Introduction
Proliferative vitreoretinopathy (PVR) is
characterized by the formation of contractile membranes in the
vitreal cavity and on both surfaces of the retina. Retinal pigment
epithelial (RPE) cells are considered to play a pivotal role in the
formation and the contraction of these membranes, and are known to
secrete a wide range of growth factors which are significant in the
progression of the disease, including transforming growth factor-β
(TGFβ), platelet-derived growth factor and fibroblast growth factor
(1). TGFβ2 is considered to be the
predominant TGFβ isoform in the posterior segment. TGFβ2 has been
identified in membranes excised from patients with PVR by
immunohistochemistry. TGFβ2 concentration is elevated in the
vitreous of patients with the disease (2,3).
Therefore, targeting of the TGFβ2 gene with a small interfering RNA
(siRNA) in RPE cells has the potential to prevent the development
of PVR.
RNA interference (RNAi) is a promising biological
strategy for the treatment of diverse diseases; however, the
therapeutic application of siRNA has been limited by its
instability and poor cellular uptake efficiency (4). If the siRNA in vivo is to
become effective with high efficiency and stability in the
long-term, a vector is required. Currently, the virus vector is the
main vector applied in RNAi research. Recombinant adeno-associated
virus (rAAV), characterized by low immunogenicity and long
exogenous gene expression time, is particularly applicable to the
gene therapy of ophthalmology. The identification of the rAAV
vector of RNAi provides the premise for the enhanced use of RNAi
technology to silence related genes in the treatment of ophthalmic
diseases (5,6). However, the solution to the low
infection rate of rAAV has not yet been elucidated (7,8). The
use of ultrasound (US) and microbubbles (MBs) has been proven to be
safe and efficient in various organs. Our previous studies
demonstrated that US with MBs enhanced rAAV transfection efficiency
in ARPE19 cells (9,10). The present study was designed to
determine whether US and/or MBs not only facilitate the delivery of
rAAV2-enhanced green fluorescent protein (EGFP) but also the
downregulation of TGFβ2 combined with rAAV2-TGFβ2 shRNA in rat RPE
cells. To the best of our knowledge, only a few studies have been
published with regard to US/MBs combined with AAV-mediated RNAi in
the sphere of ophthalmology. If successful, such an approach may
provide an alternative gene delivery method for RNAi in RPE
cell-related diseases.
Materials and methods
rAAV2-EGFP-based transfection in RPE-J
cells with US and/or US contrast agent
Cell culture
A rat RPE-J cell line was obtained from the American
Type Culture Collection (CRL-2240; ATCC, Rockville, MD, USA). The
cells were cultured to 70–80% confluence in Dulbecco’s modified
Eagle’s medium (Gibco, Grand Island, NY, USA) supplemented with 4%
fetal bovine serum at 37°C, in 5% CO2/95% air. RPE cells
were placed onto every other well of a 24-well plate at a
concentration of 1×105 cells per well prior to
infection.
US contrast agent and virus
SonoVue, a lipid-shelled US contrast agent filled
with sulfur hexafluoride gas, composed of ~2×108 per ml
MBs, and having an average diameter of 2.5–6.0 mm, was purchased
from Bracco (Milan, Italy). Perfluoropropane-albumin microspheres,
an albumin-shelled US contrast agent filled with perfluoropropane
gas, composed of ~5×108 per ml MBs, and having an
average diameter of 3–4.5 μm, was purchased from Jing Da
pharmaceutical Co., Ltd., Hu Nan, China; approval number
S20083098). Finally, rAAV2-EGFP [2×1012 vector genomes
per milliliter (vg/ml)] was purchased from Vector Gene Technology
Company Ltd. (Beijing, China). A dose of 1×104 MOI was
used (multiplicity of infection: vector genomes/number of
cells).
US exposure protocol
The volume per well was 150 μl when exposed to US
and the method of transfection configuration was the same as we
previously reported (9). A
therapeutic US device (Topteam 161, Chattanooga Medical Supply,
Inc., Chattanooga, TN, USA) was used as previously described
(9). We set the experimental
conditions as follows: frequency, 1 MHz; US intensity, 0.5 and 1
W/cm2; duration, 30, 60 and 90 sec; pulse wave with 50%
duty cycle; with or without MBs (SonoVue or
perfluoropropane-albumin microspheres; MB:cell ratio, 20:1, 40:1
and 60:1). RPE cells were infected by rAAV alone (control) or in
combination with US (rAAV+US), SonoVue (rAAV+SonoVue),
perfluoropropane-albumin microspheres (rAAV+microsphere) or
US-targeted microbubble destruction (UTMD; rAAV+US+SonoVue and
rAAV+US+microsphere group; MB:cell ratio, 20:1).
Gene transfer efficiency
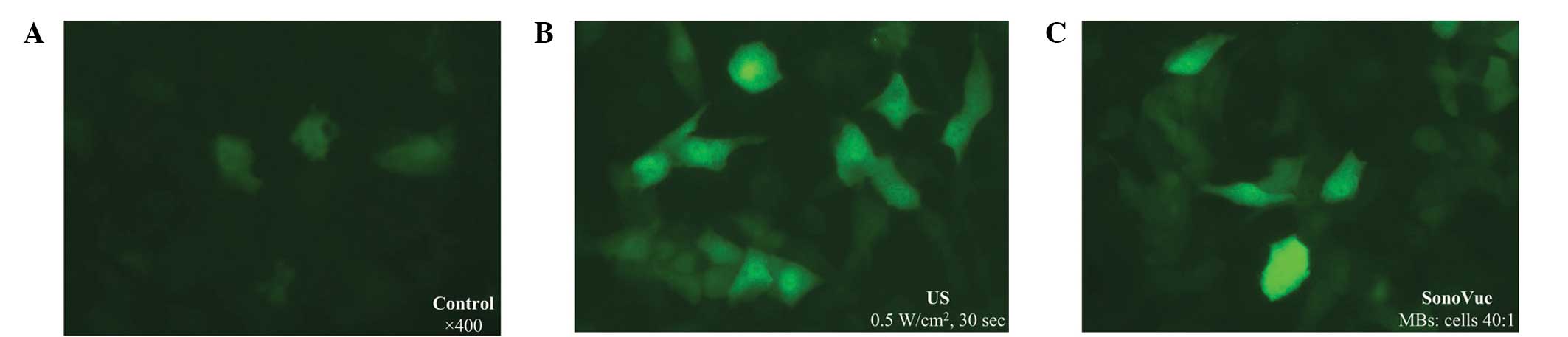
Forty-eight hours after rAAV transfection, EGFP
expression was observed and photographed using an inverted
fluorescence microscopy (Zeiss Axiovert S100, Carl Zeiss,
Oberkochen, Germany). The ratio of infected cells was examined by a
fluorescence-activated cell sorter (FACS; Epics XL, Beckman
Coulter, Miami, FL, USA).
Cell damage assay
RPE cells were placed into groups of four adjacent
holes of a 96-well plate (with every other four adjacent holes left
empty), at a concentration of 8×104 cells per well. The
cells were exposed to US (frequency, 1 MHz; US intensity, 0.5 and 1
W/cm2; duration, 30, 60 and 90 sec; 50% duty cycle) or
SonoVue (MB:cell ratio, 20:1, 40:1, 60:1 and 80:1). The volume per
well was 100 μl. Twenty-four hours after treatment and under
various exposure parameters, MTS assay (CellTiter 96®
AQueous One solution cell proliferation assay, Promega, Madison,
WI, USA) was performed to obtain the absorbance value. The
absorbance value of RPE-J cells that were not treated was used as a
control.
rAAV2-TGFβ2 shRNA-U6-EGFP-based
transfection in RPE-J cells with US or SonoVue
Design and screening of siRNAs in
RPE-J cells
The siRNA targeting the rat TGFβ2 (gene ID 81809)
coding sequence (three pairs, Table
I) and control siRNA were designed and synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China). To avoid possible
off-target effects in the rat, these target sequences had been used
to screen Gen-Bank using BLAST. To test siRNAs, RPE-J cells were
co-transfected with synthetic siRNAs and Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA). Finally, the optimal RNAi target
sequence (TGFβ2-771) was selected by the expression level of
mRNA.
 | Table IsiRNA duplexes and specificity used in
study. |
Table I
siRNA duplexes and specificity used in
study.
| siRNA | Sense strand | Antisense strand | Specificity |
|---|
| Control siRNA |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT | None |
| TGFβ2-192-siRNA |
CGGAGGUGAUUUCCAUCUATT |
CGGAGGUGAUUUCCAUCUATT | Rat |
| TGFβ2-531-siRNA |
GGGUCUUUCGCUUGCAGAATT |
UUCUGCAAGCGAAAGACCCTG | Rat |
| TGFβ2-771-siRNA |
CCUUCAUACCGUCUAAUAATT |
UUAUUAGACGGUAUGAAGGTA | Rat |
rAAV2-TGFβ2 shRNA-U6-EGFP
constructs
rAAV2-TGFβ2 shRNA-U6-EGFP was constructed by Vector
Gene Technology Company Ltd. on the basis of the TGFβ2-771 we
selected. Transgenes were driven by the U6 promoter. The titer of
genome particles was 2.7×1011 vg/ml.
rAAV2-neg-shRNA-U6-EGFP was also constructed by the same company.
The titer of genome particles was 4.4×1011 vg/ml.
US- or SonoVue-mediated rAAV2-TGFβ2
shRNA-EGFP transfected RPE cells
RPE cells were placed into a 12-well plate at a
concentration of 1×105 cells per well. The method of
transfection configuration was the same as described above. The
dose of rAAV2-TGFβ2 shRNA-EGFP and rAAV2-neg-shRNA-EGFP was
5×104 MOI.
FACS-examined EGFP expression
rAAV2-TGFβ2 shRNA-EGFP was transfected into RPE
cells with US (0.5 W/cm2 and 30 sec) or SonoVue (MB:cell
ratio, 40:1). rAAV2-TGFβ2 shRNA-EGFP transfected alone served as
the control. Forty-eight hours after transfection, EGFP expression
(ratio of positive cells and mean fluorescence density) was
examined by FACS.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of TGFβ2 gene
expression
The level of TGFβ2 mRNA in RPE-J cells was estimated
by RT-qPCR analysis 48 h after rAAV2-TGFβ2 shRNA-EGFP was
transfected with US (0.5 W/cm2 and 30 sec) or SonoVue
(MBs:cells, 40:1). RPE-J cells that were not treated were used as a
control. To determine the efficiency of gene delivery by a
combination of US or SonoVue, the cells transfected with
rAAV2-neg-shRNA-EGFP were also collected to measure the mRNA levels
48 h post-transfection. Total RNA was harvested using a TRIzol
reagent kit (Invitrogen, Karlsruhe, Germany) and then reverse
transcription to synthesize cDNA was achieved using a reverse
transcription system (Promega). RT-qPCR was performed with the
extracted total RNA using Taq PCR Master mix (Toyobo Co., Ltd.,
Osaka, Japan). Quantification using the 2−ΔΔCT
analytical method was performed in triplicate with GAPDH used for
internal standardization. Oligonucleotide primers used for the
analysis of gene expression were purchased from Sangon Co. Ltd.
(Shanghai, China), as listed in Table
II.
 | Table IIOligonucleotide primers used in
study. |
Table II
Oligonucleotide primers used in
study.
| Primer | Sense strand | Antisense strand |
|---|
| GAPDH |
GCAAGTTCAACGGCACAG |
GCCAGTAGACTCCACGACAT |
| TGFβ2 |
TGCCATCCCGCCCACTTTCTAC |
CAATCCGTTGTTCAGCCACTCT |
Statistical analysis
All values are expressed as the means ± standard
deviation. All cell viability experiments were performed in 12
parallel wells. All transfection experiments were performed in
three parallel wells. An identical procedure was followed and
repeated three times. One way analysis of variance was used to
determine the significance of the differences in multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference. The software package used was GraphPad
Prism, version 5.0 (GraphPad Software, Inc, La Jolla, CA, USA).
Results
rAAV-based transfection in RPE-J cells
with US and/or contrast agent
Gene transfer by US
The US intensities and exposure times were set at
0.5 and 1 W/cm2, and at 30, 60 and 90 sec, respectively
(50% duty cycle). Under the conditions of 0.5 W/cm2 and
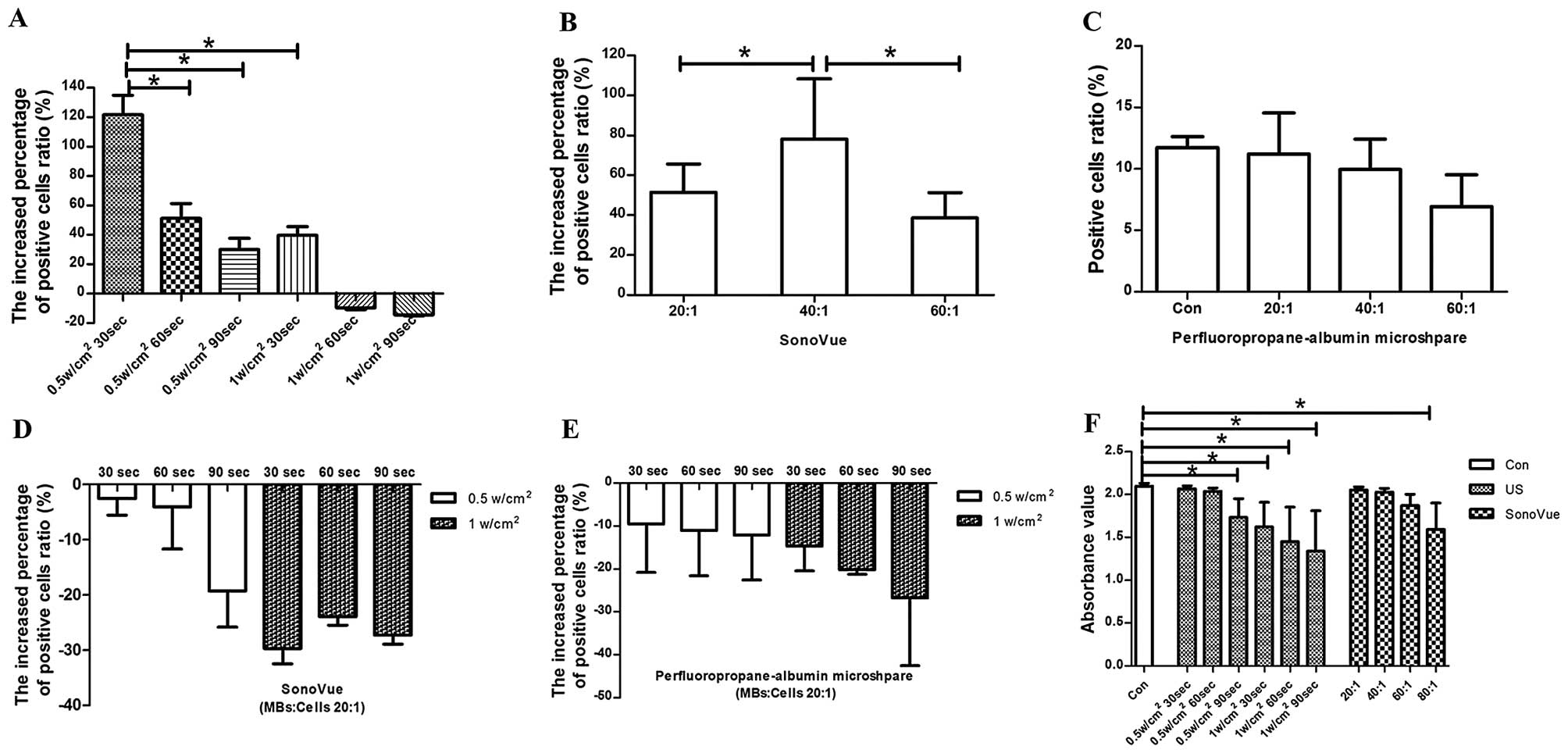
30 sec, the increased ratio of positive cells was significantly
higher than that of other groups (P<0.0001; Figs. 1A, 2A
and 2B). With US intensity of 1 W/cm2 and exposure
time of 60 and 90 sec, transfection efficiency decreased rather
than increased (Fig. 1A).
Gene transfer by SonoVue
The ratios of MBs to cells that we selected for our
study were 20:1, 40:1 and 60:1. With the ratio of 40:1, the
increased ratio of positive cells was significantly higher than
that observed in the 20:1 and 60:1 groups (P=0.011 and P<0.0001;
Figs. 1B, 2A and 2C).
Gene transfer by
perfluoropropane-albumin microspheres
The ratios of MBs to cells were 20:1, 40:1 and 60:1.
There was no difference between those groups in which cells were
treated with MBs and the control group (P>0.05, Fig. 1C).
Gene transfer by US/SonoVue or
US/perfluoropropane-albumin microspheres
With various combinations of the parameters
(intensity 0.5 and 1 W/cm2; exposure time 30, 60 and 90
sec; MB:cell ratio, 20:1) the ratios of positive cells in the UTMD
groups showed a decrease instead of an increase (Fig. 1D and E).
Cell viability assay
US intensity and exposure
duration
The US intensity was 0.5 or 1 W/cm2,
duration was 30, 60 or 90 sec and the duty cycle was 50%. Cell
viability decreased in sequence. Absorbance values under the
conditions of 0.5 W/cm2 and 90 sec, and 1
W/cm2 and 30, 60 or 90 sec were significantly lower than
those of the control group (P=0.003, P<0.0001, P<0.0001 and
P<0.0001, respectively; Fig.
1F).
Dosage of SonoVue
An MB:cell ratio of 80:1 was capable of
significantly damaging cells compared with the control group
(P<0.0001; Fig. 1F). There was
no difference between ratios of 20:1, 40:1 and 60:1 and the control
group (P>0.05).
rAAV2-TGFβ2 shRNA-EGFP-based
transfection in RPE-J cells with US or SonoVue and TGFβ2 gene
silencing evaluation
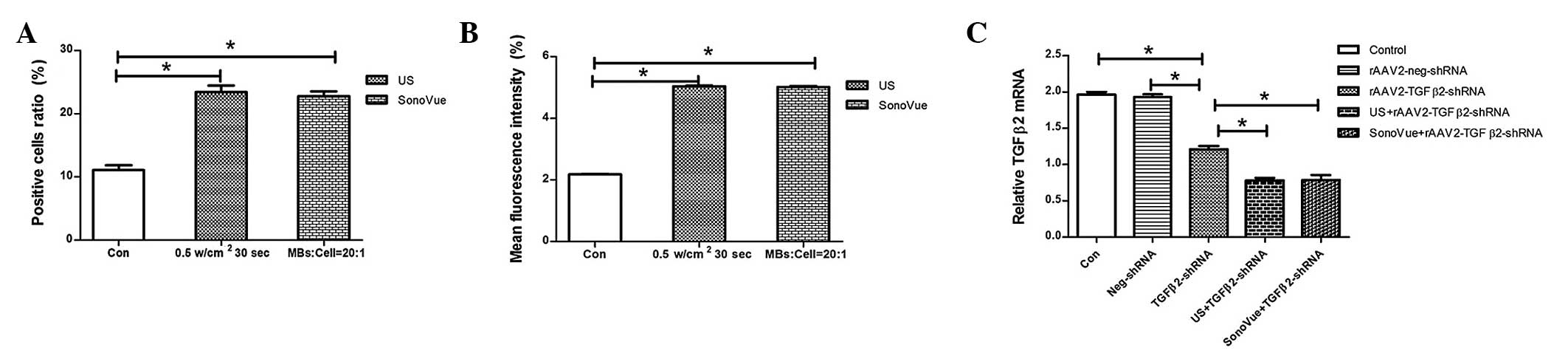
Under optimal conditions, both US (0.5
W/cm2 and 30 sec) and SonoVue (MB:cell ratio, 40:1)
significantly enhanced the rAAV2-TGFβ2 shRNA-EGFP ratio of positive
cells (P<0.0001 and P<0.0001; Fig. 3A) and mean fluorescence density
(P<0.0001 and P<0.0001; Fig.
3B) compared with those of the control group. As shown in
Fig. 3C, 48 h after transfection,
the relative TGFβ2 mRNA level in RPE-J cells of rAAV2-TGFβ2
shRNA-EGFP was markedly reduced compared with that of the control
group and rAAV2-neg shRNA-EGFP group (P<0.0001 and P<0.0001).
TGFβ2 gene silencing with a combination of US and rAAV2-TGFβ2
shRNA-EGFP resulted in a significant decrease in the mRNA
expression level compared with the rAAV2-TGFβ2 shRNA-EGFP alone
group (P<0.0001). TGFβ2 gene silencing with a combination of
SonoVue and rAAV2-TGFβ2 shRNA-EGFP also resulted in a significant
decrease in the mRNA expression level (P<0.0001). No significant
difference was observed between the control group and the
rAAV2-neg-shRNA-EGFP group (P>0.05). In addition, no significant
difference was observed between the US group and the SonoVue group
(P>0.05).
Discussion
The abnormal proliferation and migration of RPE
cells play a crucial role in the onset and development of PVR
(1). Several studies have
demonstrated that the inhibition of TGF-β has inhibitory effects on
the proliferation of RPE, the formation of ECM and collagen
contraction, and reduces the severity of PVR (11,12).
These studies also suggest that targeting of the TGFβ2 gene with a
siRNA in RPE cells has the potential to prevent the development of
PVR.
Researchers have considered RPE cells to be a
difficult cell type to transfect (13,14).
Adeno-associated virus (AAV) vectors have a number of significant
advantages over other vectors, in particular the ability to induce
long-term transgene expression in the eye and a relative lack of
pathogenicity. The identification of the AAV vector of RNAi
provides the premise for enhanced use of RNAi technology to silence
related genes in the treatment of ophthalmic diseases.
However, the transduction of AAV occurs with
relatively low efficiency, which limits its therapeutic effects.
Several methods to increase the transfection efficiency of AAV
vectors have been explored, including combinational use with an
adenovirus or a chemotherapy drug (7,8).
However, the above strategies have a number of limitations. A new
and improved method of delivery is required to improve AAV
infection with enhanced performance in RPE cells.
In 1987, Fechheimer et al firstly proposed
that US was able to promote gene transfection in vitro
(15). The mechanisms of
US-mediated transfection or UTMD enhancing gene transfection are
not clear. We performed a series of studies on UTMD enhancing gene
transfection in RPE cells (data not shown), including various
vectors (rAAV, plasmid, PEI, liposome), various genes (pDNA, siRNA)
and various cell lines (RPE-J and ARPE19 cells). Moreover, we
proved that UTMD could be used safely to enhance and accelerate
rAAV2-EGFP transgene expression of the retina (9). The goal of this feasibility study was
to assess whether US and/or MBs could not only facilitate the
delivery of rAAV2-EGFP but also downregulate TGFβ2 combined with
rAAV2-TGFβ2 shRNA in rat RPE cells.
First, we observed the efficiency (ratio of positive
cells) of UTMD combined with rAAV in driving transcription in RPE-J
cells. The results reveal that US increased rAAV transfection
efficiency. Under the conditions of 0.5 W/cm2 and 30
sec, the increased transfection efficiency was significantly higher
than that of other groups. Moreover, with an increase in US
intensity and exposure time, the transfection efficiency and cell
survival rate decreased. A ‘window’ of US intensity and exposure
time exists and affects gene transfer efficiency. Higher or lower
strength and longer or shorter exposure are not conducive to
reaching the peak of gene transfer efficiency. With an increase in
US intensity, the production probability of acoustic induced pores
increased. With a long exposure time, the biological effect of
cells increased, thereby affecting the cell survival rate. Under
the conditions of 0.5 W/cm2 and 30 sec, the energy was
sufficient for the formation of transient pores in RPE-J cells and
safe for cells.
The study demonstrated that rAAV combined with
perfluoropropane-albumin microspheres without US was unable to
increase rAAV transfection efficiency in RPE-J cells; however,
SonoVue was able to do this. The differences in the two types of
contrast agent were as follows: i) different composition of the
membranes: lipid-shelled vs. albumin-shelled; ii) different
components of wrapping gases: sulfur hexafluoride gas vs.
perfluoropropane gas; iii) different size of microbubbles: 2.5–6.0
mm vs. 3–4.5 μm. We also used two types of MBs combined with
rAAV-transfected ARPE19 cells, and neither SonoVue nor
perfluoropropane-albumin microspheres had a positive effect (data
not shown). RPE-J cells produced effective endocytosis of
phospholipid membranes using SonoVue. There are few studies on the
effect of different types of MBs on gene transfection efficiency.
One study, comparing the effect of different microbubble contrast
agents on gene transfection, demonstrated that Optison improved
pDNA transfection efficiency in the skeletal muscle of mice, but
SonoVue and Levovist did not (16). The nonspecific phagocytic ability
of cells may be the key factor in this notable phenomenon.
UTMD did not increase rAAV transfer to RPE-J cells
but led to higher cell damage. MBs acting as cavitation nuclei
effectively focus US energy and further potentiate bioeffects.
Under UTMD conditions, the energy became too much for RPE-J cells
and led to the high death rate of cells.
Whether US or MBs could damage RPE-J cells was also
evaluated. With the increase in US intensity and exposure time, the
survival rate of cells declined. High energy could lead to cell
membrane fatigue, so that the corresponding position of the cell
membrane damaged. Researchers have put most focus on whether US
exposure or US combined with MBs affected the survival rate of
cells. Few of them have paid attention to whether the US contrast
agent itself caused cell damage. In our study, with the increase in
MBs, the survival rate of cells declined. It is possible that the
increase in MB concentration affected the living environment of the
cell; for example, the osmotic pressure.
The combination of UTMD and rAAV should involve no
destruction to the rAAV activity. Our previous study reveals that,
under the UTMD conditions of intensity ≤3 W/cm2,
exposure time ≤120 sec, MB:cell ratio ≤50:1, frequency 1 MHz, 50%
duty cycle and pulse repetition frequency of 100 Hz, UTMD had no
apparent effect on the transfection ability of rAAV (9). Therefore, the parameters used in this
study were safe for rAAV and cells.
The preconditions for the realization of RNAi are
safe and efficient importation into the target cells, as well as
long-term stable effect. The first part of our study proved that US
or SonoVue facilitates rAAV delivery to RPE-J cells. The second
part of the study aimed to prove that US or SonoVue could
facilitate rAAV-TGFβ2 shRNA-EGFP delivery to RPE-J cells and
downregulate the expression of TGFβ2 mRNA efficiently. The
efficiency (ratio of positive cells and mean fluorescence density)
of US or SonoVue combined with rAAV-TGFβ2 shRNA-EGFP was observed
in driving transcription in RPE cells. US and SonoVue enhanced rAAV
transfection efficiency by increasing the transgene expression per
cell (an increase of 111.8 and 105.8%, respectively) and the
percentage of transfected cells (an increase of 131.2 and 129.8%,
respectively). Then RT-qPCR was performed to evaluate the relative
TGF mRNA levels in the groups. It was observed that the enhanced
TGFβ2 gene silencing under the condition of rAAV-TGFβ2 shRNA-EGFP
combined with US or SonoVue resulted in a significant decrease in
mRNA expression levels compared with rAAV-TGFβ2 shRNA treatment
alone.
The mechanism by which rAAV delivery efficiency is
enhanced by US and MBs is controversial (17,18).
Only two public papers have been identified from PubMed research on
this topic. One study suggested endocytosis as a mechanism that
contributed to UTMD-enhanced AAV delivery (18). The other indicated that US enabled
direct entry of the AAV vectors into the cytoplasm (17). In our study, SonoVue alone also
enhanced AAV transfection and assisted in gene silencing. Although
a study exists on the phenomenon (16), there are no studies on the
mechanism by which MBs alone enhance gene transfection efficiency.
The mechanism by which rAAV delivery efficiency is enhanced in
RPE-J cells by US or SonoVue is worthy of additional study.
In the present study, we demonstrated that
low-intensity US or an appropriate dose of SonoVue could be used
safely to increase the delivery efficiency of rAAV-TGFβ2 shRNA-EGFP
to RPE-J cells. A combination of the biological (rAAV-TGFβ2
shRNA-EGFP) and physical (US) approaches could more effectively
downregulate the mRNA expression of TGFβ2 than rAAV alone. RPE
cells in vivo were pentagonal or hexagonal, and the
morphology of RPE cells cultured in vitro was mainly
spindle. When the cells were cultured in vitro, they lost
neurohumoral regulation and the influence of adjacent cells, living
in an environment with an absence of dynamic balance. Thus, the
morphology and function would change to a certain extent. Further
studies are required to evaluate whether the delivery of rAAV-TGFβ2
shRNA to RPE cells in a rat model of PVR may be enhanced by US
and/or SonoVue.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81200700) and the Natural Science
Foundation of Shanghai (11ZR1421100).
References
|
1
|
Chen Z, Chen CZ, Gong WR, Li JP and Xing
YQ: Integrin-alpha5 mediates epidermal growth factor-induced
retinal pigment epithelial cell proliferation and migration.
Pathobiology. 77:88–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carrington L, McLeod D and Boulton M:
IL-10 and antibodies to TGF-β2 and PDGF inhibit RPE-mediated
retinal contraction. Invest Ophthalmol Vis Sci. 41:1210–1216.
2000.
|
|
3
|
Sugioka K, Kodama A, Okada K, et al:
TGF-β2 promotes RPE cell invasion into a collagen gel by mediating
urokinase-type plasminogen activator (uPA) expression. Exp Eye Res.
115:13–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee SY, Huh MS, Lee S, et al: Stability
and cellular uptake of polymerized siRNA (poly
siRNA)/polyethylenimine (PEI) complexes for efficient gene
silencing. J Control Release. 141:339–346. 2010. View Article : Google Scholar
|
|
5
|
Li Q, Dinculescu A, Shan Z, et al:
Downregulation of p22phox in retinal pigment epithelial cells
inhibits choroidal neovascularization in mice. Mol Ther.
16:1688–1694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Askou AL, Pournaras JA, Pihlmann M, et al:
Reduction of choroidal neovascularization in mice by
adeno-associated virus-delivered anti-vascular endothelial growth
factor short hairpin RNA. J Gene Med. 14:632–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang SH, Wu JH, Wu XB, et al: Distinctive
gene transduction efficiencies of commonly used viral vectors in
the retina. Curr Eye Res. 33:81–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu JH, Zhang SH, Wu XB, et al: Enhanced
transduction and improved photoreceptor survival of retinal
degeneration by the combinatorial use of rAAV2 with a lower dose of
adenovirus. Vision Res. 48:1648–1654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li HL, Zheng XZ, Wang HP, Li F, Wu Y and
Du LF: Ultrasound-targeted microbubble destruction enhances
AAV-mediated gene transfection in human RPE cells in vitro and rat
retina in vivo. Gene Ther. 16:1146–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng XZ, Wu Y, Li HL, Du LF, Wang HO and
Gu Q: Comparative analysis of the effects of ultrasound-targeted
microbubble destruction on recombinant adeno-associated virus-and
plasmid-mediated transgene expression in human retinal pigment
epithelium cells. Mol Med Rep. 2:937–942. 2009.PubMed/NCBI
|
|
11
|
Gamulescu MA, Chen Y, He S, et al:
Transforming growth factor beta2-induced myofibroblastic
differentiation of human retinal pigment epithelial cells:
regulation by extracellular matrix proteins and hepatocyte growth
factor. Exp Eye Res. 83:212–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oshima Y, Sakamoto T, Hisatomi T, et al:
Gene transfer of soluble TGF-beta type II receptor inhibits
experimental proliferative vitreoretinopathy. Gene Ther.
9:1214–1220. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sunshine JC, Sunshine SB, Bhutto I, Handa
JT and Green JJ: Poly (β-amino ester)-nanoparticle mediated
transfection of retinal pigment epithelial cells in vitro and in
vivo. PLoS One. 7:e375432012. View Article : Google Scholar
|
|
14
|
del Pozo-Rodriguez A, Delgado D, Solinis
MA, Gascon AR and Pedraz JL: Solid lipid nanoparticles for retinal
gene therapy: transfection and intracellular trafficking in RPE
cells. Int J Pharm. 360:177–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fechheimer M, Boylan JF, Parker S, Sisken
JE, Patel GL and Zimmer SG: Transfection of mammalian cells With
plasmid DNA by scrape loading and sonication loading. Proc Natl
Acad Sci. 84:8463–8467. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Liang HD, Dong B, Lu QL and
Blomley MJ: Gene transfer with microbubble ultrasound and plasmid
DNA into skeletal muscle of mice: comparison between commercially
available microbubble contrast agents. Radiology. 237:224–249.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Geers B, Lentacker I, Alonso A, et al:
Elucidating the mechanisms behind sonoporation with
adeno-associated virus-loaded microbubbles. Mol Pharm. 8:2244–2251.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin LF, Li F, Wang HP, Wei F, Qin P and Du
LF: Ultrasound targeted microbubble destruction stimulates cellular
endocytosis in facilitation of adeno-associated virus delivery. Int
J Mol Sci. 14:9737–9750. 2013. View Article : Google Scholar : PubMed/NCBI
|