Introduction
Esophageal cancer is an aggressive cancer with an
annual mortality rate almost matching incidence. At diagnosis, 54%
patients present with either localized or regional disease
(1). At the early stages of tumor
development, a variety of changes in cell-cell and cell-matrix
interactions result in abnormal cell behavior, which may induce
invasion and malignant transformation. Resistance to apoptosis
enables tumor cells to escape the effects of anticancer drugs and
natural effector cells, rendering effective treatment difficult.
Furthermore, the characteristics of this type of tumor contribute
to disease progression and increased malignancy. During the late
stages of tumor development, metastasis is the predominant
complication and renders this type of tumor difficult to control.
Therefore, elucidation of the mechanism underlying disease
progression and metastasis is urgently required.
Galectin-3, a β-galactoside-binding lectin, which
belongs to a widely distributed galectin family, contains a
carboxyl-terminal carbohydrate recognition domain with an
amino-terminal tandem repeat (2).
The protein has an extended N-terminal tail consisting of a single
polypeptide containing 8–13 consensus 9-mer amino acid repeats rich
in proline, tyrosine and glycine (3). In the past decade, galectin-3 has
been demonstrated to be widely expressed in tumor cells (4,5), and
galectin-3 expression has been shown to be involved in various
biological phenomena, including cell growth, adhesion,
differentiation, apoptosis, cancer aggressiveness and metastasis
(6,7). Recently, galectin-3 expression levels
have been demonstrated to be correlated with neoplastic
transformation in certain malignancies (6). Schoeppner et al (8) observed significantly increased
galectin-3 expression levels in high-grade dysplasia and early
invasive colon carcinoma, which exhibited a linear association with
advancing stage. Canesin et al (9) demonstrated that galectin-3 expression
contributed to disease progression and poor survival in advanced
bladder cancer patients. A study by Sakaki et al (10) indicated that the overexpression of
galectin-3 in clear cell renal cell carcinoma is a predictor of
disease progression and metastasis. Knapp et al (11) demonstrated that galectin-3
localization in benign, adjacent-benign and tumor tissues was
significantly correlated with biochemical recurrence in prostate
specimens. Braeuer et al (12) observed that increased galectin-3
expression levels in melanoma exerted profound effects on tumor
growth and metastasis. However, galectin-3 expression has been
revealed to be downregulated in other types of malignancy,
including breast, ovarian and uterine carcinomas (7,13).
The effects of galectin-3 expression on tumor
progression and metastasis in esophageal cancer, which is an
aggressive malignancy, have not yet, to the best of our knowledge,
been clarified. Therefore, in the present study, the effect of
galectin-3 on the behavior of the Eca-109 esophageal cancer cell
line was investigated.
Materials and methods
Eca-109 cell culture
Eca-109 human esophageal cancer cells were obtained
from Shandong Academy of Medical Sciences (Shandong, China). The
cells were incubated at 37°C in 5% CO2 in plastic tissue
culture flasks (Corning Inc., Acton, MA, USA) with complete
Dulbecco’s modified Eagle’s medium (DMEM)-F12 (Gibco-BRL, Carlsbad,
CA, USA) containing 10% fetal bovine serum (FBS; Gibco-BRL) and 1%
penicillin-streptomycin (HyClone; Thermo Fisher Scientific,
Rockford, IL, USA). Cobble-shaped cells began to expand after two
days. At ~80% confluence, the cells were separated by digestion
with 0.25% trypsin-EDTA (Gibco-BRL) and passaged into three plastic
tissue culture flasks in the growth medium for expansion.
Galectin-3 lentiviral vector
generation
The following galectin-3 gene sequence:
5′-CAGGAGAGTCATTGTTTGCAA-3′, with a G/C content of 42.1%, was
obtained from GenBank (Accession no. NM_02306). Shanghai Ji Kaiji
Chemical Technology Co., Ltd. (Shanghai, China) designed the GV287
AgI cleavage lentivirus recombinant target gene plasmid containing
enhanced green fluorescent protein (EGFP). This viral vector frame
sequences were designed by Shanghai Ji Kaiji Chemical Technology
Co., Ltd as viral vector for overexpression of galectin-3 in
Eca-109 cells. The viral vector frame sequences were as follows:
5′-TCAGGAGAGTCATTGTTTGCAATTCAAGAGATTGCAAACAATGACTCTCCTGTTTTTTC-3′,
5′-TCGAGA AAAAACAGGAGAGTCATTGTTTGCAATCTCTTGAAT
TGCAAACAATGACTCTCCTGA-3′. The target gene segment was ligated into
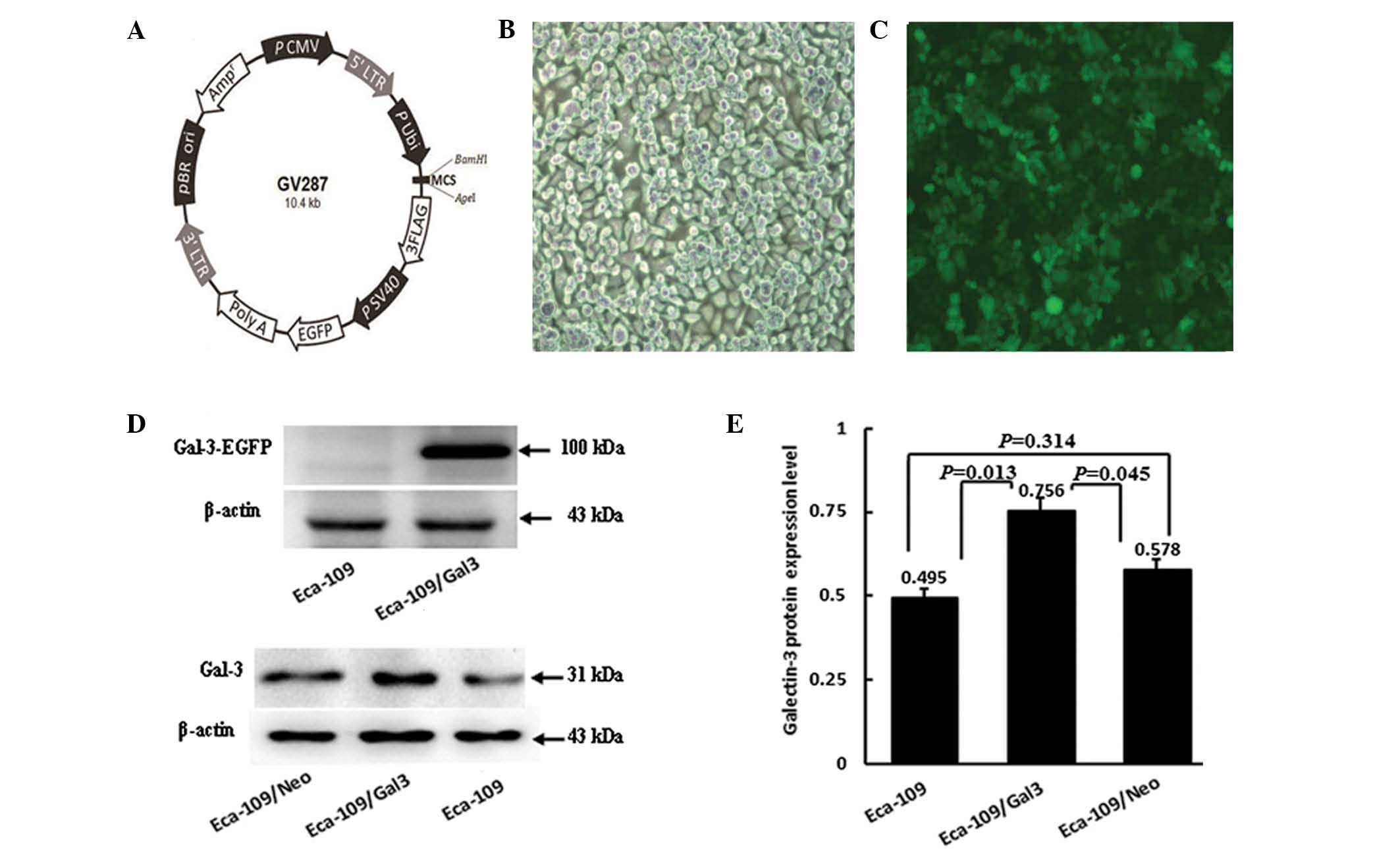
the GV287 vector (Fig. 1A;
Shanghai Ji Kaiji Chemical Technology Co., Ltd.).
Lentiviral transfection of Ecal09
cells
Eca-109 cells were transfected with the lentiviral
vector according to the lentiviral vector particle end-user
operation manual provided by Shanghai Ji Kaiji Chemical Technology
Co., Ltd. Preliminary transfection experiments were conducted to
confirm the optimal concentration of lentivirus; transfection
reagent was provided by the same company. At 80% confluence, the
Eca-109 cells were released into a single cell suspension by
digestion with trypsin-EDTA and seeded at 3,000–5,000 cells/ml) in
96-well tissue culture plates (Corning Inc.). After 24 h, the cells
were inoculated with four concentrations of lentivirus
(multiplicity of infection = 10, 20, 50 and 100, respectively) and
incubated at 37°C in 5% CO2. After 8 h, the transfection
medium was replaced with complete growth DMEM. After three days,
the optimal conditions for transfection were determined according
to the intensity of green fluorescent protein (GFP) expression
evaluated using an inverted fluorescence microscope (FSX100,
Olympus Corporation, Tokyo, Japan). On this basis, Eca-109 cells
were seeded (30,000–50,000 cells/ml) in 6-well tissue culture
plates (Corning Inc.). At 70–80% confluence, the cells were
digested and passaged into 25-cm2 cell culture flasks in
growth medium (complete DMEM -F12 containing 10% FBS) for
expansion. In addition, virus without anti-Smad was used to
transfect Eca-109 cells, serving as a negative control
(Eca-109/Neo). The recombinant galectin-3 lentiviral
vector-transfected Eca-109 cells were designated Eca-109/Gal-3.
Western blot analysis
Total protein extracts from Eca-109, Eca-109/Neo and
Eca-109/Gal-3 cells were homogenized with radioimmunoprecipitation
assay lysis solution (Sigma-Aldrich, St. Louis, MO, USA) and
centrifuged at 12,000 × g for 30 min at 4°C. The supernatant was
collected following centrifugation and protein concentrations were
determined using a Bicinchoninic Acid Protein Assay kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). Total protein extracts
were separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to a polyvinylidene
membrane (Millipore, Billerica, MA, USA). Western blot analysis was
conducted by incubating the membrane with monoclonal mouse
anti-galectin-3 antibody containing a recombinant fragment of human
galectin-3 full-length protein (1:1,000; Abcam, Cambridge, UK)
overnight at 4°C. The membranes were subsequently rinsed with wash
solution and incubated with sheep anti-rat IgG conjugated to
peroxidase (1:500; Sigma-Aldrich). Immunosignals were visualized
with the Protein Detector
5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium western
blotting kit (Beyotime Biotech., Jiangsu, China) according to the
manufacturer’s instructions. Enhanced chemiluminescence (ECL;
Millipore Corporation, Billerica, MA, USA) images were captured
with a FluorChem E instrument (Cell Biosciences, Santa Clara, CA,
USA). The quantification of each sample was conducted using
ImageQuant 5.2 software (GE Healthcare, Little Chalfont, UK). A
separate membrane was prepared using the same methods, and was
probed with mouse monoclonal anti-β-actin (1:1,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and mouse monoclonal
anti-GFP (1:1,000; CoWin Biotech Co., Ltd., Beijing, China)
antibodies.
Quantitative polymerase chain reaction
(qPCR) analysis
Reverse transcription-PCR and qPCR were used for
galectin-3 gene expression analysis. Total RNA was isolated from
cultured Eca-109 and Eca-109/Gal-3 cells using RNA-solv reagent
(Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer’s
instructions. The total RNA concentration was determined by
spectrophotometry (SPECTRA MAX190; Molecular Devices, Sunnyvale,
CA, USA). Complementary DNA (cDNA) was synthesized from 1 μg total
RNA using a Rever Tra Ace® qPCR-RT kit (Toyobo, Osaka
Japan) according to the manufacturer’s instructions.
PCR reactions were conducted using an ABI ViiA7 Dx
instrument (Life Technologies, Waltham, MA, USA) following the
manufacturer’s instructions, and performed in a 10 μl reaction
mixture containing 1 μl cDNA, 5 μl SYBR® Green (Toyobo),
1 μl of each primer and 2 μl H2O. The reaction
conditions were as follows: 10 sec at 65°C, followed by 60 cycles
of 5 sec at 60°C and 10 sec at 72°C, then 30 sec at 65°C.
Gene-specific primers were designed as determined by the following
human galectin-3 mRNA sequences in GenBank (Accession number,
NM_02306): Forward: 5′-GGTGAAGCCCAATGCAAACA-3′ and reverse:
5′-TGCAACCTTGAAGTGGTCAG-3′.
Amplification of human β-actin mRNA served as a
reference to normalize sample loading using the following primers:
Forward, 5′-TGGCACCCAGCACAATGAA-3′ and reverse:
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′.
PCR results were quantified using the ΔCt method
according to the following formula: Ratio = 2-ΔCt, where ΔCt = Ct
target gene − Ct endogenous control gene (β-actin) (14).
Cell proliferation assay
Eca-109, Eca-109/Neo and Eca-109/Gal-3 cell
suspensions (100 μl) were dispensed into 96-well round-bottomed
microtiter plates (3,000 cells/well) and incubated for 12–72 h at
37°C in 5% CO2. Subsequently, 10 μl cell counting kit-8
(CCK-8; Dojindo, Kunamoto, Japan) solution was added to each well
and the cells were incubated for a further 4 h. The absorbance was
measured at 450 nm with a spectrophotometer (Spectramax 190;
Molecular Devices, Sunnyvale, CA, USA). Growth curves were
generated from the average values of five wells in each group.
Cell apoptosis assay
Cell apoptosis was analyzed by flow cytometry
(FACSAria II; BD Biosciences, Franklin Lakes, NJ, USA). Cultured
cells were washed twice with phosphate-buffered saline (PBS) and
resuspended in binding buffer at 1×106 cells/ml. Cell
suspensions (1×105 cells/100 μl) were transferred to 5
ml culture tubes, and 5 μl Annexin V-phycoerythrin (PE;
eBioscience, San Diego, CA, USA) and 5 μl 7-amino-actinomycin
(7-AAD; eBioscience) were then added. The cells were gently
vortexed and incubated at room temperature in the dark for 15 min.
Subsequently, another 400 μl binding buffer was added. Flow
cytometry was performed within 4 h staining.
Transwell migration assay
Transwell insert (8.0 μm pore size) polycarbonate
filters (Costar®; Sigma-Aldrich) were used to examine
the effect of galectin-3 on cell migration. Serum-free medium
single-cell suspensions (5×104 cells/200 μl) were added
into the upper chamber and 500 μl complete DMEM medium was added to
the lower chamber. Following incubation for 24 h, the filters were
immersed in methanol for 15 min at room temperature, then with
0.25% crystal violet stain for 10 min at room temperature prior to
washing with water. The cells that had migrated to the lower side
of the filter were counted with an inverted fluorescence
microscope.
Matrigel invasion assay
The Transwell insert (8.0 μm pore size)
polycarbonate filters were used to investigate cell invasion. The
upper chamber was precoated with 100 μl 1:5 diluted Matrigel
(Becton-Dickinson, Franklin Lakes, NJ, USA). Serum-free medium
single-cell suspensions (1×105 cells/200 μl) were added
to the upper compartment of the precoated units. The units were
then transferred to wells containing 500 μl complete DMEM medium
and incubated for 48 h. The cells and Matrigel on the upper surface
of the membrane were removed with a cotton bud, then the membrane
was washed with PBS three times, immersed in methanol at room
temperature for 15 min and then immersed in 0.1% crystal violet
stain for 10 min at room temperature prior to washing with water.
The cells that had migrated through the pores to the lower side
were counted with an inverted fluorescence microscope.
Statistical analysis
All data were analyzed with SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA). The data are presented as the mean ± standard
deviation. Unpaired Student’s t-tests were performed for
comparisons between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Galectin-3 gene transfection
Preliminary experiments investigating the
transfection of Eca-109 cells showed that >98% the cells were
found to emit green fluorescence following transfection (Fig. 1B and C), indicating stable
expression of the galectin-3 gene.
Galectin-3 expression levels in Eca-109
cells
The levels of galectin-3 protein expression were
detected in Eca-109, Eca-109/Neo and Eca-109/Gal-3 cells by western
blotting. A 31-kDa band was detected in the Eca-109/Gal-3 group as
well as in the Eca-109 and Eca-109/Neo control groups. EGFP +
galectin-3 protein (100 kDa) was detected in Eca-109/Gal-3 cells
but not in Eca-109 cells (Fig.
1D). The protein expression levels were significantly higher in
the Eca-109/Gal-3 group than in the Eca-109 and Eca-109/Neo control
groups (P=0.013 and P=0.045, respectively), but no significant
differences were detected between the Eca-109 and Eca-109/Neo group
protein levels (P=0.314; Fig.
1E).
The expression levels of galectin-3 mRNA in the
Eca-109 cancer cells were quantified by qPCR. Galectin-3 mRNA was
detected in Eca-109, Eca-109/Neo and Eca-109/Gal-3 cells,
indicating that the galectin-3 is endogenously expressed in Eca-109
esophageal cancer cells. Compared with Eca-109 and Eca-109/Neo
cells, galectin-3 mRNA expression levels in Eca-109/Gal-3 cells
were increased 1.13- and 1.36-fold, respectively (P<0.05). No
significant differences were identified between the Eca-109 and
Eca-109/Neo cell galectin-3 mRNA expression levels (P>0.05).
Effect of galectin-3 on the proliferation
of Eca-109 cells
The effect of galectin-3 overexpression on the
proliferation of Eca-109 cancer cells was determined by the CCK-8
assay. Subsequent to culture for 12, 24, 48 and 72 h, the in
vitro growth of the Eca-109/Gal-3 group was significantly
higher than that of either the Eca-109 or Eca-109/Neo groups at all
time points (P<0.05). No significant differences were detected
between the Eca-109 and Eca-109/Neo groups at time point (12 h,
P=0.563; 24 h, P=0.917; 48 h, P=0.955; 72 h, P=0.495; Fig. 2).
Effect of galectin-3 on Eca-109 cell
apoptosis
The effect of galectin-3 overexpression on the
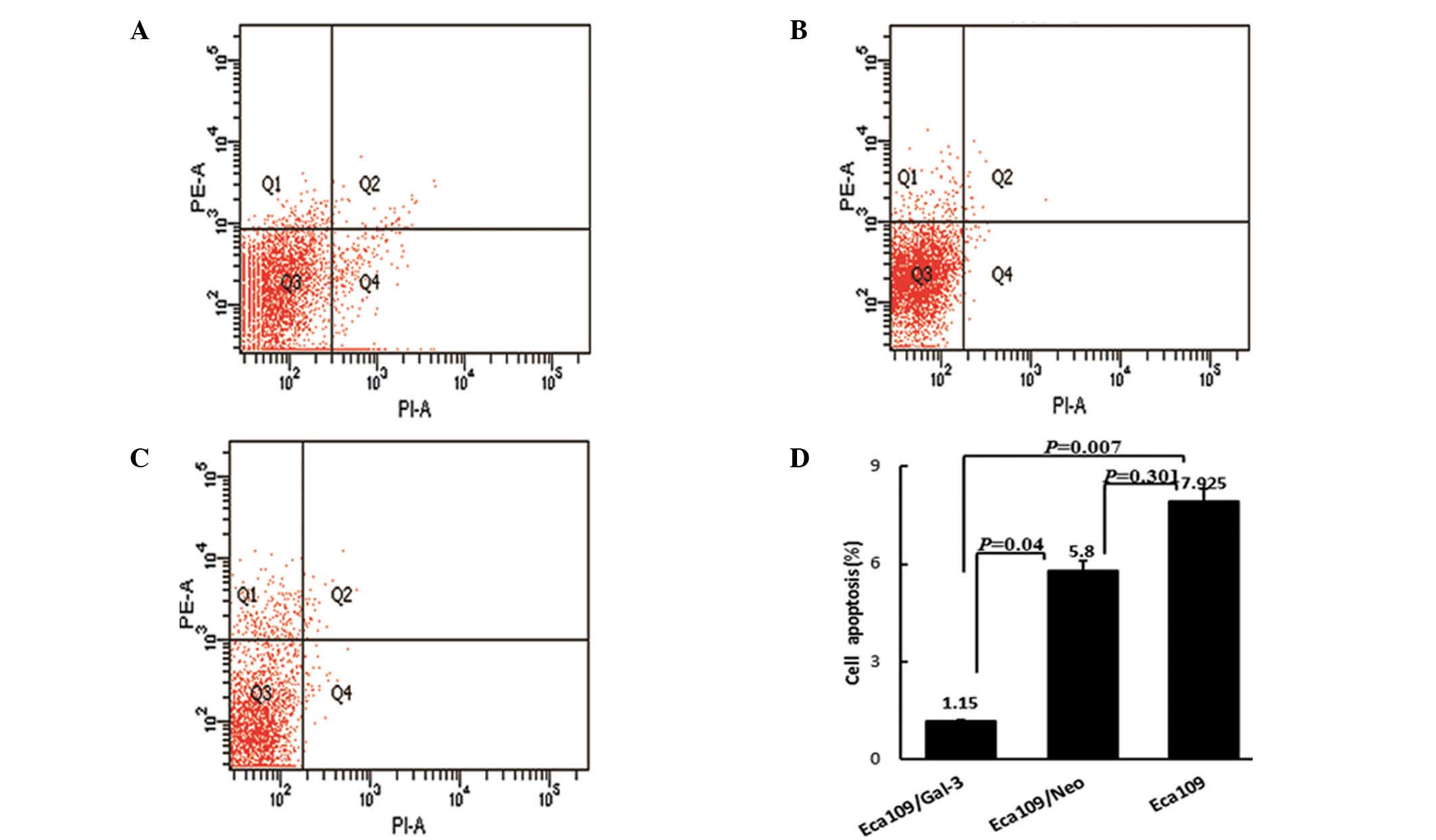
apoptosis of Eca-109 cells was determined by flow cytometric
analysis with Annexin V/PE and 7-AAD double-staining. Compared with
Eca-109 (7.9±4.4%) and Eca109/Neo cells (5.8±1.69%), the number of
apoptotic cells was significantly reduced in the Eca-109/Gal-3
group (1.2±0.26%; P=0.007 and P=0.04, respectively). No significant
differences were detected between the Eca-109 and Eca-109/Neo
groups (P=0.301; Fig. 3). These
results revealed that galectin-3 overexpression inhibited apoptosis
in Eca-109 cells.
Effect of galectin-3 on the migration and
invasion capacity of Eca-109 cells
The migration capacity of Eca-109/Gal-3 cells
(55.4±3.9), was significantly increased compared with that of
either the Eca-109 or the Eca109/Neo cells (P<0.05), while no
significant differences were observed between the migration
capacities of the Eca-109 and Eca-109/Neo cells (30.6±1.5 and
29±2.6 respectively, P>0.05).
Invasion is an important step in the movement of
tumor cells across the extracellular membrane (ECM) in tumor
metastasis. Therefore, ECMatrix served as a reconstituted basement
membrane matrix protein in invasion assays. Compared with Eca-109
and Eca-109/Neo cells, Eca-109/Gal-3 cells exhibited significantly
greater invasiveness across the ECMatrix (26.4±3.2, P<0.05). No
significant differences was detected between the Eca-109 and
Eca-109/Neo groups (14.8±2.6 and 12.4±2.3 respectively; P>0.05;
Fig. 4).
Discussion
Galectin-3, which is widely expressed in normal and
tumor cells, is associated with cell growth, adhesion,
differentiation and death, and has been observed to be expressed in
colon cancer, gastric cancer and other malignancies (4,8–13).
However, to the best of our knowledge, the galectin-3 expression
levels in esophageal cancer have not been reported thus far.
Galectin-3 is localized to the nucleus and
cytoplasm. Gaudin et al (15) reported galectin-3 to be either
exclusively cytoplasmic, predominantly nuclear or distributed
between the two compartments, depending on cell type and specific
experimental conditions. The nuclear versus cytoplasmic
distribution of galectin-3 in different cell types may reflect the
presence or absence of a potent nuclear export signal or a
compartment-specific anchor in the interacting partner. In the
present study, a 31-kDa protein band was detected in
non-transfected Eca-109 esophageal cancer cells, revealing that
galectin-3 is endogenously expressed. This was confirmed using
RT-PCR analysis of galectin-3 mRNA expression levels. However,
further investigation of primary esophageal cancer tissue is
required to determine whether this was an artifact of the Eca-109
esophageal cancer cell line used.
Metastasis is a fatal complication of malignancy.
Tumor cell metastasis from the primary to secondary sites is
associated with changes in cell adhesion, invasion and migration
that allow the survival of metastatic cells in the circulation, and
the formation of new vessels. Experimental and clinical studies
have revealed that the galectin-3 expressed in tumor cells is
important at different stages of tumorigenesis, including malignant
cell transformation, invasion and metastasis (6,7,16).
O’Driscoll et al (17)
confirmed that galectin-3 overexpression in a lung cancer cell line
significantly enhanced cell motility and invasiveness in
vitro, indicating that endogenous galectin-3 regulates cancer
cell migration.
Integrins have been observed to be expressed in
numerous cell types, and exert critical roles in inflammation,
apoptosis, proliferation, tumor cell migration and metastasis
(18). Notably, galectin-3 has
been reported to regulate tumor cell metastasis and invasion by
means of activating or expressing integrins (19). Therefore, the effects of galectin-3
on cell adhesion may be mediated by galectin-3 binding to
integrins.
Increasing evidence has demonstrated that
angiogenesis is essential for tumor growth and metastasis, and is
an important factor in cancer progression. Markowska et al
(20) reported that galectin-3
exhibited angiogenic activity in vitro, suggesting that it
modulates vascular endothelial growth factor- and basic fibroblast
growth factor-mediated angiogenesis through binding of the
carbohydrate recognition domain.
In the present study, galectin-3 overexpression was
found to be associated with enhanced migration and invasion
capacity in Eca-109 cells. This phenomenon indicates that
galectin-3 promotes esophageal cancer cell migration and invasion,
although confirmation in galectin-3 gene-silenced Eca-109
esophageal cancer cells is required.
The most extensively investigated function of
galectin-3 is the regulation of apoptosis, which is dependent on
the subcellular localization of galectin-3. The cytoplasmic
expression of galectin-3 is antiapoptotic (19). Following exposure of cells to
apoptotic stimuli, interaction with proteins, such as the
Ca2+- and phospholipid-binding synexin, is essential for
the translocation of galectin-3 to the perinuclear membrane to
inhibit changes in the mitochondrial membrane potential
thuspreventing apoptosis (21).
Therefore, the antiapoptotic activity of galectin-3 may also be
mediated by interaction with other apoptotic regulators that
function in the mitochondria.
The regulatory effects of galectin-3 on apoptosis
have been shown to be dependent on subcellular localization
(22). In the present study,
Annexin V/7-AAD double-staining was used to detect the effect of
galectin-3 on apoptosis. The results revealed that the apoptotic
rate was significantly lower in Eca-109/Gal-3 cells compared with
non-transfected Eca-109 cells, suggesting that as galectin-3
expression in Eca-109 cells is antiapoptotic, it must be
cytoplasmic. The antiapoptotic function of galectin-3 has been
documented in a series of studies. For instance, overexpression of
galectin-3 in lung cancer cells was found to be associated with
increased resistance to apoptosis compared with that of
non-transfected control cells (19). Furthermore, Yu et al
(21) demonstrated that BT549
human breast cells overexpressing galectin-3 were more resistant to
the apoptosis induced by cisplatin, nitricoxide, radiation and
anoikis than non-transfected cells. However, in the present study,
only the effect of overexpressed galectin-3 on the behavior of
human esophageal cancer Eca-109 cells was observed; the effect of a
galectin-3-silencing gene on apoptosis in Eca-109 cancer cells has
not yet been observed, although this may be analyzed in the
future.
In the present study, galectin-3 expression in
Eca-109 esophageal cancer cells was confirmed and galectin-3 was
implicated as a positive regulator of growth, migration and
invasion, and antiapoptotic effector. However, galectin-3
expression in esophageal cancer tissue remains to be confirmed. An
improved understanding of the role of galectin-3 in esophageal
cancer may provide a novel strategy for the diagnosis and prognosis
of esophageal cancer, and the development of novel therapeutic
regimens.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barondes SH, Castronovo V, Cooper DW, et
al: Galectins: a family of animal β-galactoside-binding lectins.
Cell. 76:597–598. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leffler H, Carlsson S, Hedlund M, Qian Y
and Poirier F: Introduction to galectins. Glycoconj J. 19:433–440.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lotan R, Ito H, Yasui W, et al: Expression
of a 31-kDa lactoside-binding lectin in normal human gastric mucosa
and in primary and metastatic gastric carcinomas. Int J Cancer.
56:474–480. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu FT, Hsu DK, Zuberi RI, et al:
Expression and function of galectin-3, a beta-galactoside binding
lectin, in human monocytes and macrophages. Am J Pathol.
147:1016–1028. 1995.PubMed/NCBI
|
|
6
|
Takenaka Y, Fukumori T and Raz A:
Galectin-3 and metastasis. Glycoconj J. 19:543–549. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakahara S, Oka N and Raz A: On the role
of galectin-3 in cancer apoptosis. Apoptosis. 10:267–275. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schoeppner HL, Raz A, Ho SB and Bresalier
RS: Expression of an endogenous galactose-binding lectin correlates
with neoplastic progression in the colon. Cancer. 75:2818–2826.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Canesin G, Gonzalez-Peramato P, Palou J,
et al: Galectin-3 expression is associated with bladder cancer
progression and clinical outcome. Tumour Biol. 31:277–285. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakaki M, Fukumori T, Fukawa T, et al:
Clinical significance of galectin-3 in clear cell renal cell
carcinoma. J Med Invest. 57:152–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knapp JS, Lokeshwar SD, Vogel U, et al:
Galectin-3 expression in prostate cancer and benign prostate
tissues: correlation with biochemical recurrence. World J Urol.
31:351–358. 2013. View Article : Google Scholar
|
|
12
|
Braeuer RR, Shoshan E, Kamiya T and
Bar-Eli M: The sweet and bitter sides of galectins in melanoma
progression. Pigment Cell Melanoma Res. 25:592–601. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Honjo Y, Nangia-Makker P, Inohara H and
Raz A: Downregulation of galectin-3 suppresses tumorigenicity of
human breast carcinoma cells. Clin Cancer Res. 7:661–668.
2001.PubMed/NCBI
|
|
14
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gaudin JC, Mehul B and Hughes RC: Nuclear
localisation of wild type and mutant galectin-3 in transfected
cells. Biol Cell. 92:49–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang RY, Rabinovich GA and Liu FT:
Galectins: structure, function and therapeutic potential. Expert
Rev Mol Med. 10:e172008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O’Driscoll L, Linehan R, Liang YH, et al:
Galectin-3 expression alters adhesion, motility and invasion in a
lung cell line (DLKP), in vitro. Anticancer Res. 22:3117–3125.
2002.
|
|
18
|
Hood JD and Cheresh DA: Role of integrins
in cell invasion and migration. Nat Rev Cancer. 2:91–100. 2002.
View Article : Google Scholar
|
|
19
|
Liu FT and Rabinovich GA: Galectins as
modulators of tumour progression. Nat Rev Cancer. 5:29–41. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Markowska AI, Liu FT and Panjwani N:
Galectin-3 is an important mediator of VEGF- and bFGF-mediated
angiogenic response. J Exp Med. 207:1981–1993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu F, Finley RL Jr, Raz A and Kim HR:
Galectin-3 translocates to the perinuclear membranes and inhibits
cytochrome c release from the mitochondria. A role for synexin in
galectin-3 translocation. J Biol Chem. 277:15819–15827. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haudek KC, Spronk KJ, Voss PG, et al:
Dynamics of galectin-3 in the nucleus and cytoplasm. Biochim
Biophys Acta. 1800.181–189. 2010.
|