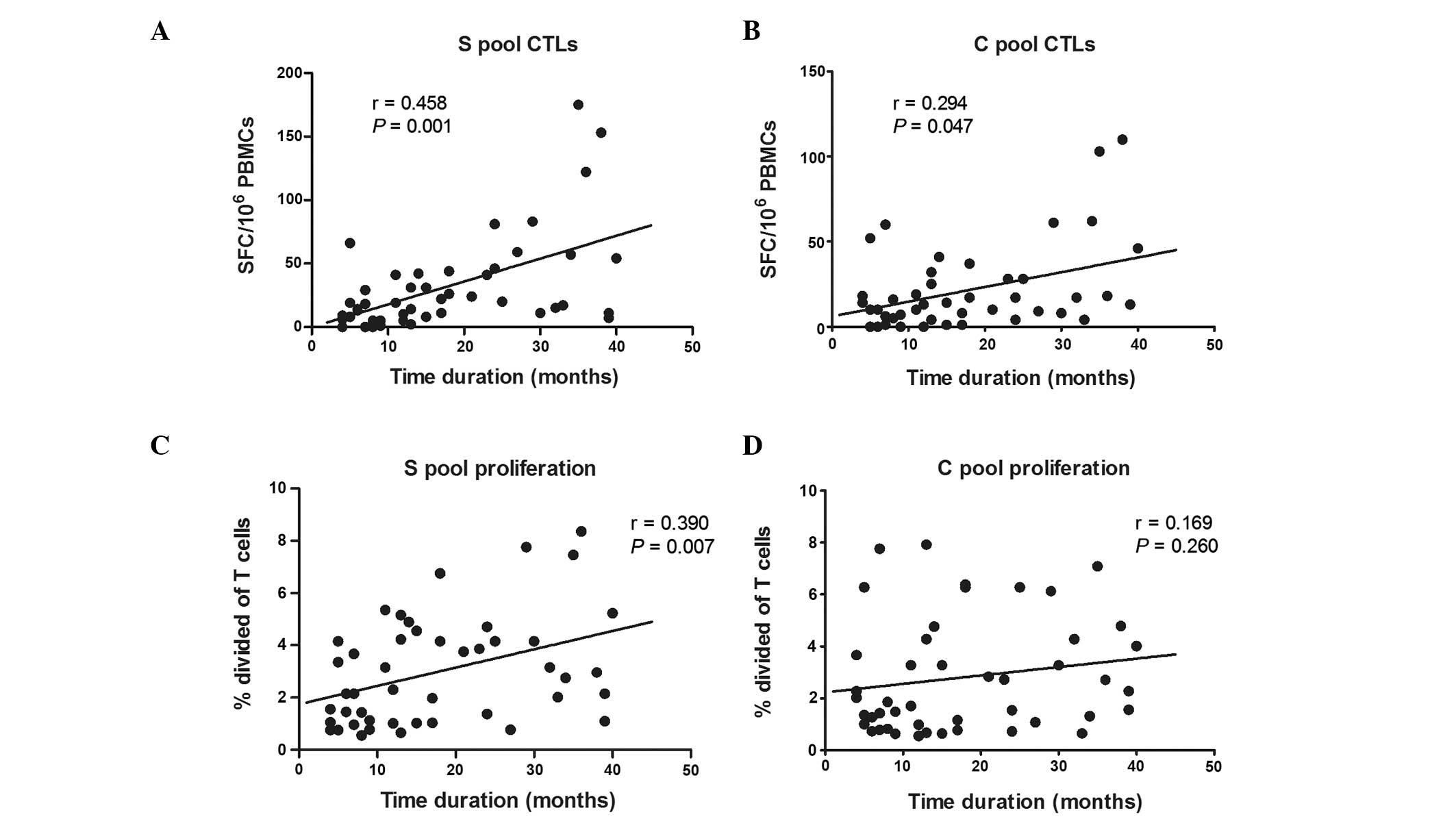
HBV-specific immune responses in relation
to the time duration of HBV DNA suppression
All the treated CHB patients were undetectable for
HBV-DNA following antiviral therapy, and the time duration of HBV
DNA suppression varied at the time of analysis (median, 15 months;
range, 4–40). The correlation between the immune responses and the
duration of HBV DNA suppression was assessed. There was a
significant positive correlation between the magnitude of
HBV-specific CTLs and the duration of HBV DNA suppression (S-pool,
r=0.458, P<0.01; C-pool, r=0.294, P<0.05), although only
S-specific T-cell proliferation was significantly correlated with
the duration of HBV DNA suppression (r=0.390, P<0.01; Fig. 5).
Correlation between the immune response
and HBV markers
In the untreated CHB patient group, the association
between the immune responses and the viral load was assessed. A
significant inverse correlation was detected between the number of
S-specific CTLs and the HBV DNA levels (r=−0.409, P<0.05;
Fig. 6A). No correlation was found
between the number of C-specific cells and HBV DNA levels (r=0.35,
P<0.05; Fig. 6B). No
correlation was detected between the immune responses and the
HBsAg, HBeAg, and ALT levels.
Discussion
The antiviral therapy currently available has become
more effective against CHB. The majority of CHB patients can
achieve persistent suppression of HBV replication following NUC
treatment. Persistent suppression of HBV replication may alleviate
liver inflammation and result in a decreased risk of liver
cirrhosis or cancer in these patients (4). The mechanisms that may contribute to
complete eradication of HBV infection include the innate immune
responses and the acquired CD4+ and CD8+
T-cell responses, particularly the HBV-specific T-cell responses
(8). These mechanisms have been
shown to be of paramount importance in evaluating the future
direction of antiviral treatment for HBV-infected patients
(12–14). It may be beneficial to identify any
immune function alterations in the patients with persistent
suppression of HBV replication following antiviral therapy that may
aid in predicting the long-term efficacy of antiviral therapy and
provide immunotherapeutic targets. To quantify the level of
functional T-cell restoration, four well-defined groups were
selected. The untreated CHB patients acted as a negative control
group, defining the basal level of impaired HBV-specific immunity
prior to antiviral therapy. The AHB patients were defined as a
positive control group who had the ability to eradicate HBV from
the body by mounting a vigorous HBV-specific immune response. The
healthy adults were defined as a positive control group who had
previously contracted HBV, but had subsequently cleared the virus
and now expressed antibodies against HBsAg and HBcAg.
The data from the ELISPOT assay provided important
information. A proportion of the untreated patients exhibited
HBV-specific CTL responses that were weak. These findings are
consistent with the majority of previous studies (7,10,14).
In the treated CHB patients, the frequency and magnitude of the
antigen-specific T cell responses were significantly higher than
those in the untreated CHB patient group. However, they were still
significantly weaker than those in the AHB patients and healthy
adults. These findings are consistent with the results from Boni
et al (10), which showed
that the T-cell responses in the NUC-treated patients with HBV DNA
suppression but HBsAg persistence were markedly stronger than in
the untreated patients with CHB, but were significantly weaker than
in the patients with HBsAg clearance and anti-HBsAg antibody
generation. As a result of the wide variability of responses among
the treated groups, certain treated patients with persistent
HBV-DNA negative status exhibited a less efficient immune response
than those of particular untreated patients.
The results from the HBV-specific T-cell
proliferation experiment were similar to the data for HBV-specific
CTL responses. The untreated CHB patients also exhibited poor
proliferative capacity of total T-cells, CD4+ T-cells
and CD8+ T-cells in response to HBV antigens. The
treated CHB patients showed an enhancement of HBV-specific
proliferation compared with the untreated patients, although the
difference between the two groups was not statistically
significant. Emerging evidence indicates that a robust, early
CD4+ T-cell response is critical in the induction of
sustained CD8+ T-cell activity (15). The present study showed that the
CD4+ T-cell made the primary contribution to the total
T-cell proliferation in the treated CHB patients and AHB patients,
who also simultaneously showed a significant enhancement of
HBV-specific CTL activity. These results suggested that the immune
status of the treated CHB patients was different from that of the
untreated CHB patients, and the enhanced CD4+ T-cell
proliferation may be important for the establishment of sustained
CD8+ T-cell activity.
The results showed that the S-specific and
C-specific CTL responses of the treated CHB patients were partly
restored, and that the HBV-specific CTL responses were
predominantly to the S-pool peptides. This finding is inconsistent
with previous study results (8),
which suggested that the T cells almost exclusively responded to
the core antigens in HBsAg seroclearance patients. The differences
among the results discussed above may be attributed to variations
among research samples with different levels of HBV control.
In the treated CHB patients, it was found that the
longer the duration of HBV DNA suppression, the stronger the
HBV-specific T-cell response was. The results demonstrate a
positive correlation between the magnitude of the HBV-specific
T-cell responses and the duration of the HBV DNA suppression. The
independent effect of the length of HBV DNA suppression on specific
immune response, found in this study, was partly in accordance with
previous studies (16,17). It is widely hypothesized that the
T-cell function exhaustion of CHB patients occurs because of the
prolonged exposure of T cells to high quantities of viral antigens
and that T-cell resting from antigenic stimulation is a crucial
requirement for restoration of a functional antiviral T-cell
response (3,5,9,18).
In this study, the results demonstrated a negative correlation
between HBV-DNA levels and HBV-specific CTL responses in untreated
CHB patients. These findings are in agreement with previous studies
(14,17) and strengthen the evidence for an
independent effect of viral load on the cellular immune
response.
The liver is the main target organ for HBV
infection, and the intrahepatic immune responses induced by HBV are
crucial for viral clearance as well as disease pathogenesis.
Previous studies in humans, chimpanzees and HBV transgenic mice
reveal that intrahepatic HBV-specific T cells, as well as natural
killer (NK) and NKT cells, are important in viral clearance and
disease pathogenesis during HBV infection. A vigorous HBV-specific
T cell response is readily detectable in the liver of AHB patients,
but due to functional or quantitative differences in this response,
chronically infected patients are unable to terminate the infection
(19,20). Our results, based on peripheral
blood samples, are in agreement with the studies already mentioned.
However, the restoration of HBV-specific immune responses detected
in the circulation only partially reflect the responses in the
liver.
In conclusion, the data indicates that the exhausted
HBV-specific immune responses are significantly restored following
the persistent suppression of HBV replication as a result of
antiviral therapy. The restoration of antiviral immunity is clearly
associated with reduced HBV DNA levels and the duration of HBV DNA
suppression, suggesting that there is a correlation between HBV
viremia and HBV-specific immune function. These findings may be
important in improving the current understanding of antiviral
therapy and for developing appropriate therapeutic strategies
against CHB.
Acknowledgements
This study was supported by the Beijing Medicine
Research and Development Fund(Beijing, China; grant no. Capital
developing 2011-2018-05).
References
|
1
|
Chao J, Song L, Zhang H, et al: Effects of
comprehensive intervention on health-related quality of life in
patients with chronic hepatitis B in China. BMC Health Serv Res.
13:3862013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang X, Zhang M, Lai Q, et al: Restored
circulating invariant NKT cells are associated with viral control
in patients with chronic hepatitis B. PLoS One. 6:e288712011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liaw YF: Impact of therapy on the outcome
of chronic hepatitis B. Liver Int. 33(Suppl 1): 111–115. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lam YF, Yuen MF, Seto WK and Lai CL:
Current antiviral therapy of chronic hepatitis B: efficacy and
safety. Curr Hepat Rep. 10:235–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Webster GJ, Reignat S, Brown D, et al:
Longitudinal analysis of CD8+ T cells specific for structural and
nonstructural hepatitis B virus proteins in patients with chronic
hepatitis B: implications for immunotherapy. J Virol. 78:5707–5719.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ribeiro RM, Germanidis G, Powers KA, et
al: Hepatitis B virus kinetics under antiviral therapy sheds light
on differences in hepatitis B e antigen positive and negative
infections. J Infect Dis. 202:1309–1318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carey I, D’Antiga L, Bansal S, et al:
Immune and viral profile from tolerance to hepatitis B surface
antigen clearance: a longitudinal study of vertically hepatitis B
virus-infected children on combined therapy. J Virol. 85:2416–2428.
2011. View Article : Google Scholar :
|
|
8
|
Liang M, Ma S, Hu X, et al: Cellular
immune responses in patients with hepatitis B surface antigen
seroclearance induced by antiviral therapy. Virol J. 8:692011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boni C, Fisicaro P, Valdatta C, et al:
Characterization of hepatitis B virus (HBV)-specific T-cell
dysfunction in chronic HBV infection. J Virol. 81:4215–4225. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boni C, Laccabue D, Lampertico P, et al:
Restored function of HBV-specific T cells after long-term effective
therapy with nucleos(t)ide analogues. Gastroenterology.
143:963–973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang Y, Ma Z, Xin G, et al: Th1 and Th2
immune response in chronic hepatitis B patients during a long-term
treatment with adefovir dipivoxil. Mediators Inflamm.
2010:1430262010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rico MA, Quiroga JA, Subirá D, et al:
Hepatitis B virus-specific T-cell proliferation and cytokine
secretion in chronic hepatitis B e antibody-positive patients
treated with ribavirin and interferon alpha. Hepatology.
33:295–300. 2001. View Article : Google Scholar
|
|
13
|
Tsai SL, Sheen IS, Chien RN, et al:
Activation of Th1 immunity is a common immune mechanism for the
successful treatment of hepatitis B and C: tetramer assay and
therapeutic implications. J Biomed Sci. 10:120–135. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Li X, Ye B, et al: Effect of
telbivudine therapy on the cellular immune response in chronic
hepatitis B. Antiviral Res. 91:23–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Janssen EM, Lemmens EE, Wolfe T, et al:
CD4+ T cells are required for secondary expansion and memory in
CD8+ T lymphocytes. Nature. 421:852–856. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Chen Y, Ma Z, et al: Effect of
regulatory T cells and adherent cells on the expansion of
HBcAg-specific CD8+ T cells in patients with chronic hepatitis B
virus infection. Cell Immunol. 264:42–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stoop JN, van der Molen RG, Kuipers EJ, et
al: Inhibition of viral replication reduces regulatory T cells and
enhances the antiviral immune response in chronic hepatitis B.
Virology. 361:141–148. 2007. View Article : Google Scholar
|
|
18
|
Wherry EJ, Blattman JN and Ahmed R: Low
CD8 T-cell proliferative potential and high viral load limit the
effectiveness of therapeutic vaccination. J Virol. 79:8960–8968.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thimme R, Wieland S, Steiger C, et al:
CD8(+) T cells mediate viral clearance and disease pathogenesis
during acute hepatitis B virus infection. J Virol. 77:68–76. 2003.
View Article : Google Scholar :
|
|
20
|
Maini MK, Boni C, Lee CK, et al: The role
of virus-specific CD8(+) cells in liver damage and viral control
during persistent hepatitis B virus infection. J Exp Med.
191:1269–1280. 2000. View Article : Google Scholar : PubMed/NCBI
|