Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide, and non-small cell lung cancer (NSCLC)
constitutes ~85% of lung cancers (1). Currently, the most effective
treatment for NSCLC is surgery. Surgery is efficient for treatment
of early-stage NSCLC, but >70% of NSCLC patients are diagnosed
at advanced stages of the disease. NSCLC patients are insensitive
to chemo- and radiotherapy, and although new advances in surgery
have made a great progress in the last decade, mortality of NSCLC
remains high (1,2). Therefore, it is urgent to identify
more efficient treatment strategies for NSCLC treatment.
Integrins are a family of transmembrane receptors
composed of two subunits, α and β. To date, there are 18α and 8β
subunits identified in mammals, and these subunits can form at
least 24 different integrin heterodimers (3). These receptors facilitate
interactions between cells and the extracellular matrix (ECM),
participate in cytoskeleton organization and play important roles
in cell signaling (4). There is
increasing evidence that integrins are involved in regulating a
diverse array of cellular functions crucial to the initiation and
progression of cancer. In addition, during progression from tumor
growth to metastasis, specific integrin signals enable cancer cells
to detach from the neighbouring cells, reorientate their polarity
during migration, and survive and proliferate in foreign
microenvironments (5,6).
Integrins αv are a subfamily of integrins,
particularly important during cancer progression, since they are
extensively expressed on the surface of most epithelial tumor
cells, and at higher levels during tumor progression and metastasis
(7–9). Epithelial to-mesenchymal transition
(EMT) is a cellular process associated with tumor progression and
metastasis. Integrin αv is lowly expressed in healthy epithelial
tissues, but upregulated during EMT. It was previously reported
that integrin αv can increase EMT in cervical squamous cell
carcinoma, and its increased expression is considered as a
prognostic factor for decreased survival (10). Since integrins induce tumor cell
growth and motility and block cell apoptosis through numerous
growth factors or cytokines, these proteins play key roles in tumor
growth and invasion, although not considered oncogenic per
se. Nip et al (11)
found that integrin αv expression is upregulated in melanoma, and
contributes to lymphatic metastasis. Hosotani et al
(12) also revealed that high
expression of integrin αv in pancreatic carcinoma is related to
MMP-2 activation and lymph node metastasis. In addition, a number
of additional tumor types have been reported to relate to integrin
αv: for example, integrin αv may modulate bone metastatic growth in
prostate cancer (13), its
activation controls metastasis in human breast cancer (14), while it is also involved in ovarian
cancer (15).
The role of integrin αv in regulating progression of
tumors has been well established. Nevertheless, the role of
integrin αv in NSCLC and the related mechanisms still remain
unclear. Therefore, in this study, we first detected the expression
of integrin αv n NSCLC and healthy lung tissues at the mRNA and
protein level, and found that integrin αv is significantly
overexpressed in human lung carcinoma compared to healthy lung
tissues. To further investigate the role of integrin αv in NSCLC,
we studied the human lung carcinoma cell line A549 that displays a
low level of integrin αv expression. By overexpressing the
integrin αv gene in A549 cells, we examined the effects of
this protein on cell proliferation and apoptosis, and investigated
the cell signaling systems related to these effects.
Materials and methods
Human lung carcinoma and healthy lung
tissues
Fifteen paired primary lung carcinoma tissues and
corresponding healthy lung tissues were collected from the Shanghai
Chest Hospital. All samples were obtained at the time of operation,
immediately snap frozen in liquid nitrogen and stored at −80°C.
Informed consent was obtained from all the patients, and the study
protocols were approved by the Ethics Committee of Shanghai
Jiaotong University (Shanghai, China)
Cell culture
The human lung carcinoma cell line (A549) was
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China) and was maintained at 37°C in a humidified
atmosphere containing 5% CO2. Cells were cultured in
Gibco® RPMI-1640 containing 10% Gibco® fetal
bovine serum (both from Thermo Fisher Scientific, Waltham, MA,
USA), 2.0 mmol/l glutamine, 100 μg/ml of ampicillin, and 100 U/ml
of streptomycin sulfate.
Plasmid construction and
transfection
The integrin αv gene was first amplified from
cDNA using polymerase chain reaction (PCR), and was then subcloned
into the hemagglutinin (HA)-pcDNA3.0 plasmid (GenePharma, Shanghai,
China) to obtain the integrin αv expression vector. PCR was
performed using the following primers: sense, 5′-CGG GAT CCA ATG
GCT GCT CCC GGG-3′, and antisense, 5′-ATT TGC GGC CGC TTA GGT TTC
AGA GTT TCC TTC G-3′, and the following cycling conditions: 94°C
for 3 min followed by 35 cycles of 94°C for 30 sec and 48°C for 3
min, followed by 72°C for 10 min. RNA was extracted using an EZNA
Total RNA kit I (Omega Bio-Tek, Inc., Norcross, GA, USA) according
to the manufacturer’s instructions. The RNA was reverse transcribed
to cDNA using PrimeScript RT reagent kit (Takara Bio, Inc., Shiga,
Japan) cDNA was obtained using a DNA Gel Extraction kit (Beyotime
Institute of Biotechnology, Haimen, China). For transfection, A549
cells were seeded into 24-well plates (2×104
cells/well), and 24 h later, transfection was carried out using the
Invitrogen™ Lipofectamine® LTX with Plus™ reagent
(Thermo Fisher Scientific) according to the manufacturer’s
specifications.
Western blot analysis
To prepare total protein extracts, cells were
collected 24 h post-transfection and lysed using RIPA buffer (0.05
M Tris-HCl, pH 7.4, 0.25% deoxycholic acid, 0.15 M NaCl, 1% NP-40,
0.5 mM DTT, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1×
proteinase inhibitor) at 4°C for 30 min. Then, the mixture was
centrifuged at 12,000 × g at 4°C for 10 min, and the supernant was
transferred to a fresh tube. For human tissues, 30–50 mg of tissue
were weighed and lysed in RIPA buffer, homogenized and centrifuged
as described above, and the supernant was collected. Total protein
was separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis, and then transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Bedford, MA, USA). After
blocking in 5% nonfat milk for 1 h at room temperature, the
membranes were incubated with the appropriate primary antibody for
2 h at 4°C overnight, followed by incubation in horseradish
peroxidase-linked secondary antibody for 1–2 h at room temperature,
and visualization with an enhanced chemiluminescence substrate (EMD
Millipore). β-actin (dilution, 1:5,000) or glyceraldehyde
3-phosphate dehydrogenase (GAPDH; 1:5,000) were used as loading
controls (both from Abcam, Cambridge, UK).
The primary antibodies used in this study, i.e.,
anti-GAPDH, -β-catenin, -integrin αv (1:1,000), -extracellular
signal regulated protein kinase 1/2 (ERK 1/2; 1:1,000) and
-phosphorylated ERK (p-ERK; 1:1,000) were purchased from Cell
Signaling Technology (Beverly, MA, USA). The anti-HA (1:1,000)
antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). The mitogen-activated protein/ERK kinase inhibitor
PD98059 was purchased from Biomol Research Laboratories (Plymouth
Meeting, PA, USA). PD98059 was dissolved in dimethyl sulfoxide
(DMSO).
Cell counting kit-8 (CCK8) assay
In order to detect the cell proliferative ability,
the CCK8 assay (Dojindo Molecular Technologies Inc., Shanghai,
China) was performed according to the manufacturer’s instructions.
Briefly, cells were cultured in 96-well plates
(5×103/100 μl/well), and 24 h later, cells were
transfected with the HA-pcDNA3.0-integrin αv or the control
HA-pcDNA3.0 plasmid (GenePharma). Then, 10 μl of CCK8 solution were
added into the cells and incubated for 0.5, 1 or 2 h. The optical
density (OD) of the samples was measured at 450 nm using an
enzyme-linked immunosorbent assay reader (Thermo Fisher
Scientific). All assays were performed in triplicate.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was isolated from human tissues using the
Invitrogen™ TRIzol reagent (Thermo Fisher Scientific) according to
the manufacturer’s instructions. The RNA was reverse transcribed to
cDNA using the Invitrogen™ M-MLV reverse transcriptase (Thermo
Fisher Scientific). qPCR was performed using the Applied
Biosystems® SYBR-Green PCR Master mix on an ABI PRISM
7900HT Fast Real-Time PCR system (both from Thermo Fisher
Scientific). qPCR reactions were carried out in a total volume of
10 μl, and following initial denaturation (95°C for 30 sec), 40
cycles were performed at 95°C for 5 sec, and 60°C for 30 sec.
Primers for the amplification of the human integrin αv gene
were: sense, 5′-CGG GTC CCG AGG GAA GT-3′, and antisense, 5′-GTG
CTG GGC TCG AAG AAG TC-3′.
Analysis of apoptosis
Cells were seeded into a 24-well plate
(2×105 cells/well) 1 day before transfection, and were
then trasfected with the HA-pcDNA3.0-integrin αv or the control
HA-pcDNA3.0 plasmid. At 24 h post-transfection, cells were
collected and washed twice with ice-cold phosphate-buffered saline
(PBS). Next, cells were resuspended in 1× binding buffer (PBS), 5
μl of FITC-Annexin V and 10 μl of propidium iodide (PI; 250 μg/ml)
were added and incubated with the cells for 10 min at room
temprerature. Then, cells were washed with PBS and examined using
flow cytometry (LSRII; BD Biosciences, Franklin Lakes, NJ, USA).
Flow cytometry data were analyzed using FlowJo software (Tree Star,
Stanford, CA, USA).
Statistical analysis
All data were expressed as the mean ± standard error
of the mean (SEM). Statistical analysis was carried out using the
SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). Student’s t-tests
were used to evaluate the differences between two groups, with
P<0.05 considered to indicate statistically significant
differences.
Results
Integrin αv is upregulated in human lung
carcinoma
To investigate the role of integrin αv in lung
carcinoma genesis, we first detected the expression level of the
integrin αv mRNA and protein in lung carcinoma tissues using
RT-qPCR and western blot analysis, respectively. The results of
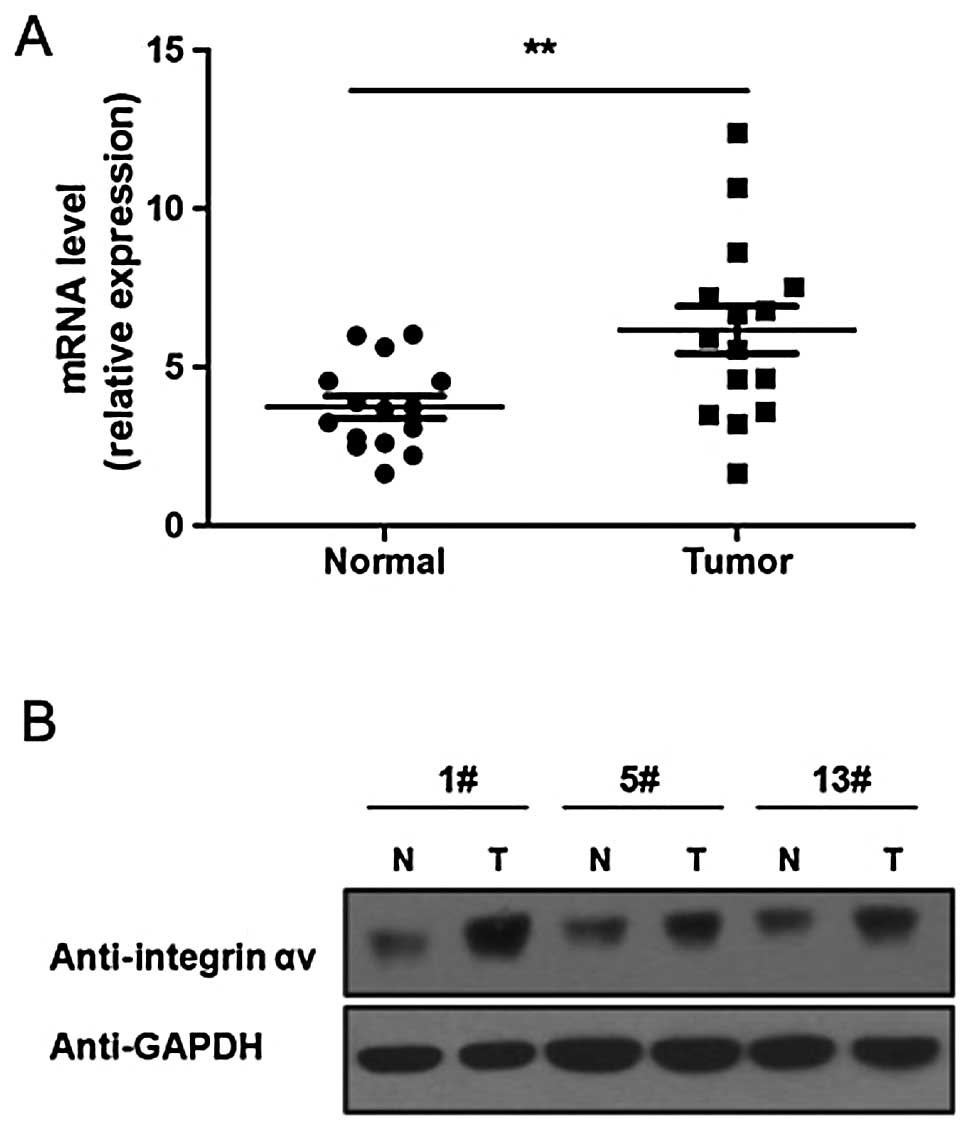
RT-qPCR showed that, compared to healthy lung tissues, the
integrin αv mRNA level is significantly increased in lung
carcinoma tissues (Fig. 1A). The
results of western blot analysis also showed that the integrin αv
protein level is higher in lung carcinoma compared to healthy
tissues (Fig. 1B).
Overexpression of integrin αv promotes
proliferation in A549 cells
Since integrin αv was found to be upregulated in
lung cancer, this protein may play important roles during lung
cancer genesis. To investigate this hypothesis, we overexpressed
and silenced the integrin αv gene in A549 cells, and then
performed a CCK8 assay to examine the effect of overexpression and
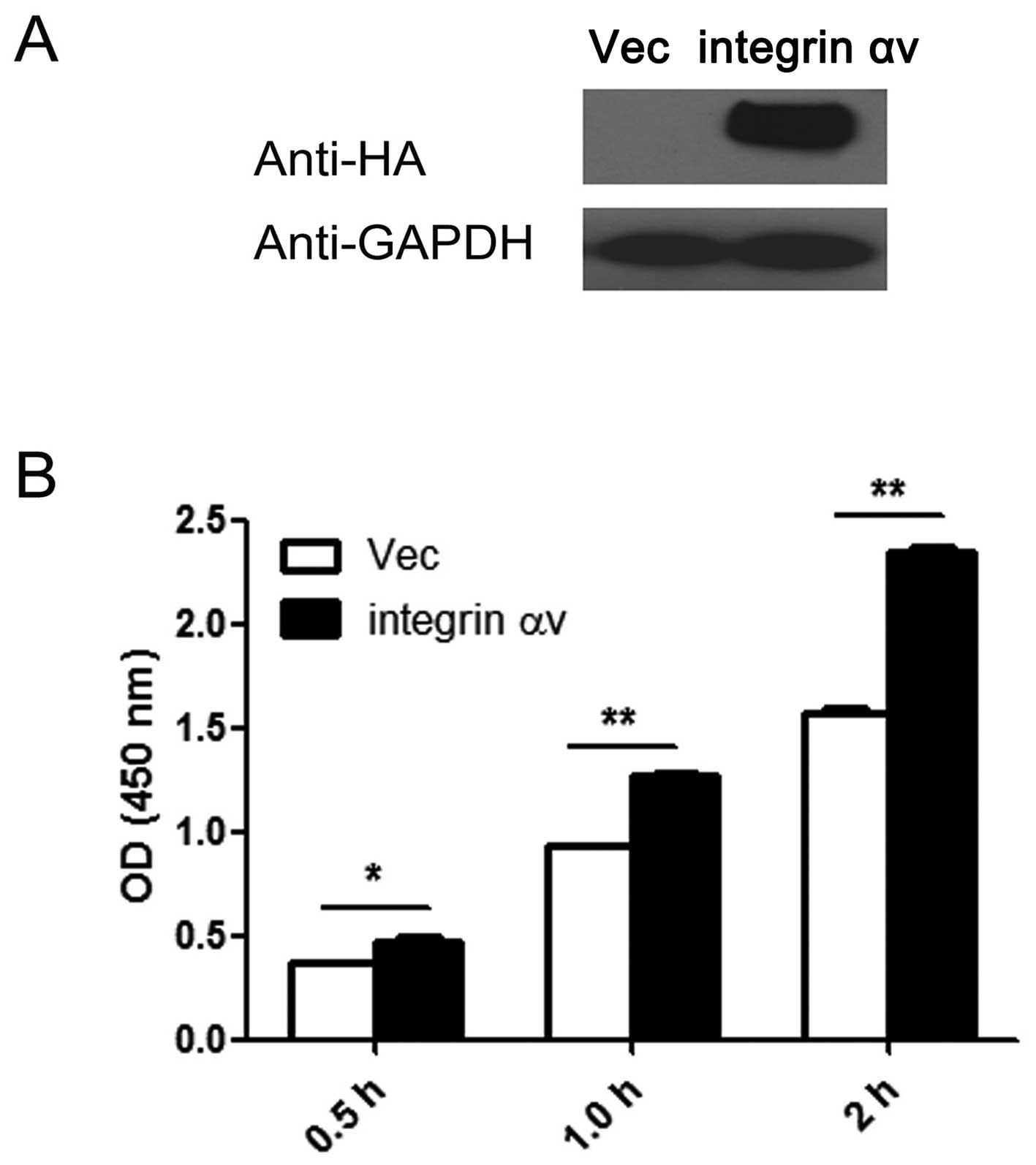
silencing on the cell proliferative ability. The amount of HA was
markedly increased following transfection, which indicates that
integrin αv is overexpressed in the A549 cells transfected
with the expression vector (Fig.
2A). The results of the CCK8 assay revealed that overexpression
of integrin αv significantly promotes cell proliferation
(Fig. 2B).
Overexpression of integrin αv inhibits
apoptosis in A549 cells
Apoptosis, also known as programmed cell death,
plays important roles in tumogenesis. Next, we investigated the
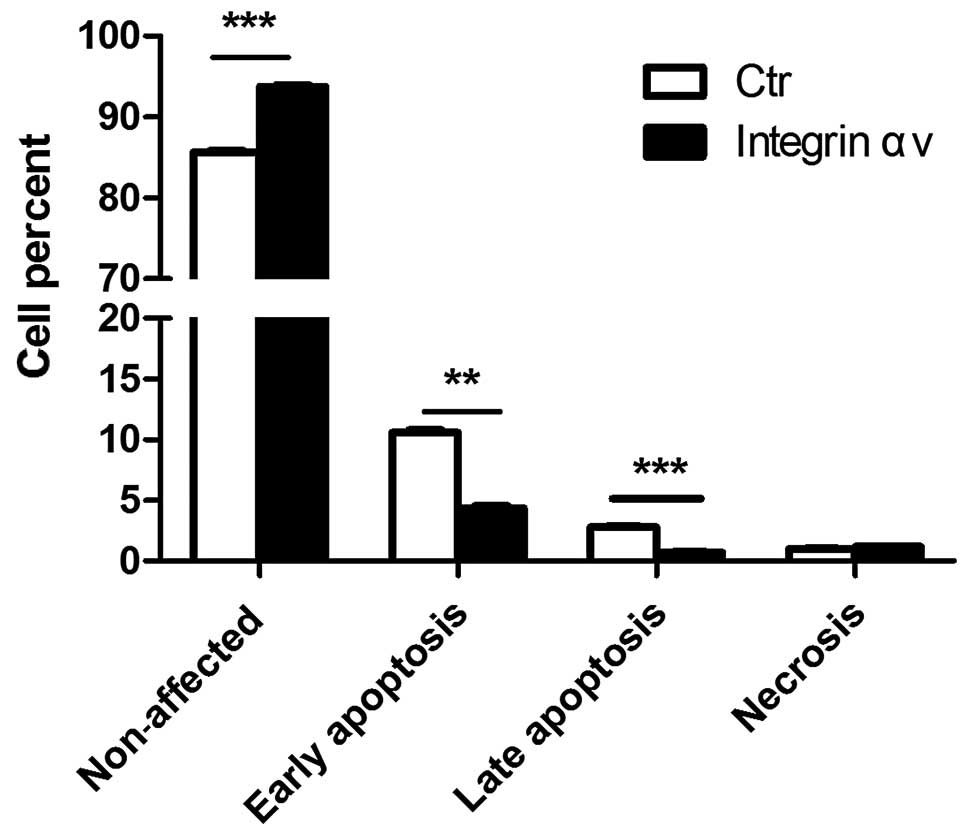
effects of integrin αv on apoptosis of A549 cells. Overexpression
of integrin αv reduced the percentage of early apoptotic
A549 cells, while the proportion of non-affected cells increased in
the HA-PCDNA3.0-integrin αv-trasfected group (Fig. 3). These results demonstrated that
integrin αv may block apoptosis of lung carcinoma cells and
thereby, contribute to tumorigenesis.
Integrin αv promotes the proliferation of
A549 cells through activation of the ERK 1/2 signaling pathway
Our results indicated that integrin αv may inhibit
apoptosis and promote proliferation of A549 cells. To further
elucidate the molecular mechanism underlying these roles, we
studied the expression of proteins of a number of important
pathways related to tumorigenesis (data not shown), and found that
the ERK 1/2 signaling pathway may be involved in the mediation of
the observed integrin αv effects. Compared to the control group,
the level of p-ERK 1/2 was higher in A549 cells overexpressing
integrin αv (Fig. 4A). To
further elucidate whether the ERK signaling pathway is involved in
the promotion of proliferation mediated by integrin αv, PD98059, a
highly specific inhibitor of the ERK signaling pathway, was used to
treat A549 cells (Fig. 4B).
Following inhibition of ERK 1/2 signaling, the increased
proliferation of A549 cells mediated by integrin αv was reduced,
while the inhibition of apoptosis was attenuated (Fig. 4C and D). These findings show that
integrin αv may function through activating the ERK 1/2 signaling
pathway in A549 cells.
Discussion
In this study, we first examined the expression of
integrin αv at the mRNA and protein level in human lung carcinoma
and healthy tissues, and found that compared to healthy lung
tissues, both the mRNA and protein levels of integrin αv are
significantly increased in NSCLC. Then, we overexpressed the
integrin αv gene in the human lung carcinoma cell line A549
to investigate the role of integrin αv in NSCLC. The results showed
that overexpression of integrin αv in A549 promotes the
proliferation of cells and reduces their apoptotic rate. Moreover,
overexpression of integrin αv in A549 cells may increase the
expression of phosphorylated ERK 1/2, which implies that integrin
αv may promote lung cancer progression through activating the ERK
1/2 signaling pathway. Following inhibition of ERK 1/2 signaling,
the promotion of proliferation of A549 cells mediated by integrin
αv was reduced, while the inhibition of apoptosis was rescued. Our
data demonstrate that the upregulation of integrin αv may
contribute to NSCLC development and/or progression through
activating the ERK 1/2 signaling pathway.
Integrins are heterodimeric transmembrane receptors
that mediate cell-matrix and cell-cell interactions. Heterodimeric
pairing of the integrin subunits α and β allows specific binding to
one or more substrates, and the heterodimer serves as an anchoring
molecule by mediating the adhesion of the cellular cytoskeleton to
the ECM. Integrin heterodimers also serve as signaling molecules,
and via their involvement in signal transduction, they control a
variety of vital cell functions such as differentiation, migration,
proliferation, apoptosis, and cell division (16,17).
Deregulation of integrin signaling may alter these processes and
eventually result in tumor formation. Numerous studies have shown
that the expression of integrins is suitable for predicting the
clinical course and for the prognosis of NSCLC. For example, it it
has been reported that upregulation of integrin α5 and β1 is
associated with poor prognosis of NSCLC patients (18,19).
Han et al (19) revealed
that the increased expression of integrin α5 and β1 significantly
correlates to lymph node metastasis of NSCLC. Adachi et al
(18) also reported that in
node-negative NSCLC patients, the overall survival rate of patients
with integrin α5-overexpressing tumors is significantly lower
compared to patients with normal integrin α5 expression; the
authors suggested that the increased expression of integrin α5 may
be a predictor of the 5-year survival rate.
It is well established that the αv integrin subunits
β1, β3, β5, β6 and β8 can pair with each other, and that αv
integrins typically recognize ligands such as vitronectin,
fibronectin and osteopontin, which contain the tripeptide
Arg-Gly-Asp (3,20). In addition, integrin αv appears to
be particularly important in tumor development. Kikkawa et
al (21) reported that
integrin αv may be involved in the early stage of liver metastasis.
Their results revealed that integrin αv promotes the extravasation
of tumor cells in liver through a process mediated by vitronectin.
In this study, we found that the mRNA expression and protein levels
of integrin αv are significantly increased in NSCLC tissues
(Fig. 1), while overexpression of
the gene in A549 cells markedly promoted cell proliferation
(Fig. 2); these results suggest
that αv integrin may be involved in the development of NSCLC, which
is in accordance with previous studies (22,23).
Metastasis is the major cause of treatment failure
and mortality in patients with malignant tumors, including NSCLC.
EMT is a process during which epithelial cells loose their
epithelial characters such as tight and adherens junction,
apical-basolateral polarity and the ability to synthesize basement
membranes, and develop a mesenchymal state. EMT is an important
phenomenon in cancer, and is involved in tumor progression and
metastasis. It has been demonstrated that integrin αv plays an
important role in EMT. Bates, and Bates and Mercurio (24,25)
conducted studies on a colon carcinoma model and found that
integrin αv is upregulated during EMT, and that increased
expression of integrin αv aresults in increased migration of the
cells. Through examining patient samples, Bates et al
(26) further showed that high
expression of integrin αv correlates to poor prognosis of colon
carcinoma. Another study by Ramos et al (27) reported that increased expression of
integrin αv in OSCC cells is involved in the initiation of EMT.
Integrin activation may lead to the activation of
downstream signal transduction events, and thus, participate in
modulating cell behavior (28). It
has been demonstrated that ERK 1/2 signaling is a major pathway by
which integrins regulate gene expression (29,30).
Activated ERK 1/2 regulates distinct transcription factors that
play an important role in physiological and pathological processes,
and numerous studies have indicated that activation of ERK 1/2 is
involved in the progression of tumors, including NSCLC (31, 32). To gain further insights into the
potential mechanism underlying the integrin αv roles in NSCLC, we
overexpressed integrin αv in A549 cells, and examined the
activation of this signaling protein. Our results showed that the
phosphorylation level of ERK 1/2 is increased in A549 cells
overexpressing integrin αv (Fig. 4). This indicates that integrin αv
may activate the ERK 1/2 signaling pathway in A549 cells.
In summary, we showed that integrin αv is
upregulated in human lung carcinoma tissues compared to healthy
ones.Overexpression of integrin αv in the lung cancer cells
A549 promoted their proliferation and restrained their apoptosis.
In addition, integrin αv was shown to increase the expression level
of phosphorylated ERK 1/2, which suggests that integrin αv may
promote lung cancer progression through activating the ERK 1/2
signaling pathway. Through inhibition of ERK 1/2 signaling, the
increased proliferation of A549 cells mediated by integrin αv was
reduced, while the inhibition of apoptosis was rescued. Our results
therefore suggest that the ERK 1/2 pathway may be a suitable
molecular target for the treatment of human lung cancer.
References
|
1
|
Pfister DG, Johnson DH, Azzoli CG, et al:
American Society of Clinical Oncology treatment of unresectable
non-small-cell lung cancer guideline: update 2003. J Clin Oncol.
22:330–353. 2004. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hynes RO: Integrins: bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giancotti FG and Ruoslahti E: Integrin
signaling. Science. 285:1028–1032. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brakebusch C, Bouvard D, Stanchi F, Sakai
T and Fässler R: Integrins in invasive growth. J Clin Invest.
109:999–1006. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo W and Giancotti FG: Integrin
signalling during tumour progression. Nat Rev Mol Cell Biol.
5:816–826. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun H, Hu K, Wu M, et al: Contact by
melanoma cells causes malignant transformation of human
epithelial-like stem cells via alpha V integrin activation of
transforming growth factor β1 signaling. Exp Biol Med (Maywood).
236:352–365. 2011. View Article : Google Scholar
|
|
8
|
Canonici A, Steelant W, Rigot V, et al:
Insulin-like growth factor-I receptor, E-cadherin and alpha v
integrin form a dynamic complex under the control of alpha-catenin.
Int J Cancer. 122:572–582. 2008. View Article : Google Scholar
|
|
9
|
Vellon L, Menendez JA and Lupu R: A
bidirectional ‘alpha(v)beta(3) integrin-ERK1/ERK2 MAPK’ connection
regulates the proliferation of breast cancer cells. Mol Carcinog.
45:795–804. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hazelbag S, Kenter GG, Gorter A, et al:
Overexpression of the alpha v beta 6 integrin in cervical squamous
cell carcinoma is a prognostic factor for decreased survival. J
Pathol. 212:316–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nip J, Shibata H, Loskutoff DJ, Cheresh DA
and Brodt P: Human melanoma cells derived from lymphatic metastases
use integrin alpha v beta 3 to adhere to lymph node vitronectin. J
Clin Invest. 90:1406–1413. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hosotani R, Kawaguchi M, Masui T, et al:
Expression of integrin alphaVbeta3 in pancreatic carcinoma:
relation to MMP-2 activation and lymph node metastasis. Pancreas.
25:e30–e35. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCabe NP, De S, Vasanji A, Brainard J and
Byzova TV: Prostate cancer specific integrin alphavbeta3 modulates
bone metastatic growth and tissue remodeling. Oncogene.
26:6238–6243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Felding-Habermann B, O’Toole TE, Smith JW,
et al: Integrin activation controls metastasis in human breast
cancer. Proc Natl Acad Sci USA. 98:1853–1858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Landen CN, Kim TJ, Lin YG, et al:
Tumor-selective response to antibody-mediated targeting of
alphavbeta3 integrin in ovarian cancer. Neoplasia. 10:1259–1267.
2008.PubMed/NCBI
|
|
16
|
Engers R and Gabbert HE: Mechanisms of
tumor metastasis: cell biological aspects and clinical
implications. J Cancer Res Clin Oncol. 126:682–692. 2000.
View Article : Google Scholar
|
|
17
|
Hood JD and Cheresh DA: Role of integrins
in cell invasion and migration. Nat Rev Cancer. 2:91–100. 2002.
View Article : Google Scholar
|
|
18
|
Adachi M, Taki T, Higashiyama M, Kohno N,
Inufusa H and Miyake M: Significance of integrin alpha5 gene
expression as a prognostic factor in node-negative non-small cell
lung cancer. Clin Cancer Res. 6:96–101. 2000.PubMed/NCBI
|
|
19
|
Han JY, Kim HS, Lee SH, Park WS, Lee JY
and Yoo NJ: Immunohistochemical expression of integrins and
extracellular matrix proteins in non-small cell lung cancer:
correlation with lymph node metastasis. Lung Cancer. 41:65–70.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takada Y, Ye X and Simon S: The integrins.
Genome Biol. 8:2152007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kikkawa H, Kaihou M, Horaguchi N, et al:
Role of integrin alpha(v)beta3 in the early phase of liver
metastasis: PET and IVM analyses. Clin Exp Metastasis. 19:717–725.
2002. View Article : Google Scholar
|
|
22
|
Jin Y, Tong DY, Chen JN, et al:
Overexpression of osteopontin, αvβ3 and Pim-1 associated with
prognostically important clinicopathologic variables in non-small
cell lung cancer. PLoS One. 7:e485752012. View Article : Google Scholar
|
|
23
|
Elayadi AN, Samli KN, Prudkin L, et al: A
peptide selected by biopanning identifies the integrin alphavbeta6
as a prognostic biomarker for nonsmall cell lung cancer. Cancer
Res. 67:5889–5895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bates RC: Colorectal cancer progression:
integrin alphavbeta6 and the epithelial-mesenchymal transition
(EMT). Cell Cycle. 4:1350–1352. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bates RC and Mercurio AM: The
epithelial-mesenchymal transition (EMT) and colorectal cancer
progression. Cancer Biol Ther. 4:365–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bates RC, Bellovin DI, Brown C, et al:
Transcriptional activation of integrin beta6 during the
epithelial-mesenchymal transition defines a novel prognostic
indicator of aggressive colon carcinoma. J Clin Invest.
115:339–347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramos DM, Dang D and Sadler S: The role of
the integrin alpha v beta6 in regulating the epithelial to
mesenchymal transition in oral cancer. Anticancer Res. 29:125–130.
2009.PubMed/NCBI
|
|
28
|
Morgan MR, Thomas GJ, Russell A, Hart IR
and Marshall JF: The integrin cytoplasmic-tail motif EKQKVDLSTDC is
sufficient to promote tumor cell invasion mediated by matrix
metalloproteinase (MMP)-2 or MMP-9. J Biol Chem. 279:26533–26539.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Faisal Khan KM, Laurie GW, McCaffrey TA
and Falcone DJ: Exposure of cryptic domains in the alpha 1-chain of
laminin-1 by elastase stimulates macrophages urokinase and matrix
metalloproteinase-9 expression. J Biol Chem. 277:13778–13786. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khan KM and Falcone DJ: Role of laminin in
matrix induction of macrophage urokinase-type plasminogen activator
and 92-kDa metalloproteinase expression. J Biol Chem.
272:8270–8275. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brognard J and Dennis PA: Variable
apoptotic response of NSCLC cells to inhibition of the MEK/ERK
pathway by small molecules or dominant negative mutants. Cell Death
Differ. 9:893–904. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gollob JA, Wilhelm S, Carter C and Kelley
SL: Role of Raf kinase in cancer: therapeutic potential of
targeting the Raf/MEK/ERK signal transduction pathway. Semin Oncol.
33:392–406. 2006. View Article : Google Scholar : PubMed/NCBI
|