Introduction
The Argonaute (Ago) proteins are defined by the
presence of Piwi and Piwi-Argnaute-Zwille domains (1), which are expressed in both
prokaryotic and eukaryotic organisms. In eukaryotes, Ago proteins
are most well known for their role in RNA silencing (2). In mammals there are eight genes
(3,4), which encode the Ago and Piwi
subfamilies (5,6). The human Piwi subfamily is comprised
of Hiwi1, Hiwi2, Hiwi3 and HILI, which are encoded for by genes on
chromosomes 12, 11, 22 and 8, respectively (7). Ago proteins, which are ubiquitously
expressed in numerous organisms, whereas in the majority of
organisms investigated so far, including humans, the expression of
Piwi proteins is restricted to the germ line, where they bind
Piwi-interacting RNAs (piRNAs) (2,5,6,8).
piRNAs are a class of small non-coding RNAs (ncRNAs), which are
involved in gene expression regulation. ncRNAs have been previously
identified in almost all eukaryotic species, including humans
(4,9,10).
In order to perform their effector functions, small ncRNAs must be
incorporated into Ago complexes, resulting in the formation of
highly specialized small-RNA-binding molecules which function in
RNA-silencing pathways.
The Piwi gene family is highly conserved and exerts
an essential role in stem cell self-renewal, gametogenesis, and RNA
interference (RNAi) in diverse organisms (11). Hiwi is involved in human germ cell
proliferation and maintenance, and the overexpression of the
molecule has been indicated as a cause of malignant testicular germ
cell tumors (11–13). The abnormal expression of Hiwi has
also been suggested to be associated with a poor prognosis in
numerous types of cancer, such as human pancreatic adenocarcinoma
(14), human esophageal squamous
cell carcinoma (15), colorectal
cancer (16) and human gastric
cancer (17). The alterations to
Hiwi mRNA expression has been previously reported to increase the
risk of tumour-related mortality in male patients with pancreatic
adenocarcinoma (14). The
cytoplasmic expression of Hiwi in esophageal cancer cells is
significantly associated with a higher histological grade, clinical
stage, and a poorer clinical outcome (15). PiwiL2 expression was shown to be
upregulated and significantly correlated with a lower degree of
differentiation, deep invasion and perineural invasion, in
colorectal cancer (16).
Furthermore, the overexpression of Hiwi in gastric cancer tissues
was shown to be similar to that of Ki-67, which is commonly used as
a marker of proliferation, and the suppression of Hiwi inhibited
the growth of gastric cancer cells and induced G2/M phase cell
cycle arrest (17). These previous
results suggest that Hiwi may be involved in the tumorigenesis of
various cancers, and may be a target for anticancer therapy.
It has been observed by immunohistochemical analysis
that Hiwi expression is significantly higher in human
hepatocellular carcinoma (HCC) tissue, as compared with adjacent
normal hepatic tissue (18). The
overexpression of intratumoral Hiwi has been associated with a
larger tumor size or intrahepatic metastasis, and was also shown to
be an independent risk factor for overall and recurrence-free
survival (19). These reports
indicate that Hiwi may have a crucial role in the carcinogenesis of
human HCC, and could serve as a potential biomarker or treatment
target for HCC. In the present study, the overexpression of Hiwi
was determined in HCC specimens, and in MHCC97L and MHCC97H HCC
cell lines. In addition, a lentivirus-mediated small hairpin RNA
(shRNA) targeting Hiwi was constructed and used to knockdown Hiwi
expression, in order to investigate the influence of Hiwi on cancer
cell proliferation and migration. It has been implied that Hiwi may
have an important role in HCC progression, and it could be a
potential target for anticancer therapy.
Materials and methods
HCC specimens, cell lines and culture
conditions
A total of 60 intratumor and 48 peritumor specimens
(used as a control; >10 mm from the tumor edge) were resected
from HCC patients. The patients were selected according to the
pathological archives from the China-Japan Union Hospital, Jilin
University (Jilin, China) between June 2006 and March 2010. All
patients provided informed consent prior to the experiment. The
fresh tissue specimens were immediately frozen in liquid nitrogen
and stored at −80°C post-resection, prior to being exposed to
radiotherapy and chemotherapy. Clinical pathological data for each
patient was available from the clinical records, and all of the
information was assessed independently by three specialists. The
present study was approved by the Medical Ethics Committee of
China-Japan Union Hospital, Jilin University. MHCC97L, MHCC97H and
HepG2 HCC cell lines, and the L02 normal hepatic cell line, were
purchased from Shanghai Bioleaf Biotech Co., Ltd. (Shanghai,
China). The cells were cultured at 37°C in a humidified atmosphere
of 5% CO2 in Dulbecco’s Modified Eagle’s medium (DMEM;
Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with
10% fetal bovine serum (FBS; Gibco-BRL, Rockville, MD, USA).
RNA isolation and quantitative polymerase
chain reaction (qPCR)
Total RNA from the HCC cell lines and tissue
specimens was extracted using the RNeasy® Plus Mini kit
(Qiagen, Valencia, CA, USA), according to the manufacturer’s
instructions. qPCR (reverse transcription reaction, 42°C for 5 min
and 95°C for 10 sec; PCR reaction, 95°C for 5 sec and 60°C for 20
sec for 40 cycles) was performed using a SYBR PrimeScript reverse
transcription-qPCR kit (Takara Biotechnology Inc., Dalian, China)
according to the manufacturer’s instructions, and β-actin was used
as an internal control. The primers for Hiwi and β-actin were
synthesized by Sangon Biotech (Shanghai, China), according to
previously reported sequences (19). The qPCR was performed with a
Lightcycler 480 II (Roche Diagnostics GmbH, Mannheim, Germany). The
data were normalized to β-actin and expressed as the fold change
over control, and calculated using the ΔΔCt method (20).
Western blot analysis
HCC specimens for western blot analysis were
homogenized prior to protein isolation. The homogenized HCC
specimens and the HCC cultured cells were collected and lysed with
ProteoJET™ Mammalian Cell Lysis reagent (Fermentas, Burlington, ON,
Canada), according to the manufacturer’s instructions. The resolved
proteins were supplemented with a protease inhibitor cocktail
(Complete Mini protease inhibitor cocktail; Roche Diagnostics
GmbH). The protein samples were then separated by SDS-PAGE
(Sigma-Aldrich, St. Louis, MO, USA), and were transferred to
polyvinylidene fluoride membranes (Pierce, Rockford, IL, USA),
which were blocked in 5% skimmed milk for 1 h at room temperature.
The membranes were then incubated with either a 1:1,000 dilution
Hiwi or a 1:3,000 dilution β-actin rabbit polyclonal antibody
(Santa Cruz Biotechnology, Santa Cruz, CA, USA), at 4°C overnight.
The membrane was then incubated with a peroxidase-conjugated
secondary antibody (1:106; Sigma-Aldrich) for 1 h at
room temperature. The blots were detected using the Enhanced
Chemiluminescence Detection system (Amersham, Uppsala, Sweden),
according to the manufacturer’s instructions, and the Hiwi level
was quantified via the relative gray value of the target band.
Lentivirus-mediated shRNA knockdown of
Hiwi expression
The shRNAs targeting Hiwi (reference sequence,
AF104260) were designed (9) by
Ambion® (Life Technologies, Carlsbad, CA, USA), and a
nonsense sequence was used as a control. The two shRNA-Hiwi
sequences and one control sequence were confirmed using Basic Local
Alignment Search Tool (BLAST) to a targeted sequence. The 9-nt
hairpin sequence (TCAAGACG) was not homologous to Hiwi and was
inserted between two shRNA-Hiwi sequences, TTTTTT, followed the
antisense strand of shRNA, and acted as a termination sequence.
BamHI and HindIII (Takara Biotechnology, Inc., Tokyo,
Japan) were used as restriction endonucleases. cDNA
oligonucleotides were synthesized and cloned into the
lentivirus-based vector pGCSIL-GFP (GeneChem Co. Ltd., Shanghai,
China). Lentiviruses were generated in HEK293T human embryonic
kidney cells (American Type Culture Collection, Rockville, MD, USA)
by co-transfection of each recombinant plasmid with a pHelper
plasmid (GeneChem Co. Ltd.). HEK293T cells were cultured at 37°C in
a humidified atmosphere of 5% CO2 in DMEM with 10% FBS.
The viral titer was quantitatively determined by counting the
number of green fluorescent protein-positive cells post-viral
infection, under a fluorescence microscope (Olympus, Tokyo, Japan).
For lentivirus transduction, MHCC97L and MHCC97H cells were
subcultured at 1×104 cells/well in 12-well culture
plates. Once the cells had reached 70% confluence, they were
transduced with the shRNA-harboring lentiviruses at a multiplicity
of infection of 10. The cells were propagated under G418 (1.5
mg/ml; Sigma-Aldrich) selection pressure.
Cell count and colony formation
assays
A cell count assay was performed as described by
previous methods (21). Briefly,
the cells were plated in 12-well plates and incubated at 37°C for
different periods of time, the cells were then trypsinized and the
number of viable cells was counted using a hemocytometer with
trypan blue staining. A total of 100 cells were seeded into a
12-well plate. After 10 days, the cells were stained with 0.5%
crystal violet (Sigma-Aldrich) in methanol for 10 min. The colonies
(>50 μm in diameter) were counted directly on the plate.
Migration and invasion assay
For the scratch assay, the cells were cultivated to
85% confluence on 12-well plates and then scratched with a 200 μl
pipette tip. The cellular growth was observed at 0 and 48 h
post-scratch. For the invasion assay, Matrigel-coated Transwell
migration chambers (Corning Costar, Cambridge, MA, USA) were used.
The cells were seeded at a density of 5×104 cells/well,
in serum-free media, in the upper chamber with the non-coated
membrane (8 μm pore size; Millipore, Zug, Switzerland). The lower
chamber contained media supplemented with 20% FBS, as a
chemoattractant. The cells in the upper chamber were discarded,
using cotton wool, following a 24 h incubation; the cells which had
migrated into the lower chamber were counted using a light
microscope.
Statistical analyses
All data are expressed as the means ± standard error
of the mean. The comparisons between two groups were conducted
using a Student’s t-test. Statistical analyses were performed using
GraphPad Prism® software version 5.0 (GraphPad Software,
La Jolla, CA, USA). A P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of Hiwi in hepatocellular
carcinoma specimens and cell lines
In order to identify a possible pro-oncogenic role
of Hiwi in HCC, the expression levels of Hiwi were determined in
both the HCC specimens and cell lines. Relative Hiwi mRNA
expression levels, determined by qPCR, were significantly
upregulated in intratumor specimens (n=60; 2.724 ± 0.185), as
compared with the peritumor specimens (n=48; 1.000 ± 0.064)
(Fig. 1A) (P<0.01). Relative
Hiwi mRNA expression levels were also found to be significantly
upregulated in MHCC97L and MHCC97H HCC cell lines, as well as
HepG2, as compared with the L02 cells (Fig. 1B; P<0.01 and P<0.05,
respectively). Relative Hiwi protein expression levels were
determined by western blot analysis. Fig. 1C shows the upregulation of Hiwi
protein expression in the intratumor specimens, as compared with
the peritumor specimens (P<0.01). Figure 1D also indicates that there was a
significant upregulation of Hiwi protein expression levels in
MHCC97L, MHCC97H and HepG2 cells, as compared with L02 cells
(P<0.01 and P<0.05, respectively). These results provide
further evidence that Hiwi expression is upregulated in HCC tissue
specimens and cell lines.
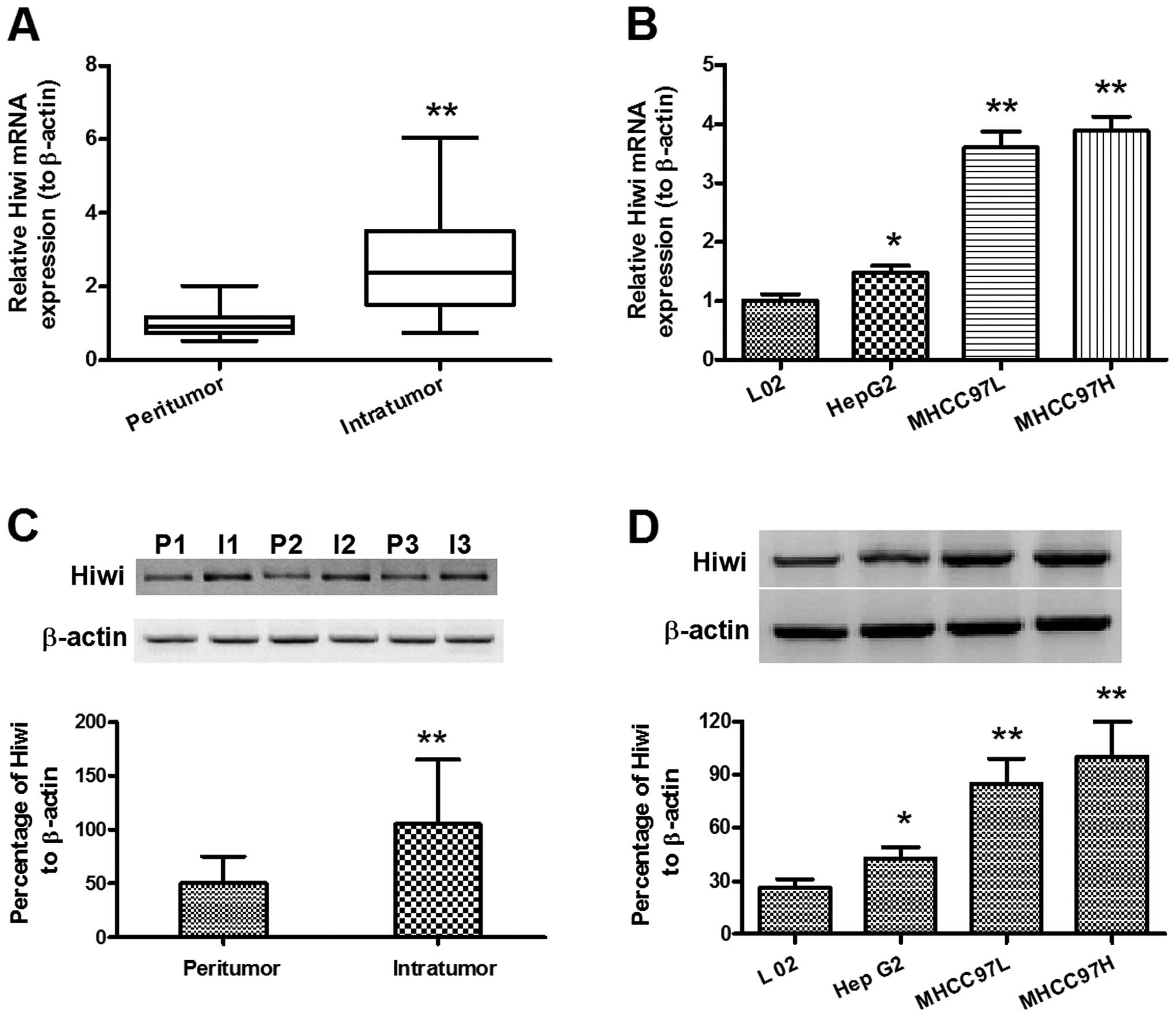 | Figure 1Overexpression of Hiwi in
hepatocellular carcinoma specimens and cell lines. (A) Relative
mRNA expression levels of Hiwi in the intratumor (n=60) and
peritumor (n=48) tissues of hepatocellular carcinoma (HCC), as
determined by quantitative polymerase chain reaction. (B) Relative
mRNA expression levels of Hiwi in HepG2, MHCC97L, and MHCC97H HCC
cell lines, as compared with L02, normal hepatic cells. (C)
Overexpression of relative Hiwi protein expression levels in the
intratumor (n=42) and peritumor (n=27) tissues of HCC, as
determined by western blot analysis. (D) Overexpression of relative
Hiwi protein expression levels in HepG2, MHCC97L, and MHCC97H HCC
cell lines, as compared with L02, normal hepatic cells, as
determined by western blot analysis. The data represent the means ±
standard error of the mean. *P<0.05,
**P<0.01. I, intratumor; P, peritumor. |
Hiwi knockdown in MHCC97L and MHCC97H HCC
cell lines, by lentivirus-mediated RNAi
To further identify the pro-oncogenic role of Hiwi
in HCC, Hiwi expression was knocked down by lentivirus-mediated
shRNA. The recombinant lentiviruses (Lenti-Hiwi-shRNA-1 and
Lenti-Hiwi-shRNA-2) and the control lentivirus (Lenti-shRNA-Con)
were constructed with pRNAT-U6.1 vector, the cDNA sequence of
Hiwi-shRNA-1, Hiwi-shRNA-2 or the control were inserted into the
multiple cloning site, between the U6 and CMV promoters (Fig. 2A). The lentiviruses were packaged
by transfecting the recombinant plasmid into HEK293T cells. The
MHCC97L and MHCC97H cells were then transduced with the lentivirus
(Lenti-Hiwi-shRNA-1, Lenti-Hiwi-shRNA-2, or Lenti-shRNA-Con), and
the cells were selected for by G418 selection pressure, following
three serial passages. The relative Hiwi mRNA and protein
expression levels were determined in both cell lines, transduced
with either shRNA-Hiwi or shRNA-Con, by qPCR and western blot
analysis. As shown in Fig. 2B and
C, the Hiwi mRNA expression levels in both cell lines were
significantly reduced when transduced with either shRNA-Hiwi-1 or
shRNA-Hiwi-2, as compared with shRNA-Con, post 24 h growth
(P<0.05 and P<0.01, respectively). Western blot analysis also
indicated reduced Hiwi protein expression levels in both cell lines
post 48 h growth (Fig. 2D and E).
These results indicated that lentivirus-mediated RNAi could
efficiently and specifically suppress Hiwi expression in both
MHCC97L and MHCC97H HCC cells.
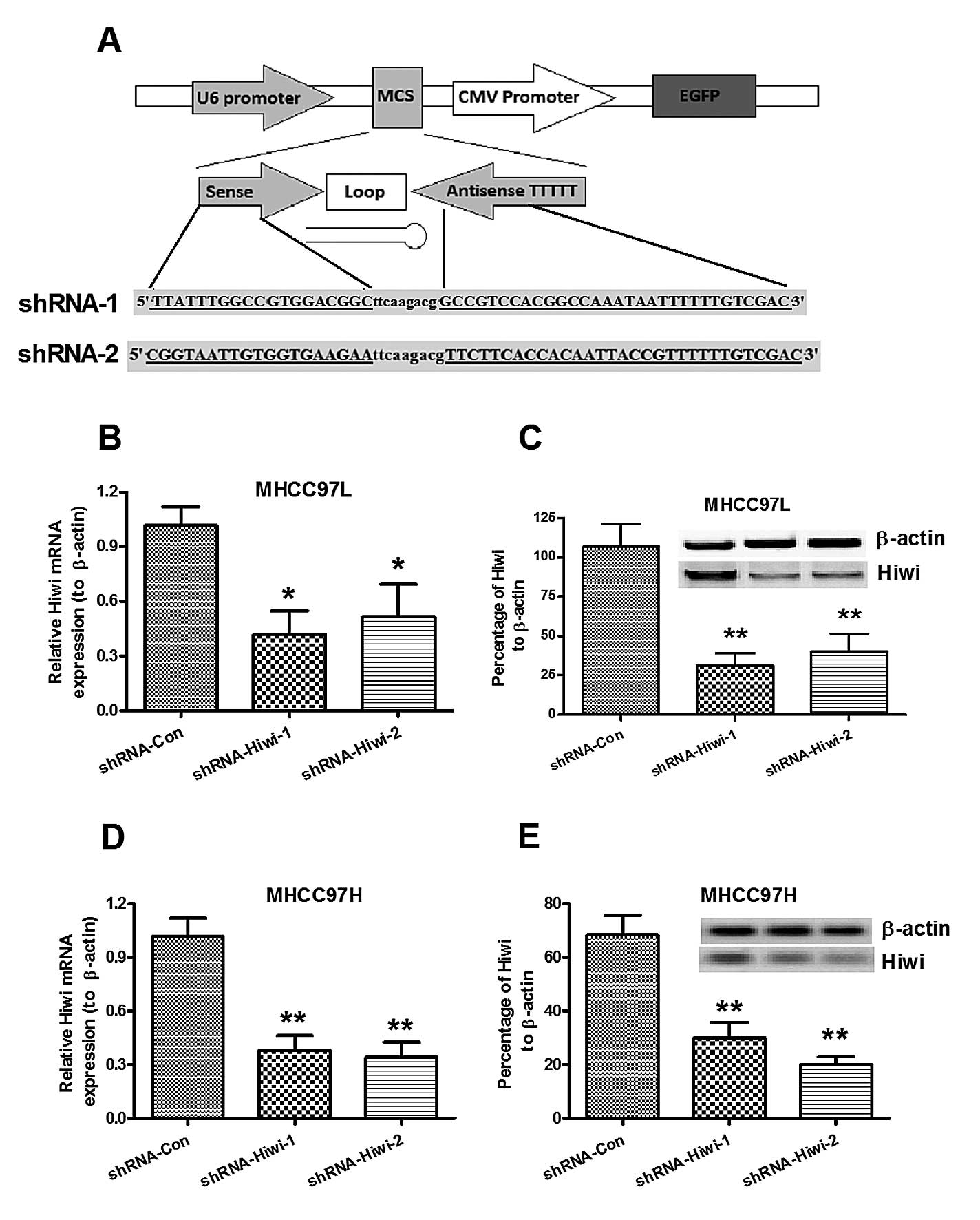 | Figure 2Suppression of Hiwi expression in
MHCC97L and MHCC97H hepatocellular carcinoma (HCC) cell lines,
mediated by lentivirus-mediated RNA interference. (A) Schematic
diagram of the lentivirus vector, and structure of the cDNA
template of the small hairpin RNA (shRNA) targeting Hiwi. The
length of the palindrome sequence (19 or 20 nucleotide sense strand
and antisense strand) was separated by a 9 nucleotide spacer,
followed by four continuous thymines (T), as a termination signal.
Two restriction enzymes (BamHI and XhoI) were added
to the two termination ends. (B and C) Relative Hiwi mRNA
expression levels in (B) MHCC97L or (C) MHCC97H HCC cells 24 h
post-infection of lentivirus, expressing control or Hiwi shRNA, as
determined by quantitative polymerase chain reaction. (D and E)
Relative Hiwi protein expression levels in (C) MHCC97L or (E)
MHCC97H HCC cells 48 h post-infection of lentivirus, expressing
control or Hiwi shRNA, as determined by western blot analysis.
β-actin was used as an internal control. The data represent the
means ± standard error of the mean of three independent
experiments. *P<0.05, **P<0.01,
compared to the control group. CMV, cytomegalovirus; MCS, multiple
cloning site; EGFP, enhanced green fluorescent protein. |
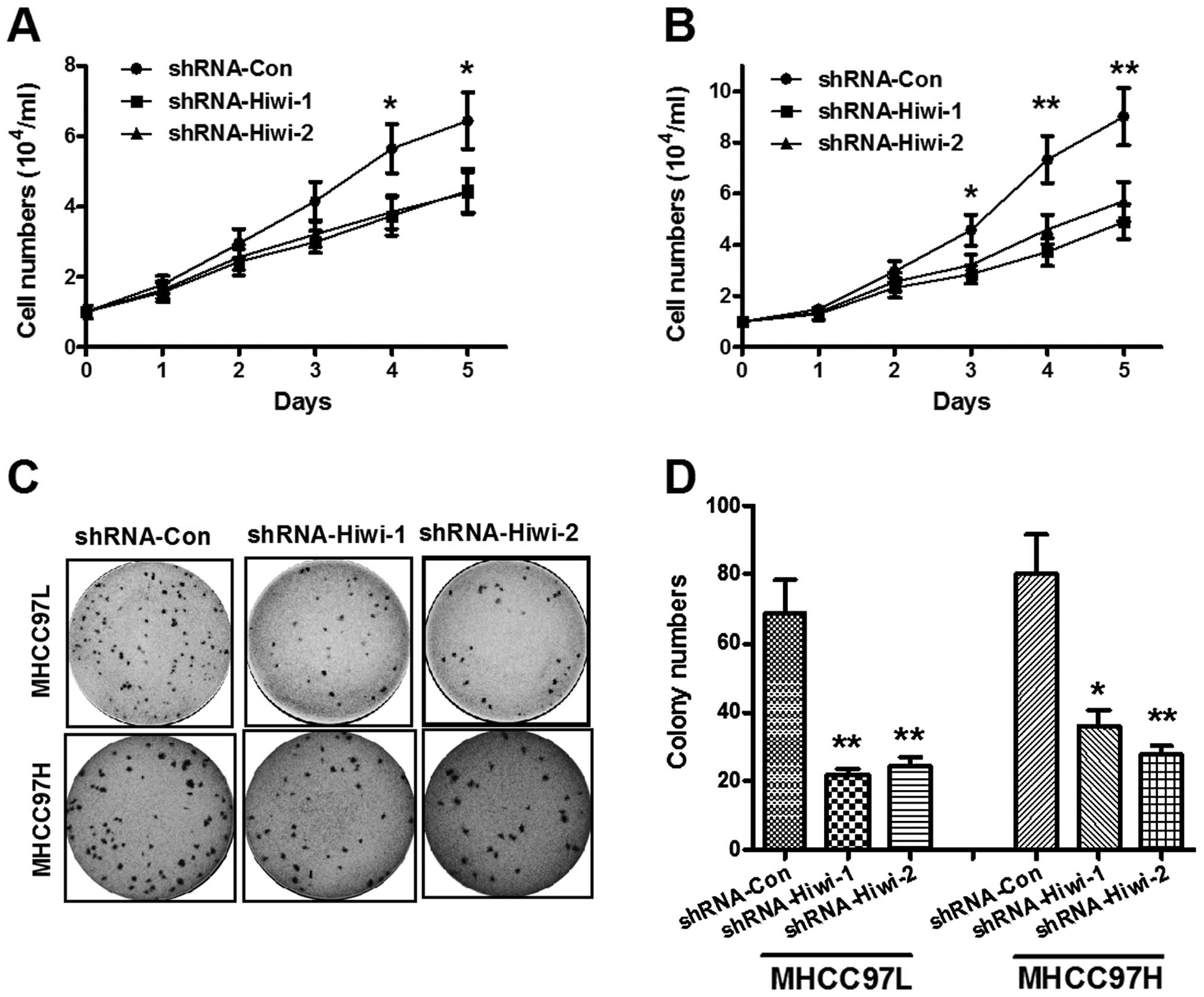
Hiwi knockdown inhibits HCC cell
proliferation in vitro
The effects of Hiwi expression knockdown on the
growth of HCC cells were assessed. The growth of HCC cells in
vitro was measured by cell count and colony formation assays.
The cell count assay was performed daily for five days. As shown in
Fig. 3A, Hiwi silencing inhibited
MHCC97L cell proliferation in a time-dependent manner. As compared
with the control shRNA group, the cell numbers in the
Hiwi-shRNA-1/2 groups (3.72 ± 0.54×104 and 3.84 ±
0.48×104, respectively) were significantly reduced four
days post-inoculation as compared with the control group (5.64 ±
0.70×104) (P<0.05), and five days post-inoculation:
4.45 ± 0.62×104 for shRNA-Hiwi-1 and 4.38 ±
0.59×104 for shRNA-Hiwi-2, as compared with the control
(6.43 ± 0.82×104) (P<0.05). The reduction of Hiwi
protein expression levels was more significant in the MHCC97H
cells. Fig. 3B demonstrated that
from three days post-inoculation, both Hiwi-shRNAs significantly
inhibited HCC cell growth (P<0.05); the growth rate was more
significantly reduced four and five days post-inoculation in
response to both of the Hiwi-shRNAs (P<0.01). Furthermore, the
colony forming abilities of both MHCC97L and MHCC97H cells
transduced with either control shRNA or Hiwi-shRNA1/2 lentivirus,
was evaluated. As shown in Fig. 3C and
D, the colony numbers of MHCC97L or MHCC97H cells in the
Hiwi-shRNA-1 and Hiwi-shRNA-2 groups were significantly reduced, as
compared with the control shRNA group (P<0.01). These results
provide further evidence of the role of Hiwi in the promotion of
HCC cellular growth.
Hiwi knockdown decreases cell migration
and invasion of HCC cell growth in vitro
It is well known that cell migration is responsible
for tumor metastasis (22). To
further identify the pro-oncogenic function of Hiwi, the
differences in the migration processes of MHCC97L and MHCC97H cells
transduced with either control shRNA or Hiwi-shRNA-1 or 2 was
determined by scratch assay. The MHCC97L or MHCC97H cells
transduced with Hiwi-shRNA-1 or 2 exhibited significantly slower
migration, as compared with the cells transduced with control shRNA
(Fig. 4A and B) (P<0.01 and
P<0.05, respectively). Furthermore, the invasive capabilities of
the cells was determined using a Matrigel-coated Transwell assay,
the invasive cell number was quantified in Fig. 4C. Consistent with the findings of
the scratch assay, MHCC97L and MHCC97H cells transduced with
Hiwi-shRNA1 or 2 had a significant reduction in cell invasive
ability, as compared with the control shRNA-treated cells. These
results indicated that Hiwi knockdown by shRNA may reduce the
migration of HCC cells in vitro.
 | Figure 4Hiwi silencing decreases cell
migration and invasion of hepatocellular carcinoma (HCC) cells
in vitro. (A) Migration of both MHCC97L and MHCC97H HCC
cells, after Hiwi expression was silenced by RNA interference, by
cell scratch assay. Cell migration was observed 0 and 48 h
post-scratch. Solid lines show the edges of the wound at the start
of experiments. (B) The migration was calculated by the rate of
cells filling the scratched area (left three columns, MHCC97L
cells; right three columns, MHCC97H cells). (C) Cell invasion was
determined by Matrigel-coated Transwell assay. The cells that
crossed the Matrigel-coated filter were fixed, stained and counted
(left three columns, MHCC97L cells; right three columns, MHCC97H
cells). The data represent the means ± standard error of the mean
of three independent experiments. Statistical significance was
shown as *P<0.05, **p<0.01. D, day;
shRNA, small hairpin RNA. D, day; Con, control. |
Discussion
HCC is one of the most common types of cancer and is
characterized by high malignancy, particularly in Africa and Asia,
where it is the second most common cause of cancer mortality in
China (23), with ~600,000 deaths
each year worldwide (24). The
predisposing factors for HCC include chronic infection with
hepatitis B and C virus, alcohol abuse, and aflatoxin intake
(25,26), which all may induce cirrhosis,
posing the highest risk of HCC development; 80% of HCCs develop
from cirrhotic livers (27). The
activation of oncogenes and inactivation of tumor suppressor genes
have been identified as being associated with carcinogenesis and
the progression of HCC. Numerous genes have been identified which
are differentially expressed in HCC tumor tissue, as compared with
paratumor tissue, which are oncogenic or tumor suppressive. These
genes include IGF2, FAT10, SCARA5, DLK1, p53 and Zinc finger
protein 267 (28–33). Previously, Hiwi has been indicated
as being significantly overexpressed in human HCC tissue, as
compared with adjacent normal hepatic tissue (18). Hiwi has also been shown to be an
independent risk factor for overall survival and recurrence-free
survival rates (19) in patients
with HCC. These results imply that Hiwi may be an oncogenic
regulator of human HCC.
In the present study, the overexpression of Hiwi in
HCC specimens and cell lines was confirmed, at both the mRNA and
protein expression levels. Both qPCR and western blot analysis
revealed a significantly upregulated level of Hiwi expression in
intratumor specimens, as compared with peritumor specimens. Hiwi
overexpression was also identified in MHCC97L and MHCC97H HCC cell
lines, as well as HepG2, as compared with the L02 normal hepatic
cells. To further identify the role of Hiwi in HCC, a
lentivirus-mediated shRNA was used to knockdown Hiwi expression.
The influence of the Hiwi knockdown was determined on the
proliferation and migration of HCC cells. The results demonstrated
that the Hiwi expression in both MHCC97L and MHCC97H cells was
significantly reduced following Hiwi-specific shRNA transduction,
at both the mRNA and protein level. These results indicated that
lentivirus-mediated shRNA may efficiently and specifically suppress
Hiwi expression in both MHCC97L and MHCC97H cells.
The influence of the Hiwi knockdown on the
proliferation and migration of HCC cells was evaluated. The cell
count assay showed that cell proliferation was significantly
reduced, in a time-dependent manner, in MHCC97L and MHCC97H cells
in the shRNA-Hiwi-1 or 2 groups, as compared with cells in the
shRNA-Con group. The colony formation assay also indicated that the
colony numbers of MHCC97L or MHCC97H cells in both the Hiwi-shRNA-1
and Hiwi-shRNA-2 groups were significantly lower, as compared with
the shRNA-Con group. These results indicated that the
shRNA-mediated Hiwi knockdown, resulted in growth inhibition of the
HCC cells. The influence of Hiwi knockdown on the cell migration
and invasion of HCC cells was determined by scratch and Transwell
assays. The results demonstrated that the Hiwi knockdown in MHCC97L
or MHCC97H cells, by Hiwi-shRNA-1 or 2, significantly reduced the
migratory capacity of both cells. Hiwi-shRNA1 or 2 transduction
also significantly reduced the invasiveness of MHCC97L and MHCC97H
cells. These results indicated that Hiwi knockdown by shRNA reduced
the migratory capacity of HCC cells in vitro.
In conclusion, the present study confirmed the
overexpression of Hiwi in HCC specimens and cell lines, at both the
mRNA and protein expression levels. It was also identified that
Hiwi expression knockdown, in MHCC97L and MHCC97H HCC cell lines,
by lentivirus-mediated RNAi resulted in inhibition of cellular
growth, migration and invasion in vitro. The results of the
present study implied that Hiwi has an oncogenic role in HCC.
References
|
1
|
Bohmert K, Camus I, Bellini C, Bouchez D,
Caboche M and Benning C: AGO1 defines a novel locus of Arabidopsis
controlling leaf development. EMBO J. 17:170–180. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hall TM: Structure and function of
argonaute proteins. Structure. 13:1403–1408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carmell MA, Xuan Z, Zhang MQ and Hannon
GJ: The Argonaute family: tentacles that reach into RNAi,
developmental control, stem cell maintenance, and tumorigenesis.
Genes Dev. 16:2733–2742. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meister G and Tuschl T: Mechanisms of gene
silencing by double-stranded RNA. Nature. 431:343–349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hutvagner G and Simard MJ: Argonaute
proteins: key players in RNA silencing. Nat Rev Mol Cell Biol.
9:22–32. 2008. View
Article : Google Scholar
|
|
6
|
Peters L and Meister G: Argonaute
proteins: mediators of RNA silencing. Mol Cell. 26:611–623. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Höck J and Meister G: The Argonaute
protein family. Genome Biol. 9:2102008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Houwing S, Kamminga LM, Berezikov E, et
al: A role for Piwi and piRNAs in germ cell maintenance and
transposon silencing in Zebrafish. Cell. 129:69–82. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elbashir SM, Harborth J, Lendeckel W,
Yalcin A, Weber K and Tuschl T: Duplexes of 21-nucleotide RNAs
mediate RNA interference in cultured mammalian cells. Nature.
411:494–498. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sana J, Faltejskova P, Svoboda M and Slaby
O: Novel classes of non-coding RNAs and cancer. J Transl Med.
10:1032012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiao D, Zeeman AM, Deng W, Looijenga LH
and Lin H: Molecular characterization of hiwi, a human member of
the piwi gene family whose overexpression is correlated to
seminomas. Oncogene. 21:3988–3999. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Skotheim RI, Kraggerud SM, Fossa SD, et
al: Familial/bilateral and sporadic testicular germ cell tumors
show frequent genetic changes at loci with suggestive linkage
evidence. Neoplasia. 3:196–203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Summersgill B, Osin P, Lu YJ, Huddart R
and Shipley J: Chromosomal imbalances associated with carcinoma in
situ and associated testicular germ cell tumours of adolescents and
adults. Br J Cancer. 85:213–220. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grochola LF, Greither T, Taubert H, et al:
The stem cell-associated Hiwi gene in human adenocarcinoma of the
pancreas: expression and risk of tumour-related death. Br J Cancer.
99:1083–1088. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He W, Wang Z, Wang Q, et al: Expression of
HIWI in human esophageal squamous cell carcinoma is significantly
associated with poorer prognosis. BMC Cancer. 9:4262009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oh SJ, Kim SM, Kim YO and Chang HK:
Clinicopathologic implications of PIWIL2 expression in colorectal
cancer. Korean J Pathol. 46:318–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Sun Y, Guo J, et al: Expression of
hiwi gene in human gastric cancer was associated with proliferation
of cancer cells. Int J Cancer. 118:1922–1929. 2006. View Article : Google Scholar
|
|
18
|
Jiang J, Zhang H, Tang Q, Hao B and Shi R:
Expression of HIWI in human hepatocellular carcinoma. Cell Biochem
Biophys. 61:53–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao YM, Zhou JM, Wang LR, et al: HIWI is
associated with prognosis in patients with hepatocellular carcinoma
after curative resection. Cancer. 118:2708–2717. 2012. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Li Z, Tian T, Lv F, et al: Six1 promotes
proliferation of pancreatic cancer cells via upregulation of cyclin
D1 expression. PLoS One. 8:e592032013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu C, Liu S, Fu H, et al: MicroRNA-193b
regulates proliferation, migration and invasion in human
hepatocellular carcinoma cells. Eur J Cancer. 46:2828–2836. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aravalli RN, Steer CJ and Cressman EN:
Molecular mechanisms of hepatocellular carcinoma. Hepatology.
48:2047–2063. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schafer DF and Sorrell MF: Hepatocellular
carcinoma. Lancet. 353:1253–1257. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thorgeirsson SS and Grisham JW: Molecular
pathogenesis of human hepatocellular carcinoma. Nat Genet.
31:339–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iizuka N, Oka M, Yamada-Okabe H, et al:
Comparison of gene expression profiles between hepatitis B virus-
and hepatitis C virus-infected hepatocellular carcinoma by
oligonucleotide microarray data on the basis of a supervised
learning method. Cancer Res. 62:3939–3944. 2002.PubMed/NCBI
|
|
29
|
Oliva J, Bardag-Gorce F, French BA, et al:
Fat10 is an epigenetic marker for liver preneoplasia in a
drug-primed mouse model of tumorigenesis. Exp Mol Pathol.
84:102–112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang J, Zheng DL, Qin FS, et al: Genetic
and epigenetic silencing of SCARA5 may contribute to human
hepatocellular carcinoma by activating FAK signaling. J Clin
Invest. 120:223–241. 2010. View
Article : Google Scholar :
|
|
31
|
Huang J, Zhang X, Zhang M, et al:
Up-regulation of DLK1 as an imprinted gene could contribute to
human hepatocellular carcinoma. Carcinogenesis. 28:1094–1103. 2007.
View Article : Google Scholar
|
|
32
|
Okada T, Iizuka N, Yamada-Okabe H, et al:
Gene expression profile linked to p53 status in hepatitis C
virus-related hepatocellular carcinoma. FEBS Lett. 555:583–590.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schnabl B, Valletta D, Kirovski G and
Hellerbrand C: Zinc finger protein 267 is up-regulated in
hepatocellular carcinoma and promotes tumor cell proliferation and
migration. Exp Mol Pathol. 91:695–701. 2011. View Article : Google Scholar : PubMed/NCBI
|