Introduction
According to the World Heath Organization, gastric
cancer is the second most common cause of cancer mortality
worldwide, with 870,000 novel cases occurring annually (1). The invasion and metastatic spread of
gastric cancer is associated with patient survival and prognosis
(2). Despite the optimization of
surgery, radiotherapy and chemotherapy treatments, survival rates
of patients with advanced gastric cancer have remained poor
(3).
Toll-like receptors (TLRs) are transmembrane
receptors, which are mainly expressed in immune and epithelial
cells and have an important role in conferring innate immunity
(4,5). Members of the TLR family comprise ≥13
associated genes, TLR1-TLR13 (6).
TLR9 is one of the most important members of the TLR family
(7). This previous study indicated
that high expression of TLR9 occurred not only in immune cells, but
also in numerous types of cancer cell, including breast, brain,
ovarian, gastric, lung and prostate cancer cells (7). The role of TLR9 expressed by tumor
cells in the evasion of immune surveillance was demonstrated in
animal experiments and indicated that TLR9 stimulation may lead to
tumor progression, inflammation and enhanced cell survival
(8). TLR9 was demonstrated to be
expressed by gastric epithelial cells in the human stomach and has
critical roles in the development and progression of gastric cancer
(9,10). TLR9-1486C polymorphism carriers
have been found to be associated with an increased risk and poorer
prognosis of patients with gastric carcinoma in the Chinese
population (11). Ligands binding
to TLR9 activate multiple signaling factors, including nuclear
factor kappa B (NFκB) and result in increased production of
inflammatory mediators which leads to a higher risk of developing
chronic inflammatory diseases and cancer (12). NFκB is a transcription factor
involved in conferring innate and adaptive immunity and has a
crucial role in mediating inflammation against infectious
molecules. The NFκB signaling pathway likely links chronic
inflammation and tumor development, a hypothesis which was
supported by the observation that constitutively active NFκB is
detected in numerous human malignancies (13). A recent study directly demonstrated
that NFκB had a pivotal role in TLR-induced tumorigenesis when TLRs
were activated (14). In a
previous study by our group, it was revealed that TLR9 was
expressed in gastric cancer and associated with a high degree of
tumor differentiation (15). These
findings may be useful in identifying potential prognostic markers.
However, at present, the mechanism of regulation of TLR9 expression
and its specific role in gastric cancer cells remain to be
elucidated.
The purpose of the present study was therefore to
investigate the expression of TLR9 in human gastric cancer cells
and to analyze its potential association with gastric cancer cell
proliferation and migration. The non-specific inhibitor of TLR9,
chloroquine (CQ), was applied to gastric cancer MGC803 cells at
various time-points to evaluate the involvment the of the TLR9/NFκB
signaling pathway in gastric cancer cell migration and the
proliferation of tumor cells using the wound healing assay and MTT
analysis. The mRNA expression levels of tumor progression- and
migration-associated factors, including matrix metalloproteinase-2
(MMP-2), MMP-7 and cyclooxygenase-2 (COX-2) were also examined by
reverse transcription polymerase chain reaction (RT-PCR). The
results of the present study may provide an experimental basis for
the development of clinical immunology treatments for gastric
cancer.
Materials and methods
Chemicals
The full phosphorothioated CpG-oligodeoxynucleotide
(ODN)2006 and primers were synthesized by SBS Genetech Co., Ltd.
(Beijing, China). Mouse monoclonal antibodies (mAbs) against TLR9,
fluorescein isothiocyanate (FITC)-conjugated TLR9, GAPDH, NFκB and
immunoglobulin G (IgG)2α isotype control were purchased from Abcam
(Shanghai, China). The Annexin V-FITC/propidium iodide (PI) kit was
obtained from Bender (Shenzen, China). TRIzol, RPMI-1640 and fetal
bovine serum (FBS) were from Gibco-BRL (Invitrogen Life
Technologies, Beijing, China). CQ was purchased from Sigma-Aldrich
(St. Louis, MO, USA). The reverse transcription kit was purchased
from TransGen Biotechnology Co., Ltd. (Beijing, China).
Bicinchoninic acid (BCA) and enhanced chemiluminescence (ECL) kits
were purchased from Pierce Biotechnology, Inc. (Rockford, IL, USA).
A Nuclear Extract kit was obtained from Active Motif (Carlsbad, CA,
USA). Protease inhibitor mixture was bought from Roche Diagnostics
(Basel, Switzerland).
MGC803 cell line and cell culture
The gastric cancer cell line MGC803 (Cell Bank,
Shanghai, China) was preserved in the Ningxia Key Laboratory of
Cerebrocranial Diseases. The cells were cultivated in RPMI-1640
medium which contained 10% FBS (pH 7.2), 100 U/ml penicillin and
100 g/ml streptomycin (Invitrogen Life Technologies) in 5%
CO2 at 37°C.
Flow cytometry
MGC803 cells were detached with 2.5 g/l trypsin
(containing 0.02% EDTA; Invitrogen Life Technologies) and washed
with cold phosphate-buffered saline (PBS). Mouse IgG2α anti-human
TLR9 mAb (dilution, 1:50) or the appropriate isotypic control mAb
were used at 0.5 mg/106 cells for 30 min on ice.
Following washing with cold PBS, cells were stained with
FITC-conjugated anti-mouse antibody (dilution, 1:50) and analyzed
using a BD FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA).
Cells were gated using forward versus side scatter to exclude dead
cells and debris. Fluorescence of 104 cells per sample
was measured in logarithmic mode for visual inspection of the
distributions and in linear mode for quantifying the expression of
the relevant molecules by calculating the mean fluorescence
intensity.
Western blot analysis
Nuclear extracts were prepared using a Nuclear
Extract kit according to the manufacturer’s instructions. Total
cell extracts were prepared by lysing the cells in buffer
containing 1% NP40, 150 mM NaCl, 50 mM Tris-HCl, a protease
inhibitor mixture, 50 mM NaF and 1 mM Na3VO4
for phosphatase inhibition. The protein concentration in each
sample was determined using a BCA Protein Assay kit. A total of 40
μg protein was loaded onto pre-casted 10% Bis-Tris Gels (Sigma,
Beijing, China) and subjected to SDS-PAGE. Transferring to a
nitrocellulose membrane was performed for 3 h at 4°C and 60 V using
a wet transfer system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Equal protein loading was confirmed with ponceau staining
(Pierce Biotechnology, Inc.) (16). Membranes were blocked in 5% nonfat
dry milk in 0.1% Tween-20-Tris buffered saline (TBST; pH 7.4)
overnight at 4°C. Anti-TLR9 (mouse monoclonal to TLR9; dilution,
1:100) and anti-GAPDH (mouse monoclonal to GAPDH; dilution,
1:1,000) antibodies were incubated overnight at 4°C in 1% nonfat
dry milk in TBST. Membranes were washed three times and incubated
with appropriate goat anti-mouse secondary Abs (dilution, 1:1,000)
for 1 h at room temperature. Following three washes in TBST, the
membranes were developed with ECL detection reagents and exposed to
Hyperfilm ECL.
RT-PCR
Total RNA was isolated using the RNeasy Mini kit
(Qiagen, Beijing, China). DNA was removed from total RNA using the
DNA-free kit (Turbo, Shanghai, China). A total of 1 μg RNA was used
to synthesize cDNA using the Advantage RT-for-PCR kit (Clontech,
Wuhan, China). A total of 50 ng cDNA was subsequently used for PCR.
The TLR-9 and β-actin primers were as follows: TLR9 forward,
5′-GGACACTCCCAGCTCTGAAG-3′ and reverse, 5′-TTGGCTGTGGATGTTGTTGT-3′;
β-actin forward, 5′-TAGAGATTGGAGGTTGTTCCT-3′ and reverse,
5′-TCCACCAACTAAGAACGGCC-3′. PCR was performed as follows: 94°C for
5 min, 35 cycles of denaturation at 94°C for 30 sec, annealing at
55°C for 30 sec, extension at 72°C for 30 sec and a single
extension at 72°C for 5 min.
MGC803 cells were treated with CpG-ODN2006
(5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′) or CQ for 24 h, prior to total RNA
extraction using TRIzol. Primers and annealing temperatures were as
follows: MMP-2 forward, 5′-CTTCCAAGTCTGGAGCGATGT-3′ and reverse,
5′-TACC GTCAAAGGGGTATCCAT-3′ (annealing temperature, 65°C); MMP-7
forward, 5′-CGGGGTACCATAATGTCCTGAATGA TACC-3′ and reverse,
5′-CCCAAGCTTTGCCGTCCAGAGAC AATTG-3′ (annealing temperature, 66°C);
COX-2 forward, 5′-GCCTGAATGTGCCATAAGACTGAC-3′ and reverse,
5′-AAACCCACAGTGCTTGACACAGA-3′ (annealing temperature, 62°C); NFκB
p65 forward, 5′-GTTCACAGACCTG GCATCCGT-3′ and reverse,
5′-GAGAAGTCCATGTCCGCA ATG-3′ (annealing temperature, 57°C); β-actin
forward, 5′-TGGCACCCAGCACAATGAA-3′ and reverse,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ (annealing temperature, 60°C). PCR
was performed as follows: 94°C for 3 min, followed by 35 cycles of
94°C for 30 sec, annealing at different temperatures for 30 sec and
extension at 72°C for 30 sec, followed by a final cycle of 72°C for
10 min. Results were analyzed with BandScan 5.0 line image analysis
software (Glyko, Novato, CA, USA) to calculate relative mRNA
expression levels.
Cell proliferation analysis
The anti-proliferative effects of CQ on MGC803 cells
were examined by MTT colorimetric assay. Cells were seeded in
96-well plates at a density of 5×104 cells per well for
24 h, prior to exposure to the indicated concentrations of CQ for
24, 48 and 72 h, respectively. RPMI-1640 was used as a negative
control. MTT was dissolved at a concentration of 5 mg/ml in sterile
PBS at room temperature. Following removal of the medium, 20 μl was
added to each well followed by 4 h of incubation. The MTT solution
was aspirated and the purple formazan crystals produced by the
mitochondrial dehydrogenase enzymes were dissolved in DMSO. The
optical density (OD) of each well was measured at 570 nm on an
ELISA reader (Bio-Rad, Beijing, China).
Wound healing assays
MGC803 cells were seeded into six-well culture
plates (1×105 cells per well) and cultured for 24 h. The
medium was subsequently removed and replaced with 20 μg/ml CpG-ODN
1816 RPMI-1640 medium or CQ at a concentration of 100 or 200 μg/ml
once the cells had reached 80% confluence. The supernatant was
discarded, scratches were produced in the confluent layer of cells
using a sterile scraping cutter and the ability of cells to heal
the scratch was analyzed at 12, 24 and 36 h. Images were captured
of wound healing assays using an inverted microscope (CKX41SF;
Olympus Corporation, Tokyo, Japan).
Statistics
Statistical differences were determined using
Student’s t-test for paired samples or by one-way analysis of
variance followed by Student’s t-test with the Bonferroni
correction (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference between
values.
Results
TLR9 mRNA and protein is expressed in
MGC803 cells
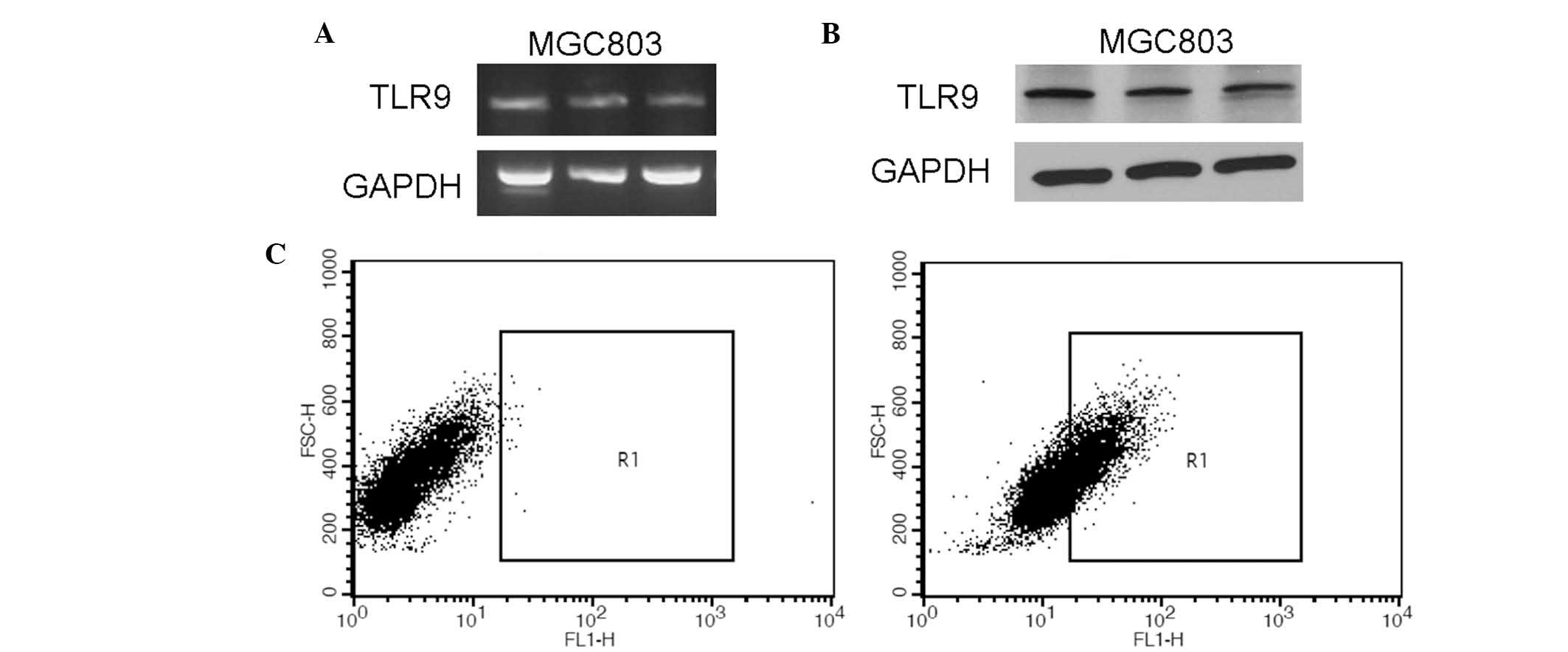
The expression of TLR9 mRNA in the gastric cancer
MGC803 cell line was analyzed by RT-PCR (Fig. 1A). The results revealed that TLR9
mRNA was expressed in MGC803 cells. To confirm the RT-PCR findings,
the protein expression levels of TLR9 in MGC803 cells were examined
by western blot analysis (Fig. 1B)
and flow cytometric analysis (Fig.
1C). Analysis revealed that TLR9 protein was expressed in
MGC803 cells.
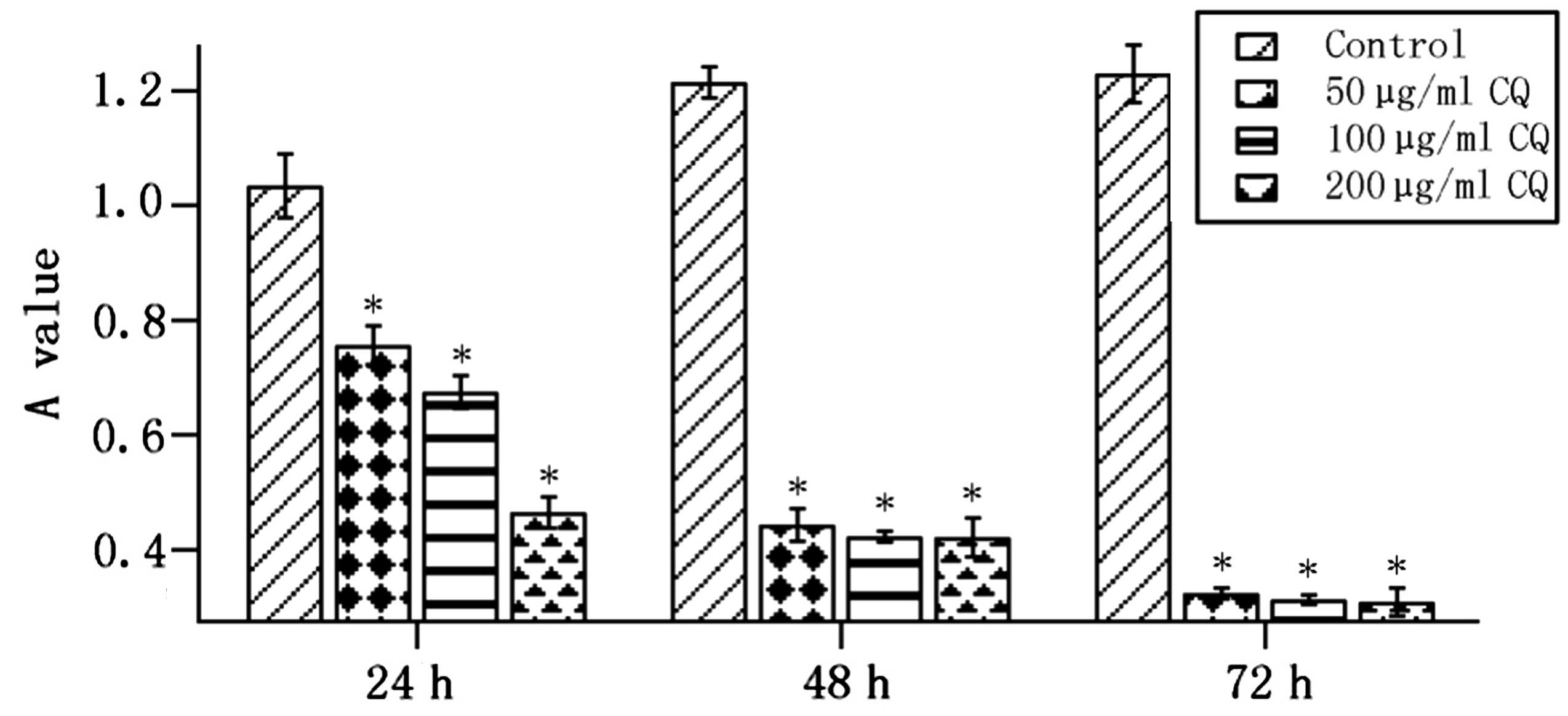
CQ inhibits MGC803 cell growth
When cells were treated with various concentrations
of CQ (50, 100 or 150 μg/ml) for 24, 48 and 72 h, cell
proliferation was significantly inhibited in all CQ-treated groups
compared with that of the control group (P<0.05; Fig. 2).
CQ inhibits MMP-2, MMP-7, COX-2 and NFκB
p65 mRNA expression
MGC803 cells were treated with various
concentrations of CQ, in order to investigate the effects of TLR9
on MMP-2, MMP-7, COX-2 and NFκB p65 gene expression. CQ inhibited
mRNA expression, while CpG-ODN enhanced mRNA expression of all four
factors (P<0.05; Fig. 3).
 | Figure 3CQ inhibits mRNA expression levels of
MMP-2, MMP-7, COX-2 and NFκB p65, detected by reverse transcription
polymerase chain reaction. (A) MMP-2. (B) MMP-7. (C) COX-2. (D)
NFκB p65. (E) Analysis of A, B C and D. Lanes, left to right: 1,
marker; 2, 10 μg/ml CpG-ODN; 3, 20 μg/ml CpG-ODN; 4, 50 μg/ml CQ;
5, 100 μg/ml CQ; 6, 200 μg/ml CQ. *P<0.05 vs.
control. CQ, chloroquine; MMP, matrix metalloproteinase; COX-2,
cyclooxygenase-2; NFκB, nuclear factor-κB; CpG-ODN,
CpG-oligodeoxynucleotide. |
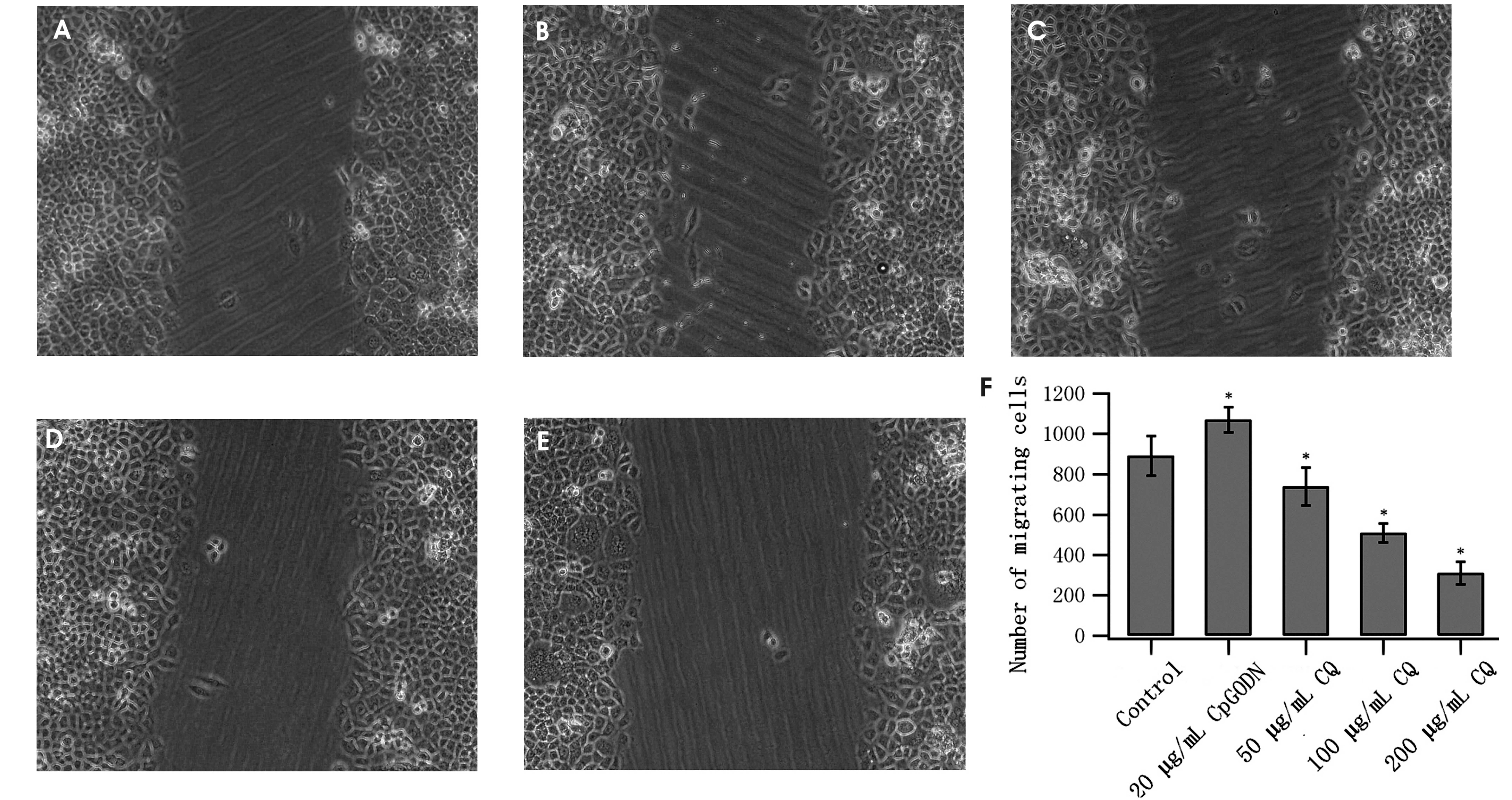
CQ inhibits MGC803 cell migration
The wound healing assay results indicated that
following CQ treatment, cell migration to the damaged zone
decreased and that the numbers of migrated cells following 36 h
treatment were markedly decreased, except at a concentration of 50
μg/ml CQ. Conversely, CpG-ODN treatment promoted MGC803 cell
migration (Fig. 4).
Discussion
Gastric cancer is one of the leading causes of
mortality worldwide (17). Due to
gastric cancer being prone to relapse and metastasis, the
identification of prognostic factors is essential for improvement
of the traditional risk classification system currently used in
gastric cancer.
TLRs are important innate immunity regulators that
may be activated upon recognition of bacterial and viral ligands,
known as pathogen-associated molecular patterns (18). TLR9 is expressed in dendritic cells
and various tissue types. TLR9 was found to be expressed in various
cancer cell lines and human tumors, including non-small cell lung
cancer, glioma and prostate cancer (19,20).
TLR4 and TLR9 are known to be expressed by gastric epithelial cells
in the human stomach (9,10). In the present study, TLR9 was
demonstrated to be expressed and functional in gastric carcinoma
cells. TLR9 recognizes unmethylated CpG-ODNs that are abundant in
bacterial DNA, leading to NFκB activation (21,22).
Participation of the NFκB signaling pathway in carcinogenesis
differs between organs, cells and models. The crosstalk between an
inflammatory cell and a neoplastic cell, which is instigated by the
activation of NFκB, is critical for tumor organization (23). NFκB activation initiates the
transcription of numerous cytokine genes involved in inflammation,
evasion of apoptosis, tumor formation and transformation, including
MMPs, COX and TNF-α (7,24,25).
MMP-2 has been shown to have an important role in cancer metastasis
(26,27). MMP-7 inhibits apoptosis of cancer
cells, reduces cell adhesion and induces angiogenesis, making it
easier for the cancer cells to invade small blood vessels and
lymphatic tubes and metastasize (28,29).
COX-2 has been detected in various tumor tissues, including
pancreatic cancer, colorectal carcinoma and non-small cell lung
cancer and is positively correlated with tumor invasion and
lymphatic metastasis (30,31). In the present study, CQ, the
non-specific inhibitor of TLR9, was demonstrated to decrease NFκB
p65 mRNA expression levels and attenuate the bioactivity of NFκB
p65. CQ also reduced mRNA expression of MMP-2, MMP-7 and COX-2,
suggesting that the TLR9/NFκB signaling pathway may be involved in
cancer occurrence and migration. However, elucidation of the
specific roles of these three factors in gastric cancer requires
further study.
In conclusion, in the present study, MGC803 gastric
cancer cells were found to express TLR9 and the TLR9/NFκB signaling
pathways were involved in cancer cell migration. The non-specific
inhibitor of TLR9, CQ, inhibited cancer cell migration. Therefore,
the TLR9/NFκB signaling pathway may be involved in gastric cell
carcinogenesis and may represent an important therapeutic target in
gastric cancer.
Acknowledgements
The authors would like to thank all the members of
the Immunology Lab (Ningxia Medical University, Yinchuan, China)
for their help. The present study was supported by the Ningxia
Natural Science Foundation Program (nos. NZ0991, NZ11100 and
NZ14057), the National Natural Science Foundation of China (nos.
31060140, 31260243 and 31460257) and was sponsored by the Program
for New Century Excellent Talents in University, State Education
Ministry (Beijing, China), which was awarded to Dr Yin Wang.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: Globocan 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Taghizadeh-Kermani A, Yahouiyan SZ,
AliAkbarian M and Seilanian Toussi M: Prognostic significance of
metastatic lymph node ratio in patients with gastric cancer: an
evaluation in North-East of Iran. Iran J Cancer Prev. 7:73–79.
2014.PubMed/NCBI
|
|
3
|
D’Angelo G, Di Rienzo T and Ojetti V:
Microarray analysis in gastric cancer: A review. World J
Gastroenterol. 20:11972–11976. 2014. View Article : Google Scholar
|
|
4
|
Aderem A and Ulevitch RJ: Toll-like
receptors in the induction of the innate immune response. Nature.
406:782–787. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeda K and Akira S: Toll-like receptors
in innate immunity. Int Immunol. 17:1–14. 2005. View Article : Google Scholar
|
|
6
|
Oldenburg M, Krüger A, Ferstl R, Kaufmann
A, Nees G, Sigmund A, et al: TLR13 recognizes bacterial 23S rRNA
devoid of erythromycin resistance-forming modification. Science.
337:1111–1115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Merrell MA, Ilvesaro JM, Lehtonen N, Sorsa
T, Gehrs B, Rosenthal E, Chen D, Shackley B, Harris KW and Selander
KS: Toll-like receptor 9 agonists promote cellular invasion by
increasing matrix metalloproteinase activity. Mol Cancer Res.
4:437–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berger R, Fiegl H, Goebel G, Obexer P,
Ausserlechner M, Doppler wW, et al: Toll-like receptor 9 expression
in breast and ovarian cancer is associated with poorly
differentiated tumors. Cancer Sci. 101:1059–1066. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmausser B, Andrulis M, Endrich S, Lee
SK, Josenhans C, Müller-Hermelink HK, et al: Expression and
subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9
on the gastric epithelium in Helicobacter pylori infection. Clin
Exp Immunol. 136:521–526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmausser B, Andrulis M, Endrich S,
Müller-Hermelink HK and Eck M: Toll-like receptors TLR4, TLR5 and
TLR9 on gastric carcinoma cells: an implication for interaction
with Helicobacter pylori. Int J Med Microbiol. 295:179–185. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Xue L, Yang Y, Xu L and Zhang G:
TLR9 promoter polymorphism is associated with both an increased
susceptibility to gastric carcinoma and poor prognosis. PLoS One.
8:e657312013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wagner H: The immunobiology of the TLR9
subfamily. Trends Immunol. 25:381–386. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koti M, Gooding RJ, Nuin P, Haslehurst A,
Crane C, Weberpals J, et al: Identification of the
IGF1/PI3K/NFkB/ERK gene signalling networks associated with
chemotherapy resistance and treatment response in high-grade serous
epithelial ovarian cancer. BMC Cancer. 13:5492013. View Article : Google Scholar
|
|
14
|
Zhan Z, Xie X, Cao H, Zhou X, Zhang XD,
Fan H, et al: Autophagy facilitates TLR4- and TLR3-triggered
migration and invasion of lung cancer cells through the promotion
of TRAF6 ubiquitination. Autophagy. 10:257–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma YJ, Cui L, Zhang YL and Li XP:
Correlations of TLR9 to clinicopathologic features of gastric
cancer. J Ningxia Med Univ. 34:126–128. 2012.
|
|
16
|
Romero-Calvo I, Ocón B, Martínez-Moya P,
Suárez MD, Zarzuelo A, Martínez-Augustin O and de Medina FS:
Reversible Ponceau staining as a loading control alternative to
actin in Western blots. Anal Biochem. 401:318–320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li MZ, Deng L, Wang JJ, Xiao LB, Wu WH,
Yang SB and Li WF: Surgical outcomes and prognostic factors of T4
gastric cancer patients without distant metastasis. PLoS One.
9:e1070612014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang JQ, Jeelall YS, Ferguson LL and
Horikawa K: Toll-like receptors and cancer: MYD88 mutation and
inflammation. Front Immunol. 5:367–377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ilvesaro JM, Merrell MA, Swain TM,
Davidson J, Zayzafoon M, Harris KW and Selander KS: Toll like
receptor-9 agonists stimulate prostate cancer invasion in vitro.
Prostate. 67:774–781. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren T, Xu L, Jiao S, Wang Y, Cai Y, Liang
Y, Zhou Y, Zhou H and Wen Z: Tlr9 signaling promotes tumor
progression of human lung cancer cell in vivo. Pathol Oncol Res.
15:623–630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krieg AM: CpG motifs in bacterial DNA and
their immune effects. Annu Rev Immunol. 20:709–760. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Latz E, Schoenemeyer A, Visintin A,
Fitzgerald KA, Monks BG, Knetter CF, et al: TLR9 signals after
translocating from the ER to CpG DNA in the lysosome. Nat Immunol.
5:190–198. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maeda S: NF-κB, JNK, and TLR signaling
pathways in hepatocarcinogenesis. Gastroenterol Res Pract.
367694:2010.
|
|
24
|
Kakiuchi Y, Tsuji S, Tsujii M, Murata H,
Kawai N, Yasumaru M, et al: Cyclooxygenase-2 activity altered the
cell-surface ca- rbohydrate antigens on colon cancer cells and
enhanced liver metastasis. Cancer Res. 62:1567–1572.
2002.PubMed/NCBI
|
|
25
|
Chen R, Alvero AB, Silasi DA, Steffensen
KD and Mor G: Cancers take their Toll - the function and regulation
of Toll-like receptors in cancer cells. Oncogene. 27:225–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jezierska A and Motyl T: Matrix
metalloproteinase-2 involvement in breast cancer progression: a
mini-review. Med Sci Monit. 15:RA32–RA40. 2009.PubMed/NCBI
|
|
27
|
Sims JD, McCready J and Jay DG:
Extracellular heat shock protein (Hsp)70 and Hsp90α assist in
matrix metalloproteinase-2 activation and breast cancer cell
migration and invasion. PLoS One. 6:e188482011. View Article : Google Scholar
|
|
28
|
Okayama H, Kumamoto K, Saitou K, Hayase S,
Kofunato Y, Sato Y, et al: CD44v6, MMP-7 and nuclear Cdx2 are
significant biomarkers for prediction of lymph node metastasis in
primary gastric cancer. Oncol Rep. 22:745–755. 2009.PubMed/NCBI
|
|
29
|
Yeh YC, Sheu BS, Cheng HC, Wang YL, Yang
HB and Wu JJ: Elevated serum matrix metalloproteinase-3 and -7 in
H. pylori-related gastric cancer can be biomarkers correlating with
a poor survival. Dig Dis Sci. 55:1649–1657. 2010. View Article : Google Scholar
|
|
30
|
Sugie S, Tsukino H, Mukai S, Akioka T,
Shibata N, Nagano M and Kamoto T: Cyclooxygenase 2 genotypes
influence prostate cancer susceptibility in Japanese men. Tumour
Biol. 35:2717–2721. 2014. View Article : Google Scholar
|
|
31
|
Lu J, Li XF, Kong LX, Ma L, Liao SH and
Jiang CY: Expression and significance of cyclooxygenase-2 mRNA in
benign and malignant ascites. World J Gastroenterol. 19:6883–6887.
2013. View Article : Google Scholar : PubMed/NCBI
|