Introduction
Colorectal cancer (CRC) is a prevalent type of
cancer, which has a high mortality rate worldwide (1). In Europe and the USA, CRC is the
third most common type of human cancer and the second leading cause
of cancer-associated mortality (2,3). In
China, the incidence of CRC has risen steadily over the last few
decades, with increasing morbidities in younger patients (<50
years) (4). Recent cancer
statistics have indicated that CRC accounts for ~9% of all
cancer-associated mortalities (5).
The survival rate of CRC is higher with earlier diagnoses followed
by treatment with surgical resection; however, the long-term
survival and prognosis of the patients at stages III and IV remain
poor (6). Genes associated with
mutations in TP53 (7),
KRAS (8,9) and BRAF (9,10) as
well as defective DNA mismatch repair (11) have been investigated for their
prognostic and predictive value in CRC; however, the application of
these markers requires validation in clinical practice and further
evaluation. Sensitive biomarkers enable an early diagnosis and
prognosis prediction; therefore, novel factors for predicting tumor
recurrence and prognosis following surgery are urgently
required.
The Wnt signaling pathway and its downstream
components have a role in the regulation of cancer progression
through numerous processes, including tumor initiation, tumor
growth, cell senescence, cell death, differentiation and metastasis
(12). The Wnt signaling pathway
molecule Nemo-like kinase (NLK) is a member of the
extracellular-signal regulated kinase/mitogen-activated protein
kinase (MAPK) and cyclin-dependent kinase families (13). NLK was reported to induce apoptosis
and inhibit androgen receptor-mediated transcriptional activity in
prostate cancer cells (14).
However, NLK also contributes to tumor growth via the activation of
cell cycle proteins cyclin D1 and cyclin-dependent kinase 2 in
human hepatocellular carcinoma (15). NLK was also demonstrated to induce
apoptosis in glioma cells via activation of caspases (16). These previous studies have
indicated that NLK may be a critical regulator of tumor growth and
development. In the present study, reverse transcription
quantitative polymerase chain reaction (RT-qPCR) and
immunohistochemical analysis were used to determine whether there
was an association between NLK expression and the clinical outcome
of CRC patients.
Materials and methods
Tissue specimens and patient
information
A total of 406 clinical specimens were collected
from the medical records of patients with CRC who underwent surgery
at the Department of Gastrointestinal Surgery of Qianfoshan
Hospital of Shandong Province and the Department of Digestive
Diseases of Shandong Provincial Hospital Affiliated to Shandong
University (Shandong, China). All specimens were archived under
protocols approved by the institutional review boards of Shandong
University and written informed consent was obtained from the
patients. The group was composed of 172 males and 234 females with
a mean±standard error of the mean age of 64.8±17.1 (range, 23–91)
years. The diagnoses were confirmed by two pathologists and based
on the tumor, node, metastasis classification system: 48 cases at
stage I, 162 cases at stage II, 160 cases at stage III and 36 cases
at stage IV. Among these patients, 132 had lymph node metastases
(LNM). The follow-up of CRC patients post-surgery was performed
according to the National Comprehensive Cancer Network Practice
guidelines. Overall survival (OS) and disease-free survival (DFS)
rates were defined as the interval from the initial surgery to
clinically or radiologically proven recurrence/metastasis and
mortality, respectively.
The patients were enrolled in the present study
between 2006 and 2009. The follow-up for all cases was terminated
in February 2012. During survival analysis, cases were regarded as
censored data when patients were lost to follow-up or succumbed to
their disease.
RNA extraction and RT-qPCR
Total RNA extraction of 50 paired freshly frozen
primary tumor and adjacent normal mucosa (10 cm away from the
original tumor site) of CRC specimens were performed according to
the manufacturer’s instructions (Qiagen, Shanghai, China). A
Reverse Transcription kit (Qiagen) was used to reverse transcribe
total RNA according to the manufacturer’s instructions.
Quantitative PCR was performed using a SYBR Green PCR kit (Thermo
Scientific, Waltham, MA, USA) according to the manufacturer’s
instructions. The human NLK gene was amplified using a commercial
NLK qPCR Primer Pair (NM_016231; OriGene, Rockville, MD, USA) and
β-actin (HP204660; OriGene) was used as the internal control.
Cycling conditions were as follows: Denaturation (5 min at 93°C)
followed by 40 cycles of denaturation (30 sec at 93°C), annealing
(15 sec at 58°C) and elongation (1 min at 72°C). Each reaction was
performed in triplicate and the 2−ΔΔCt method was used
to calculate relative expression.
Western blot analysis
Western blot analysis was performed as previously
described (17). Monoclonal human
anti-NLK antibodies (1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA) and monoclonal anti-β-actin antibodies (1:2,000;
Beyotime Biotechnology, Jiangsu, China) were used as primary
antibodies. Immunoreactive bands were detected using a
Phototope-horseradish peroxidase western blot detection kit (Cell
Signaling Technology, Inc.). For densitometric analysis, NLK
protein bands on the blots were measured using Image J software
(National Institutes of Health, Bethesda, MD, USA) following
normalization to the corresponding β-actin expression levels.
Immunohistochemical analysis
Paraffin-embedded sections fixed in formalin were
deparaffinized, rehydrated and incubated with 3% hydrogen
peroxidase (Qiagen). The sections were then heated in a microwave
oven (1,000 Watts 8503; Kenmore, Chicago, IL, USA) for 3 min at
100°C for antigen retrieval. Slides were incubated with blocking
serum (Qiagen) and primary antibodies for NLK (1:100) overnight at
4°C. The immunohistochemical reaction was visualized using 0.05%
diaminobenzidine followed by counterstaining with hematoxylin.
Sections were then examined and analyzed using a microscope (Leica
M80; Leica Microsystems, Wetzlar, Germany). Negative control
sections were incubated with preimmune rabbit serum (Qiagen)
instead of the primary antibodies.
Immunostaining was defined independently using two
pathologists blinded to the clinical data and scored by multiplying
the staining intensity and the percentage of the stained tumor
cells. Staining intensity was graded from 0–3 and the percentage of
the stained tumor cells was graded as follows: 0, <5%; 1, 5–25%;
2, 26–50%; 3, 51–75%; and 4, >75%. Final scores ranged from 0 to
12. Samples with overall scores from 0–4 were defined as negative
expression, while the samples with scores 5–12 were grouped and
defined as positive expression (18). Specimens with inconsistent scores
were re-evaluated by two pathologists until an agreement was
reached.
Statistical analysis
For categorical variables, values are expressed as
the numerical count and the χ2 test or Fisher’s exact
test were used to determine the statistical significance of
differences between NLK and clinicopathological variables.
Kaplan-Meier curves with log-rank tests represented the cumulative
survival rate for OS and DFS using NLK expression levels. The Cox
proportional hazards model was used to calculate univariate and
multivariate hazard ratios for the study variables. P<0.01 was
considered to indicate a statistically significant difference
between values. All statistical analyses were performed using the
SPSS 17.0 statistical software package (SPSS, Inc., Chicago, IL,
USA).
Results
NLK upregulation in CRC tissues
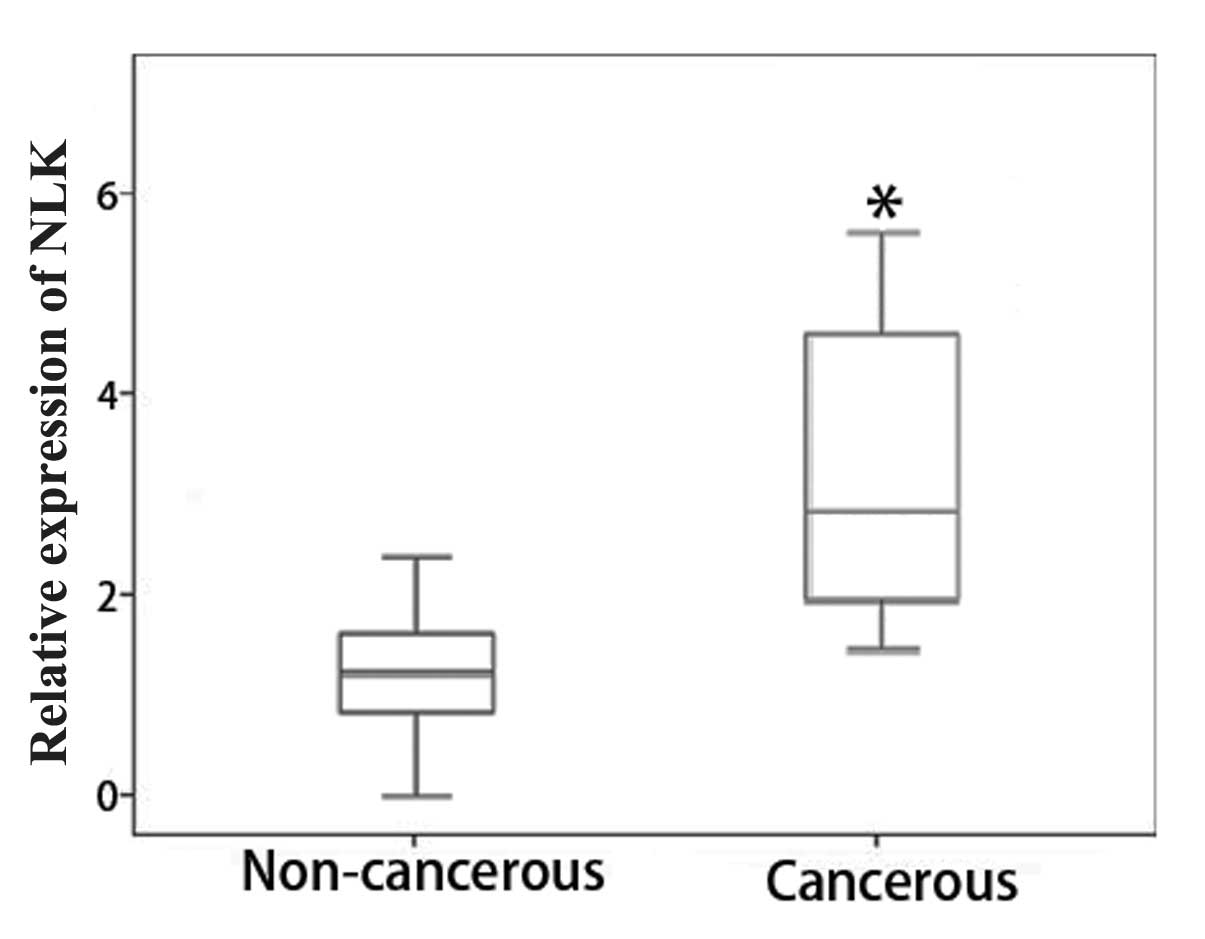
Among the 50 paired specimens available for RT-qPCR
analysis, the relative expression levels of NLK mRNA showed a
minimum of a two-fold increase in 78.0% of tumor tissues compared
to those of the adjacent normal mucosa (Fig. 1). This therefore suggested that NLK
expression was upregulated in CRC tissues.
In addition, western blot analysis was used to
confirm these results in the examined 50 paired tumors and
corresponding normal tissues. The positive rate of NLK expression
was 66.0% in CRC tissues and 18.0% in the matched non-cancerous
normal tissues; therefore, NLK expression was significantly higher
in CRC tissues than that in the matched normal colorectal tissues
(P<0.01) (Fig. 2A and B).
Correlation between NLK expression and
clinicopathological features in CRC
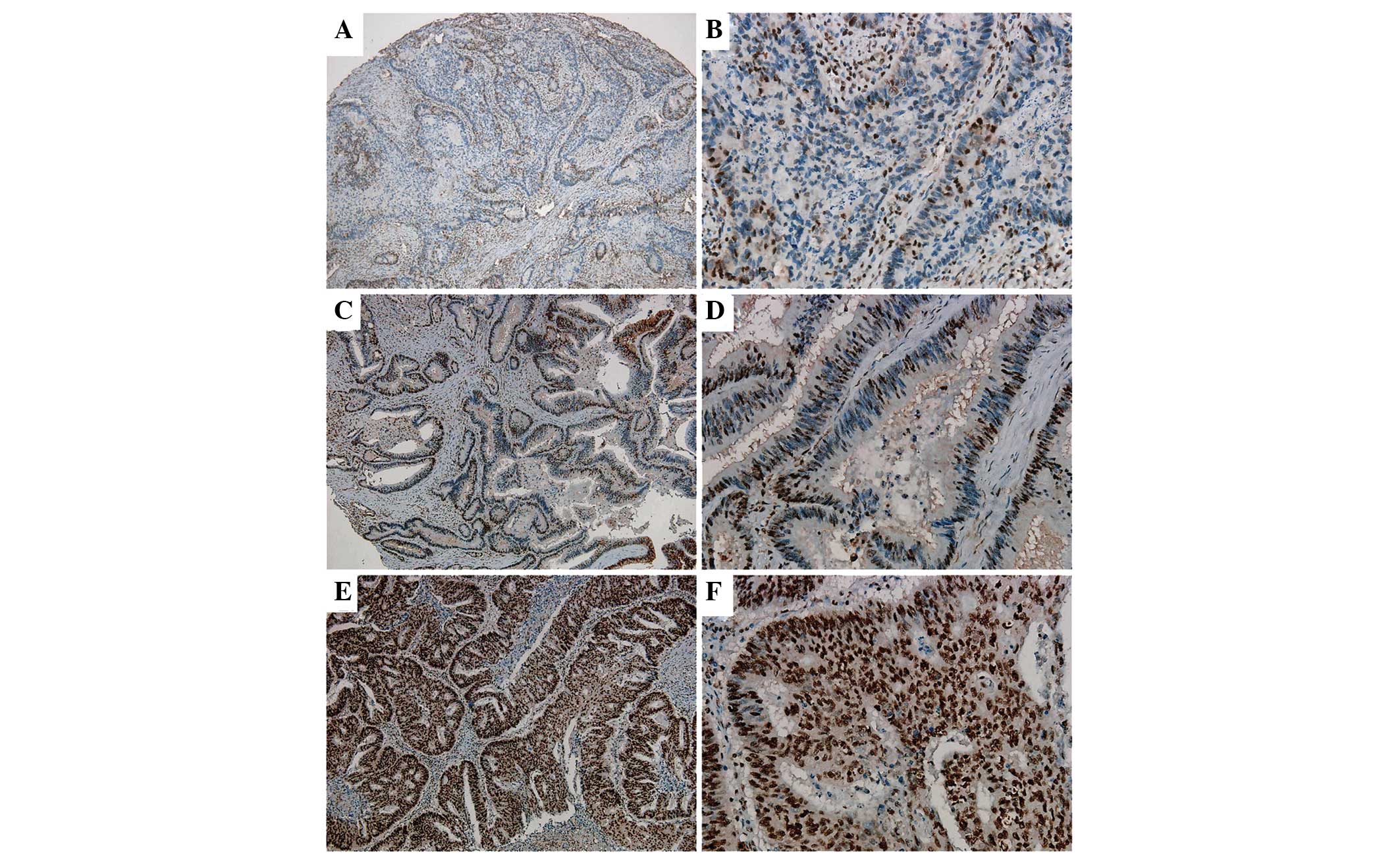
In order to further analyze the clinical and
pathological features of NLK expression, immunohistochemical
analysis was used to detect NLK protein expression in 406 cases of
CRC and paired adjacent noncancerous tissues (Fig. 3). The results demonstrated that
89.7% of non-cancerous specimens were negative for NLK expression;
by contrast, 57.6% of CRC specimens exhibited positive NLK
expression. In addition, among the 132 LNM tissues, 90.9% displayed
positive NLK expression (Table
I).
 | Table INLK expression in adjacent normal
mucosa, cancerous tissues and LNM tissues. |
Table I
NLK expression in adjacent normal
mucosa, cancerous tissues and LNM tissues.
| | Expression of
NLK | |
|---|
| |
| |
|---|
| Tissue sample | n | Negative, n (%) | Positive, n (%) | P-value |
|---|
| Normal mucosa | 406 | 364 (89.7) | 42 (9.9) | <0.001a |
| Cancerous | 406 | 172 (42.4) | 234 (57.6) | <0.001a |
| LNM | 132 | 12 (9.1) | 120 (90.9) | <0.001a |
The correlations between NLK protein expression and
clinicopathological features are shown in Table II. The positive expression of NLK
was significantly correlated with the depth of tumor invasion, LNM,
distant metastasis, vascular invasion and histological
differentiation. No significant correlations were observed between
NLK expression and age or gender. NLK expression levels were found
to be significantly higher in the nodal metastasis than those of
the CRC and noncancerous tissues (P<0.001). These data indicated
that increased NLK expression may correlate with CRC
metastasis.
 | Table IINLK expression and clinicopathological
characteristics in colorectal cancer. |
Table II
NLK expression and clinicopathological
characteristics in colorectal cancer.
| NLK protein
expression | |
|---|
|
| |
|---|
| Variable | Negative (n=172) | Positive (n=234) | P-value |
|---|
| Age |
| <65 | 76 | 86 | 0.586 |
| ≥65 | 96 | 148 | |
| Gender |
| Male | 72 | 100 | 0.374 |
| Female | 100 | 134 | |
| pT stage |
| pT1 | 8 | 8 | <0.001a |
| pT2 | 34 | 12 | |
| pT3 | 72 | 80 | |
| pT4 | 58 | 134 | |
| pN stage |
| pN0 | 166 | 50 | 0.001a |
| pN1 | 2 | 120 | |
| pN2 | 4 | 64 | |
| M stage |
| M0 | 170 | 50 | <0.001a |
| M1 | 2 | 15 | |
| Vessel invasion |
| No | 168 | 200 | 0.018a |
| Yes | 4 | 34 | |
| Differentiation |
| Good | 102 | 96 | 0.0015a |
| Moderate/poor | 70 | 140 | |
NLK expression and survival analysis
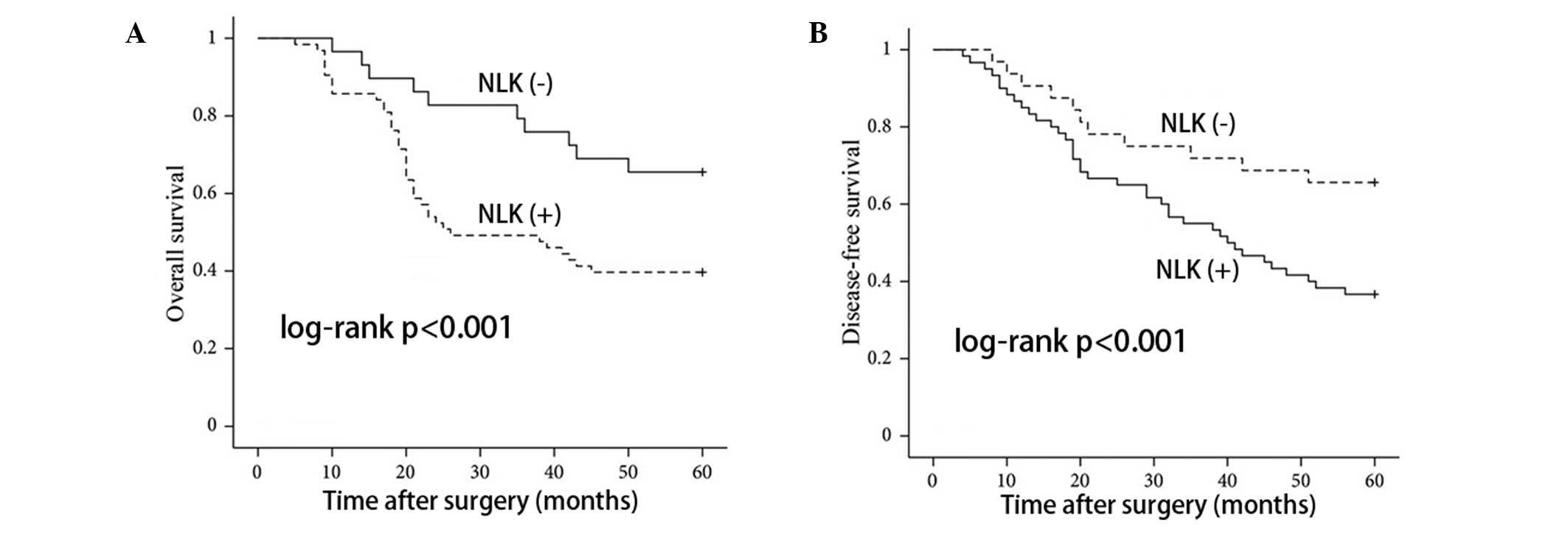
In order to assess the possible associations between
NLK expression and CRC patient survival, Kaplan-Meier curves using
log-rank tests for OS and DFS were performed. As shown in Fig. 4, patients with positive NLK
expression showed decreased rates of OS and DFS, respectively. In
addition, patients with positive NLK expression had a higher
recurrence rate than patients with negative expression (P<0.001;
data not shown). As shown in Table
III, univariate analysis revealed that OS as well as DFS were
significantly associated with advanced tumor stage, lymph node
metastasis, distant metastasis, histological differentiation,
vascular invasion and NLK expression. Multivariate analysis was
performed using the Cox proportional hazards model, and the results
demonstrated that positive NLK expression remained a significant
independent prognostic factor for OS [hazard ratio (HR)=0.035; 95%
confidence interval (CI)=0.02–0.19; P<0.001] and DFS (HR=0.033;
95% CI=0.007–0.09; P<0.001).
 | Table IIIUnivariate and multivariate analysis
of survival in 406 colorectal cancer patients. |
Table III
Univariate and multivariate analysis
of survival in 406 colorectal cancer patients.
| OS | DFS |
|---|
|
|
|
|---|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age |
| <65 | 1 | | | | 1 | | | |
| ≥65 | 1.003
(0.61,1.58) | 0.73 | | | 0.93
(0.59,1.55) | 0.69 | | |
| Gender |
| Male | 1 | | | | 1 | | | |
| Female | 0.74
(0.43,1.19) | 0.46 | | | 0.729
(0.44,1.42) | 0.53 | | |
| pT stage |
| pT1 | 1 | | 1 | | 1 | | 1 | |
| pT2 | 0.36
(0.07,1.51) | 0.18 | 0.96
(0.21,4.14) | 0.96 | 0.39
(0.09,1.57) | 0.16 | 1.26
(0.22,5.67) | 0.88 |
| pT3 | 0.09
(0.02,0.41) | 0.001 | 0.27
(0.06,1.18) | 0.08 | 0.08
(0.04,0.44) | 0.001 | 0.36
(0.05,1.46) | 0.25 |
| pT4 | 0.34
(0.19,0.56) | <0.001 | 0.29
(0.15,0.53) | <0.001 | 0.32
(0.14,0.57) | <0.001 | 0.42
(0.27,0.84) | 0.016 |
| pN stage |
| pN0 | 1 | | 1 | | 1 | | 1 | |
| pN1 | 0.06
(0.04,0.12) | <0.001 | 0.68
(0.14,3.09) | 0.64 | 0.06
(0.02,0.18) | <0.001 | 3.16
(0.67,13.7) | 0.19 |
| pN2 | 0.28
(0.19,0.53) | <0.001 | 0.38
(0.40,0.69) | <0.001 | 0.282
(0.14,0.85) | <0.001 | 0.38
(0.46,0.67) | 0.001 |
| M stage |
| M0 | 1 | | 1 | | 1 | | 1 | |
| M1 | 0.07
(0.04,0.15) | <0.001 | 0.19
(0.08,0.41) | <0.001 | 0.05
(0.02,0.08) | <0.001 | 0.043
(0.01,0.16) | <0.001 |
| Vessel
invasion |
| No | 1 | | 1 | | 1 | | 1 | |
| Yes | 0.19
(0.08,0.43) | <0.001 | 0.72
(0.34,1.42) | 0.38 | 0.01
(0.07,0.26) | <0.001 | 0.35
(0.17,0.74) | 0.006 |
|
Differentiation |
| Good | 1 | | 1 | | 1 | | 1 | |
| Moderate/poor | 0.14
(0.07,0.235) | <0.001 | 0.37
(0.16,0.75) | 0.007 | 0.18
(0.06,0.27) | <0.001 | 0.355
(0.13,0.78) | 0.012 |
| NLK status |
| Negative | 1 | | 1 | | 1 | | 1 | |
| Positive | 0.04
(0.01,0.09) | <0.001 | 0.035
(0.02,0.19) | <0.001 | 0.034
(0.02,0.08) | <0.001 | 0.033
(0.007,0.09) | <0.001 |
Discussion
The results of the present study have demonstrated
the correlation between elevated NLK expression and human CRC
prognosis; of note, correlations were observed between positive NLK
expression and aggressive features of CRC, including tumor depth,
lymph node metastasis and distant metastasis. This therefore
indicated the potential use of NLK as a tumor biomarker for CRC;
furthermore, these results suggested that NLK may be used as a
novel prognostic marker for more aggressive phenotypes of CRC
patients following surgical resection.
The NLK gene, which encodes a proline-directed MAPK
family member, was identified in 1994 (19). Functional analyses have
demonstrated that NLK contributed to numerous signaling pathways
via its ability to phosphorylate diverse transcription factors
(20). NLK was reported to be a
pivotal regulator of the Wnt/β-catenin signaling pathway (21). In addition, NLK has also been shown
to regulate the activity of multiple transcription factors,
including NF-κB, Smads and p53 (22). Therefore, the reported involvement
of NLK in numerous signaling pathways has demonstrated its vital
role in mediating cell signals. Studies of NLK function in human
cancers have also confirmed the role of NLK in cell growth and
proliferation. In addition, NLK was reported to function as a tumor
suppressor gene or an oncogene in different types of cancers; for
example, data have demonstrated that NLK expression was decreased
during prostate cancer progression and indicated that NLK inhibited
androgen receptor (AR) expression and subsequent AR-mediated
transcription as well as promoted apoptosis in prostate cancer cell
lines (14). In a previous study,
clinicopathological analysis revealed that NLK expression levels
were significantly higher in human glioma tissues compared with
those of lower grade tumors and the survival rate of glioma
patients expressing low levels of NLK was significantly decreased
compared to that of patients with gliomas expressing high levels of
NLK (16). Conversely, NLK was
reported to act as an oncogene in certain types of tumors. A
previous study demonstrated the mitogenic potential of NLK in
hepatocellular carcinomas through siRNA-mediated disruption of NLK,
which was shown to inhibit proliferation of Hep3B cells and arrest
cell cycle transition (15). These
discrepancies may be due to the different pathologies of the types
of tumors being studied. In the present study, NLK expression was
shown to be significantly upregulated in CRC tissues compared to
that of the paired non-cancerous samples, indicating the
involvement of NLK in CRC progression.
A recent study reported that NLK overexpression was
associated with the progression of gallbladder cancer and that NLK
may have potential for use as a prognostic marker (23). Therefore, the present study aimed
to analyze the correlations between NLK expression and the
clinicopathological features of CRC patients as well as clinical
outcome. These results revealed that positive NLK expression was
significantly correlated with the depth of tumor invasion, lymph
node metastasis, distant metastasis, histological differentiation,
vascular invasion and advanced tumor stage. Kaplan-Meier survival
analysis demonstrated that positive NLK expression was negatively
correlated with the decreased overall survival rate of CRC
patients. Of note, Cox multivariate analysis revealed that NLK
expression was an independent factor in predicting OS and DFS for
CRC patients. In conclusion, the results of the present study
indicated that NLK may have a crucial role in promoting the
aggressive phenotypes of CRC and therefore may have the potential
for use as a prognostic marker of CRC.
Acknowledgements
The present study was supported by a grant from the
Natural Science Foundation of Shandong Province (no.
ZR2011HQ054).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gaedcke J, Grade M, Camps J, Søkilde R,
Kaczkowski B, Schetter AJ, Difilippantonio MJ, Harris CC, Ghadimi
BM, Møller S, et al: The rectal cancer microRNAome - microRNA
expression in rectal cancer and matched normal mucosa. Clin Cancer
Res. 18:4919–4930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang S, Cui Y, Weng Z, Gong X, Chen M and
Zhong B: Changes on the disease pattern of primary colorectal
cancers in Southern China: a retrospective study of 20 years. Int J
Colorectal Dis. 24:943–949. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Speetjens FM, Zeestraten EC, Kuppen PJ,
Melief CJ and van der Burg SH: Colorectal cancer vaccines in
clinical trials. Expert Rev Vaccines. 10:899–921. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diep CB, Thorstensen L, Meling GI,
Skovlund E, Rognum TO and Lothe RA: Genetic tumor markers with
prognostic impact in Dukes’ stages B and C colorectal cancer
patients. J Clin Oncol. 21:820–829. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Walther A, Johnstone E, Swanton C, Midgley
R, Tomlinson I and Kerr D: Genetic prognostic and predictive
markers in colorectal cancer. Nat Rev Cancer. 9:489–499. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roth AD, Tejpar S, Delorenzi M, Yan P,
Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C,
et al: Prognostic role of KRAS and BRAF in stage II and III
resected colon cancer: results of the translational study on the
PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 28:466–474.
2010. View Article : Google Scholar
|
|
10
|
Rajagopalan H, Bardelli A, Lengauer C,
Kinzler KW, Vogelstein B and Velculescu VE: Tumorigenesis: RAF/RAS
oncogenes and mismatch-repair status. Nature. 418:9342002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
French AJ, Sargent DJ, Burgart LJ, Foster
NR, Kabat BF, Goldberg R, Shepherd L, Windschitl HE and Thibodeau
SN: Prognostic significance of defective mismatch repair and BRAF
V600E in patients with colon cancer. Clin Cancer Res. 14:3408–3415.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar
|
|
13
|
Ishikawa T, Shimizu D, Kito A, Ota I,
Sasaki T, Tanabe M, Yamada A, Arioka H, Shimizu S, Wakasugi J, et
al: Breast cancer manifested by hematologic disorders. J Thorac
Dis. 4:650–654. 2012.PubMed/NCBI
|
|
14
|
Emami KH, Brown LG, Pitts TE, Sun X,
Vessella RL and Corey E: Nemo-like kinase induces apoptosis and
inhibits androgen receptor signaling in prostate cancer cells.
Prostate. 69:1481–1492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung KH, Kim JK, Noh JH, Eun JW, Bae HJ,
Xie HJ, Ahn YM, Park WS, Lee JY and Nam SW: Targeted disruption of
Nemo-like kinase inhibits tumor cell growth by simultaneous
suppression of cyclin D1 and CDK2 in human hepatocellular
carcinoma. J Cell Biochem. 110:687–696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui G, Li Z, Shao B, Zhao L, Zhou Y, Lu T,
Wang J, Shi X, Wang J, Zuo G, et al: Clinical and biological
significance of nemo-like kinase expression in glioma. J Clin
Neurosci. 18:271–275. 2011. View Article : Google Scholar
|
|
17
|
Wang S, Wu X, Chen Y, Zhang J, Ding J,
Zhou Y, He S, Tan Y, Qiang F, Bai J, et al: Prognostic and
predictive role of JWA and XRCC1 expressions in gastric cancer.
Clin Cancer Res. 18:2987–2996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weichert W, Röske A, Gekeler V, Beckers T,
Ebert MP, Pross M, Dietel M, Denkert C and Röcken C: Association of
patterns of class I histone deacetylase expression with patient
prognosis in gastric cancer: a retrospective analysis. Lancet
Oncol. 9:139–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishitani T and Ishitani S: Nemo-like
kinase, a multifaceted cell signaling regulator. Cell Signal.
25:190–197. 2013. View Article : Google Scholar
|
|
20
|
Ota S, Ishitani S, Shimizu N, Matsumoto K,
Itoh M and Ishitani T: NLK positively regulates Wnt/β-catenin
signalling by phosphorylating LEF1 in neural progenitor cells. EMBO
J. 31:1904–1915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishitani T: Context-dependent dual and
opposite roles of nemo-like kinase in the Wnt/β-catenin signaling.
Cell Cycle. 11:1743–1745. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yasuda J, Yokoo H, Yamada T, Kitabayashi
I, Sekiya T and Ichikawa H: Nemo-like kinase suppresses a wide
range of transcription factors, including nuclear factor-κB. Cancer
Sci. 95:52–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li M, Zhang S, Wang Z, Zhang B, Wu X, Weng
H, Ding Q, Tan Z, Zhang N, Mu J, et al: Prognostic significance of
nemo-like kinase (NLK) expression in patients with gallbladder
cancer. Tumour Biol. 34:3995–4000. 2013. View Article : Google Scholar : PubMed/NCBI
|