Introduction
There are thousands of patients suffering from
articular cartilage defects worldwide, creating a great economic
burden on society. The repair of articular cartilage defects
remains a challenge for orthopedists, although a series of methods
have been developed to attempt to solve these problems, including
chondrocyte transplantation, cartilage transplantation and
artificial joint replacement (1).
Cartilage tissue engineering provides a novel method for the repair
of articular cartilage defects, an alternative to the traditional
‘Traumatic Restorative Treatment’ mode, which results in the
treatment of lesions at the cost of creating another lesions; for
example, the reconstruction of the ear in microtia patients where
the costal cartilage was excised and used for reconstruction of the
ear (2). Adipose-derived stem
cells (ADSCs) are a notable alternative source of seed cells for
cartilage tissue engineering. They can be easily collected by
liposuction and have the potential to differentiate into various
types of mesenchymal cell, including osteoblast, chondrocyte and
muscle cells (3–5). Yao et al (6) reported ectopic bone formation in
adipose-derived stromal cell-seeded osteoinductive calcium
phosphate scaffolds. Additionally, Yoon et al (7) demonstrated enhanced cartilage
formation via three-dimensional cell engineering of human ADSCs.
Besides the seeding cells, the biomaterial scaffold is also
essential to the success of cartilage tissue engineering
applications. Platelet-rich plasma (PRP) is blood plasma with
increased levels of platelets. As a concentrated source of
autologous platelets, PRP contains a high level of several growth
factors and other cytokines that have been widely used in
orthopedic surgery and regenerative medicine (8–10).
The significant advantages of PRP over other biomaterials are its
autologous source and high concentration of growth factors,
including transforming growth factor (TGF) β, insulin-like growth
factor (IGF) and vascular endothelial growth factor (8,11).
Previously, PRP was utilized in bone graft augmentation in oral and
maxillofacial surgery, and the results indicated significantly
improved bone density and fusion rates in the mandible (12). PRP has potential uses in tissue
healing and regeneration. Coviello et al (13) evaluated PRP wound healing benefits
in multiple myeloma (MM) patients that developed osteonecrosis of
the jaw (ONJ) following surgical tooth extraction, and the results
indicated that PRP improves wound healing in MM
bisphosphonate-associated ONJ. Also, Driver et al (14) reported autologous PRP gel treatment
of nonhealing diabetic foot ulcers, and observed that PRP
contributed to the healing process of these chronic wounds. In
vitro studies have confirmed that PRP enhances the
proliferation of a variety of human cell types. In addition, a
number of studies indicated that PRP increases cell growth and the
synthesis of extracellular matrix. Lucarelli et al (15) observed that 10% PRP promotes bone
marrow-derived stem cell proliferation, and Akeda et al
(16) also reported that PRP
stimulates porcine chondrocyte proliferation and matrix
biosynthesis.
In the current study, the effect of PRP on the
proliferation and chondrogenic differentiation of ADSCs was
investigated in order to evaluate the potential application of
ADSCs and PRP in the repair of articular cartilage defects. The
concentration of different growth factors and the proliferation of
ADSCs were analyzed in the present study, and the expression levels
of selected cartilage-specific genes were evaluated in order to
specifically explore the chondrogenic differentiation of ADSCs in
the presence or absence of PRP. The present study further
investigated the in vivo neocartilage formation of ADSCs in
PRP gel when co-cultured with chondrocytes.
Materials and methods
Tissue samples
The adipose tissue samples were obtained from the
lipoaspirates of patients who had undergone liposuction (mean age,
27 years; range, 20–35 years). Chondrocytes were isolated from
articular cartilage slices of patients (mean age, 58 years; range,
49–62 years) undergoing total knee arthroplasty at Shanghai 6th
People’s Hospital (Shanghai, China) from August 2012 to August
2013.
Cell harvest and culture
ADSCs from lipoaspirates and chondrocytes from
articular cartilage were isolated and expanded as previously
described (5,17). ADSCs and chondrocytes at passage 3
were used in the present study.
PRP preparation
Human PRP was harvested from the peripheral blood
using a continuous two-step sedimentation process as previously
described (18). Briefly, 10 ml
peripheral blood was harvested from cubital fossa venipuncture into
vacuum tubes and centrifuged at 200 × g for 10 min. Next, the
plasma was separated into PRP by further centrifugation at 200 × g
for 10 min. To activate the PRP, 10% thrombin solution (v/v, 1,000
U/ml in 100 mM CaCl2) was added to the PRP to yield PRP
gel. Soluble PRP releasates from the clotted preparations were
isolated by centrifugation (1,000 × g for 5 min).
Detection of growth factors
It is established that growth factors are important
in the proliferation and chondrogenic differentiation of ADSCs,
thus an enzyme-linked immunosorbent assay (ELISA) was utilized to
quantitatively analyze the concentrations of the growth factors
TGF-β, platelet-derived growth factor BB (PDGF-BB), IGF and
epidermal growth factor (EGF) in the whole blood and in the
activated PRP.
Measurement of cell proliferation
The ADSCs were cultured under four different
conditions as follows: 10% fetal bovine serum (FBS), 5% PRP (PRP
releasate in serum-free medium), 10% PRP and 20% PRP. The
proliferation of ADSCs was measured by a cell counting kit assay
(CCK8; Dojindo, Rockville, MD, USA) following culture in different
concentrations of PRP on days 1, 3, 5 and 7 in vitro, as
described in a previous study (19).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Cells from the four groups were harvested at days 1,
3 and 7 then dissolved in TRIzol (Sigma-Aldrich, St. Louis, MO,
USA) to extract the total RNA according to previously described
methods (20). In brief, Total RNA
was extracted using Trizol according to the manufacturer’s
instructions. 1 μg RNA was reverse-transcribed into cDNA using
Superscript II reverse transcriptase (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
cDNA amplification was performed in a thermocycler (Biometra T3000;
Biometra, Göttingen, Germany) using Taq polymerase supplied with
KCl buffer and 1.5mM MgCl2 (Invitrogen Life Technologies) at 94°C
for 1 min, 58°C for 30 sec and 72°C for 1 min. PCR products were
resolved on 1.5% agarose gel (Invitrogen Life Technologies) run in
1× Tris borate-EDTA buffer. The expression levels of the genes were
quantified in duplicates, using SYBR Green Master Mix (Applied
Biosystems, Foster City, CA, USA). PCR reactions were run on an ABI
7900HT RT-PCR system (Applied Biosystems) and the SDS software,
version 2.1 (Applied Biosystems) was used to analyze the results.
Gene expression was analyzed via comparative CT Method
(ΔΔCT) and were normalized to 18s rRNA. qPCR was used to
detect expression levels of cartilage-specific genes (type II
collagen, sox-9 and aggrecan) in order to evaluate the level of
chondrogenic differentiation. The primer sequences are presented in
Table I.
 | Table IPrimers used for quantitative
polymerase chain reaction. |
Table I
Primers used for quantitative
polymerase chain reaction.
| Gene | Primer | Product (bp) |
|---|
| Type II collagen |
F-CTGGACTAGTGGGTCCCAGG
R-CCTCTCCTTGCTCACCCTTG | 111 |
| Sox-9 |
F-GGCTCGGACACAGAGAACAC
R-GTGCGGCTTATTCTTGCTCG | 195 |
| Aggrecan |
F-GGTGTAGGCACCTCCTTTCC
R-GAAAGGGTGAGGGGTGTCAG | 106 |
| β-actin |
F-CTTCCAGCCTTCCTTCCTGG
R-CTGTGTTGGCGTACAGGTCT | 110 |
Construction of ADSC/PRP composites
The ADSCs at passage 3 were used for constructing
ADSC/PRP composites. Briefly, the cells were counted and
resuspended in PRP, at a cell density of 5.0×106/ml.
Next, 0.15 ml of 10% thrombin solution (v/v, 1,000 U/ml in 100 mM
CaCl2; Sigma-Aldrich) was added to the cell/PRP
suspension to activate the PRP and form ADSC/PRP composites. The
ADSC/PRP composites were divided into two groups: The experimental
group (ADSC/PRP composite co-culture with chondrocytes); and the
control group (ADSC/PRP composites alone). In the experimental
group, the ADSC/PRP composites were cultured in high-glucose
HyClone Dulbecco’s modified Eagle’s medium containing 10% FBS (GE
Healthcare Bio-Sciences, Piscataway, PA, USA) in a 0.4-μm
Transwell® chamber (Corning Life Sciences, Corning, NY,
USA) with adherent chondrocytes cultured in the plate below the
membrane, while ADSC/PRP composites in the control group were
cultured in the medium alone. After 21 days, the composites were
implanted subcutaneously into BALB-c nude mice (four weeks old;
weight, 20 g). Animals were sacrificed by cervival dislocation and
the samples were harvested at 8 weeks post-implantation. Mice were
purchased from the Shanghai Animal Center (Shanghai, China) and all
animal experimental procedures in the present study were approved
by the Ethics Committee of Shanghai Jiao Tong University School of
Medicine (Shanghai, China). Mice were housed at 21°C in regular
light (06:30–19:30 h)-dark (19:30–06:30 h) cycles with food and
water ad libitum at the Animal Care and Veterinary Services
Facility, according to the guidelines of the International Council
for Laboratory Animal Science. They were acclimated for 2 weeks
prior to the experiment.
Histological examination
Specimens were fixed in neutral-buffered formalin
overnight at 4°C, embedded in paraffin and sectioned (5-μm
thickness). The cross-sections were stained with hematoxylin/eosin
and safranin-O dyes, which indicate production of
glycosaminoglycan, then observed under an inverted phase contrast
microscope (TS100; Nikon, Tokyo, Japan).
Immunohistochemical analysis
Collagen II immunohistochemical staining was
performed to evaluate the chondrogenic differentiation of ADSC/PRP
composites. The sections were immersed in phosphate-buffered saline
(PBS) containing 1% goat serum at room temperature for 2 h to block
non-specific reactions. Subsequently, the sections were incubated
in PBS containing 1% bovine serum albumin (BSA) and collagen type
II monoclonal clonal mouse anti-rabbit antibody (DAB; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight. Following
washing with PBS three times, the samples were incubated in PBS
containing 3% BSA. Finally, the samples were incubated in PBS
containing 1% BSA and horseradish peroxidase (HRP)-conjugated
anti-mouse immunoglobulin G antibody (1:200; Santa Cruz
Biotechnology, Inc.) at 25°C for 4 h, followed by color development
with diaminobenzidine tetrahydrochloride (Santa Cruz Bitoechnology,
Inc.) (21).
Biochemical and biomechanical assay
After 8 weeks of in vivo culture, the
compressive modulus, glycosaminoglycan (GAG) content and total
collagen content were determined according to previously described
methods (22). In brief, a
biomechanical analyzer (Instron, Canton, MA, USA) was used for
biomechanical test, in which a constant compressive strain rate of
1 mm/min was applied until a maximal force of 100 N was achieved
and thus a force-displacement curve was obtained. The compressive
modulus of tested tissue was calculated based on the
force-displacement curve.
Statistical analysis
Data are presented as the mean ± standard deviation.
The data were analyzed by one-way analysis of variance using SPSS
17.0 software (International Business Machines, Armonk, NY, USA).
P<0.05 was considered to indicated a statistically significant
difference.
Results
Quantification of growth factors
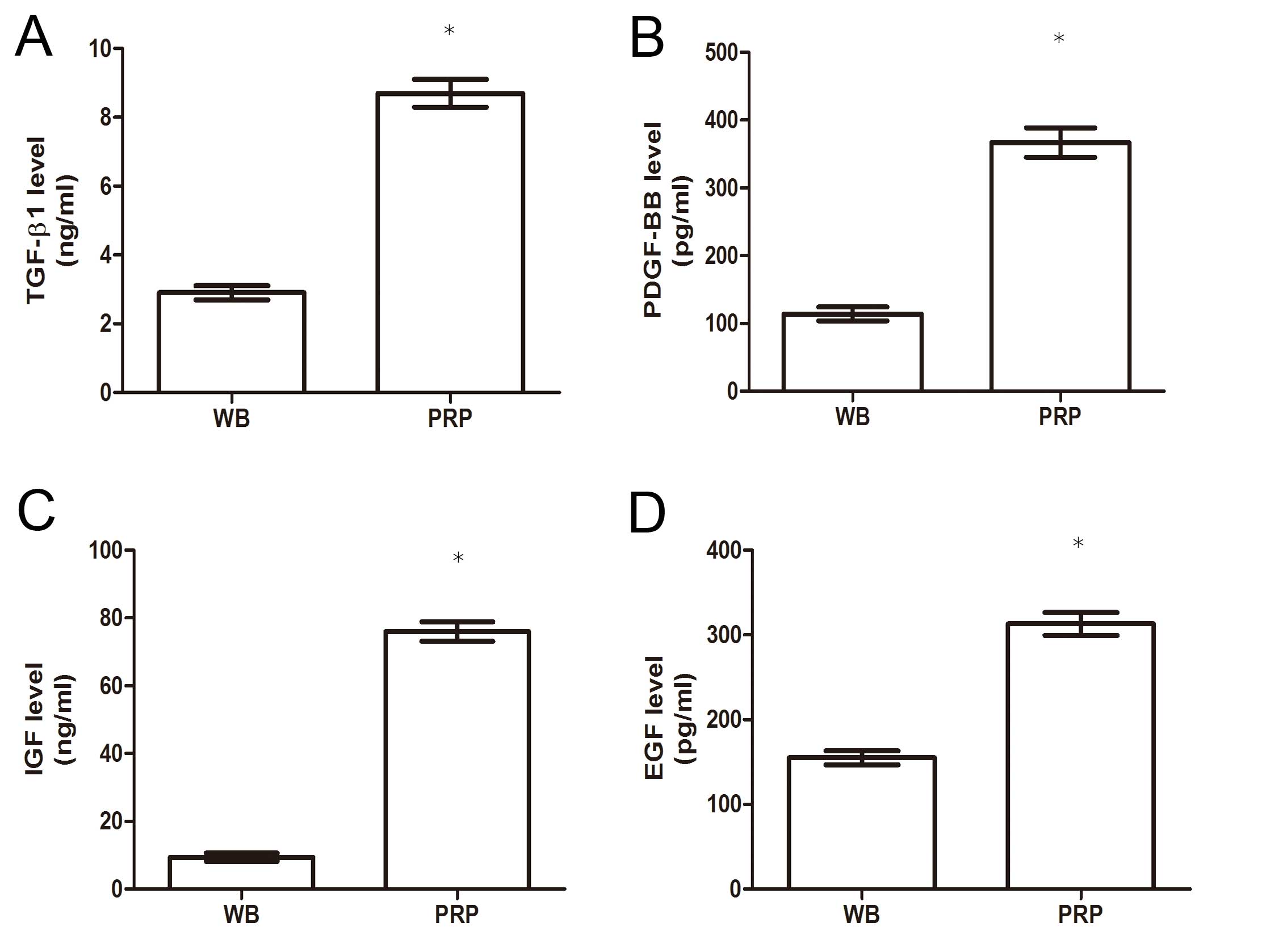
As presented in Fig.
1, the levels of the following growth factors exhibited a
significant difference between the two groups: TGF-β1 (8.70±1.10
ng/ml in PRP vs. 2.90±0.51 ng/ml in whole blood); PDGF-BB
(367.00±53.21 pg/ml vs. 114.43±25.35 pg/ml); IGF (76.00±8.22 ng/ml
vs. 9.40±1.60 ng/ml); and EGF (313±33.65 pg/ml vs. 155±23.98
pg/ml), indicating that activated PRP contained higher levels of
the growth factors than whole blood.
ADSC proliferation in different
concentrations of PRP
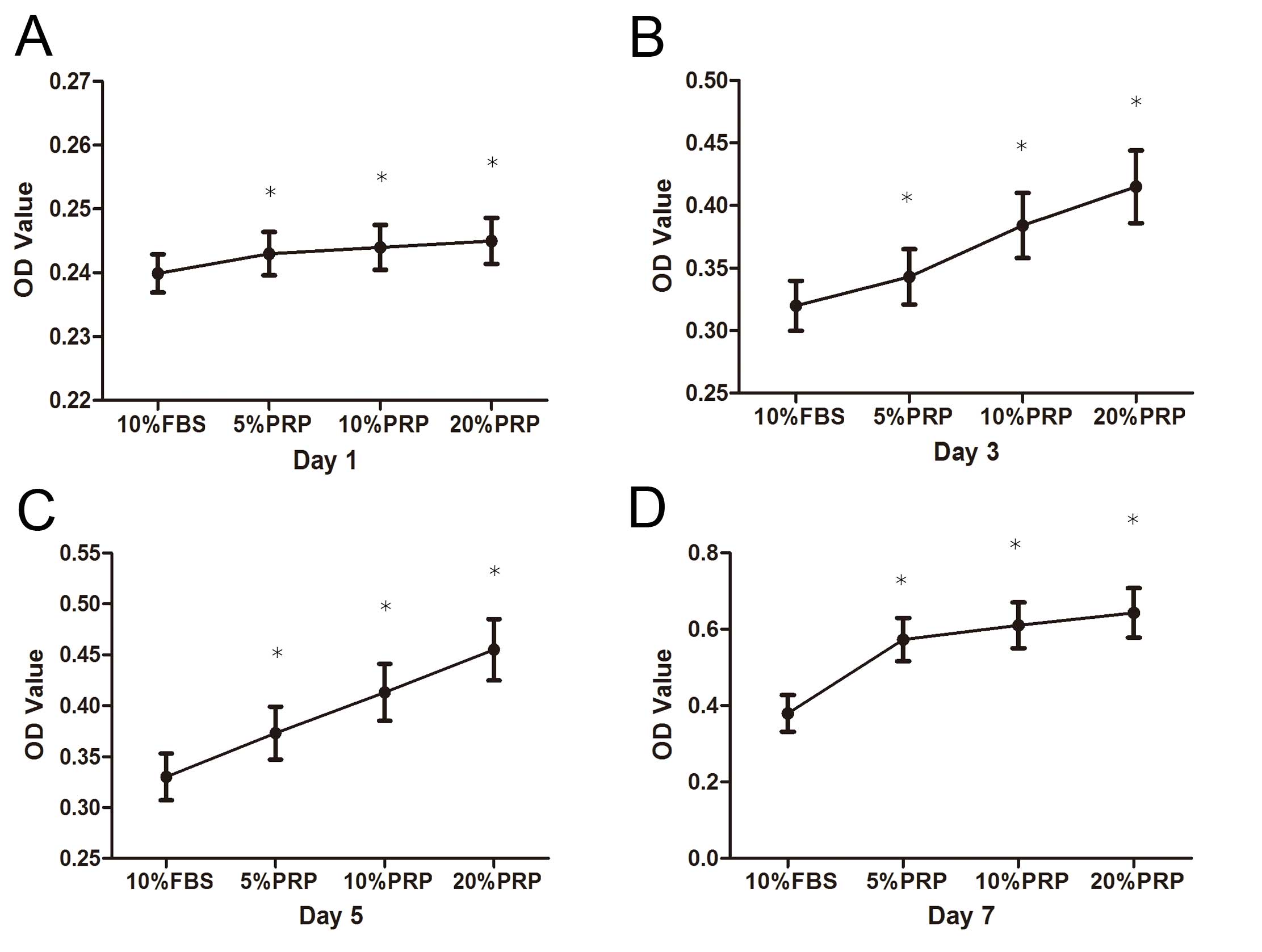
Fig. 2 indicates
the results of the cell proliferation assay. A significant increase
in the number of ADSCs over the 7-day in vitro culture
period was observed. In addition, there was a general increase in
cell number with increasing concentrations of PRP, and 20% PRP had
the strongest effect on ADSC proliferation.
Expression of cartilage-specific
genes
Expression levels of chondrogenic
differentiation-related genes were significantly increased
following PRP incubation. Collagen II, sox-9 and aggrecan were
upregulated in a dose- and time-dependent manner (Fig. 3).
In vivo result of ADSC/PRP
composites
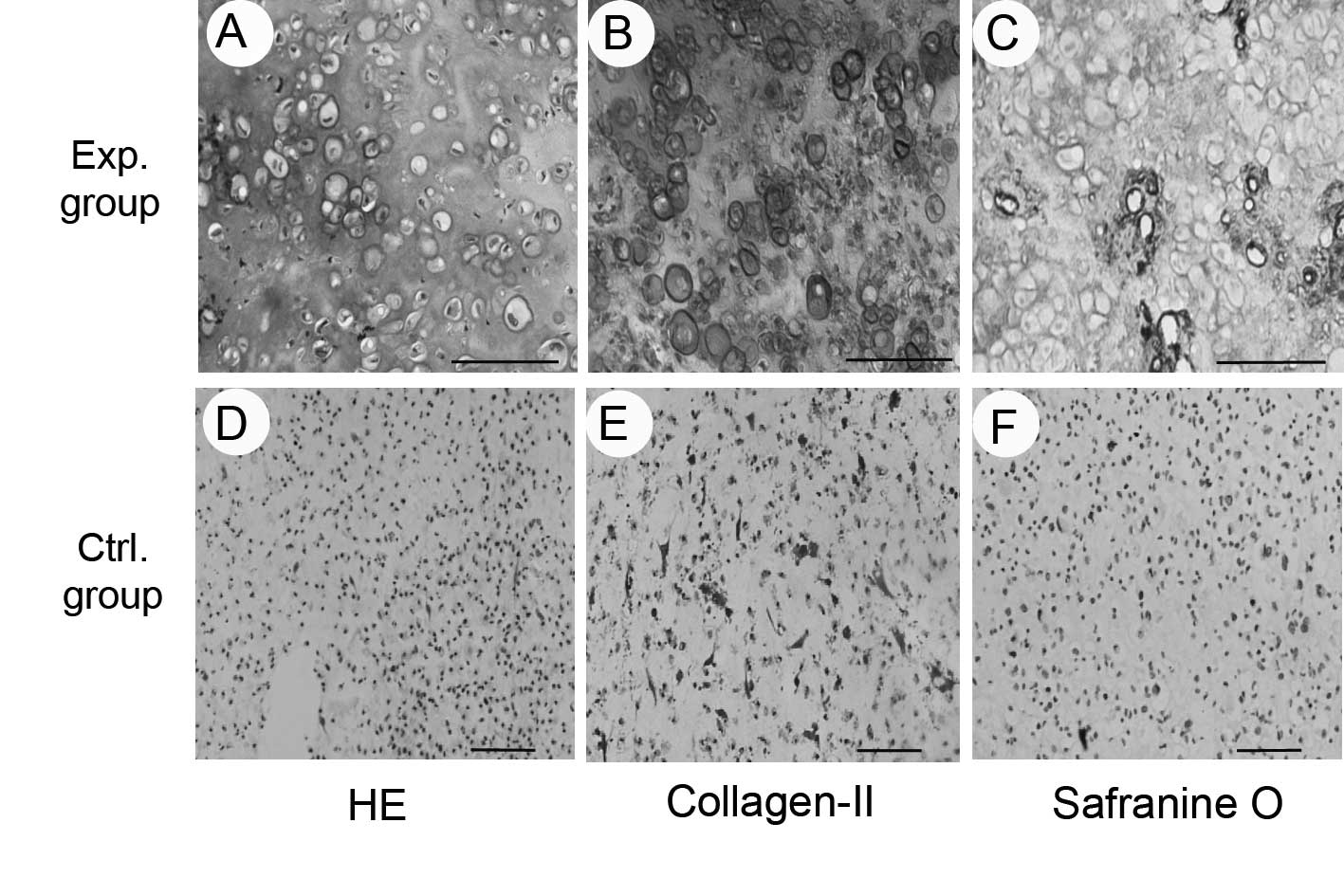
Eight weeks subsequent to subcutaneous implantation,
the ADSC/PRP composites in the experimental group presented an
ivory-whitish cartilage-like appearance, while the composites in
the control group had shrunk and become fibrous.
The results of histological and immunohistochemical
examinations showed that the composites in the experimental group
formed cartilage-like tissue with obvious lacuna-like structures,
and positive staining for safranin O, indicating the production of
glycosaminoglycan, and type II collagen (Fig. 4). The results of the biochemical
and biomechanical assays indicated that the GAG and total collagen
content, in addition to the compressive modulus, were significantly
increased in the PRP-treated groups when compared with the control
group (P<0.05; Fig. 5).
Discussion
Cartilage tissue engineering provides a potential
approach for the repair of articular cartilage defects (23). Cartilage tissue engineering
requires three key elements: A seeding cell source, a
three-dimensional biomaterial scaffold and a chondrogenic
microenvironment (24). Adult stem
cells may be ideal donor cells in cartilage regeneration, and
previous studies on the bone marrow-derived stem cells (BMSCs) have
been conducted (25). Liu et
al (24) constructed mature
engineered cartilage in vitro and in vivo using BMSCs
(26). However, the limited supply
of BMSCs restricts the clinical application of BMSC-based
engineered cartilage tissue. By contrast, adipose tissue, with its
abundant sources and easy methods of acquisition, in addition to
possessing multilineage differentiation potential and high
proliferation potential in vitro, is becoming an attractive
seeding cell source for tissue engineering (27). Animal and clinical studies have
previously demonstrated that ADSCs were able to repair damaged
skeletal tissue or bone defects. Declercq et al (28) constructed bone grafts engineered
from human ADSCs in dynamic three-dimensional environments, and
Guasti et al (29) reported
chondrogenic differentiation of ADSCs within nanocaged POSS-PCU
scaffolds. In the current study, the chondrogenic differentiation
of ADSCs was also demonstrated.
The other key aspect of cartilage tissue engineering
is the biomaterial scaffold. The quality of the scaffold
contributes greatly to the pattern of cell growth, proliferation
and differentiation on the scaffold and the success of tissue
regeneration. Previously, PRP, as autologous blood plasma
containing high levels of growth factors, has been widely used in
regenerative medicine, promoting the proliferation and
differentiation of a variety of cell types, including chondrocytes
and mesenchymal stem cells (MSCs) (15,16).
In the present study, the levels of the different growth factors
were measured in whole blood and PRP, and the results indicated
that the concentrations of growth factors in PRP were significantly
higher than the levels in whole blood. PRP, as a concentrated
source of autologous platelets, contains several different growth
factors and the release of growth factors occurs following the
activation of PRP. Conventionally, the addition of calcium and/or
thrombin to PRP is used to promote the release of the α granules
from platelets, and this process creates a PRP gel that contains
high concentrations of growth factors, such as TGF-β1, IGF, EGF and
PDGF-BB.
The release of growth factors contributes to cell
proliferation and differentiation. The growth curve of ADSCs in
different levels of PRP indicates that PRP promotes ADSC
proliferation, and ADSCs in PRP exhibited increased growth rates
than those in 10% FBS. Kakudo et al (30) demonstrated that ADSCs more rapidly
proliferate in 5% activated PRP than in 20% activated PRP. However,
the present results indicated that ADSC proliferation increased in
a PRP concentration-dependent manner.
Additionally, PRP stimulates ADSC proliferation and
chondrogenic differentiation of ADSCs in vitro. The current
in vitro findings suggested that cartilage-specific genes,
such as collagen II, sox-9 and aggrecan were also significantly
higher in the PRP-treated cells compared with the controls.
However, the results also indicated that the ADSC/PRP composites
alone were not able to form neo-cartilage 8 weeks following
subcutaneous implantation. This indicates that the expression of
cartilage-specific genes was reduced and suggests that the
expression of growth factors and other cytokines was decreased, and
were therefore unable to promote the chondrogenic differentiation
of ADSCs. Notably, ADSCs in the PRP gel formed neo-cartilage in
vivo when co-cultured with chondrocytes, while ADSCs combined
with PRP gel alone formed fibrous tissue in vivo. A previous
study reported that chondrocytes promoted the chondrogenic
differentiation of MSCs. Xue et al (21) demonstrated that chondrocytes
promoted the stable subcutaneous chondrogenesis of bone
marrow-derived stromal cells. This suggests that PRP may promote
the chondrogenic differentiation of ADSC in a chondrogenic
microenvironment and particularly in an articular microenvironment,
indicating that ADSCs combined with PRP gel would be an ideal
therapy for the repair of articular cartilage defects.
In conclusion, the current study identified that
inactivated PRP significantly enhances human ADSC proliferation
in vitro. In addition, it was demonstrated that inactivated
PRP promoted the expression of cartilage-specific genes, including
collagen II, sox-9 and aggrecan in vitro. ADSCs combined
with PRP gel formed mature engineered cartilage tissue in
vivo following co-culture with chondrocytes, providing a
potential novel method for the treatment of cartilage defects.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81071452 and 81271961).
References
|
1
|
Mera H, Itokazu M and Wakitani S:
Cartilage repair and regenerative medicine; past, present, and
future. Clin Calcium. 23:1715–1722. 2013.(In Chinese). PubMed/NCBI
|
|
2
|
Kristiansen M, Öberg M and Wikström SO:
Patients’ satisfaction after ear reconstruction with autologous rib
cartilage. J Plast Surg Hand Surg. 47:113–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu G, Cheng Y, Guo S, et al:
Transplantation of adipose-derived stem cells for peripheral nerve
repair. Int J Mol Med. 28:565–572. 2011.PubMed/NCBI
|
|
4
|
Cho JW, Kang MC and Lee KS: TGF-β1-treated
ADSCs-CM promotes expression of type I collagen and MMP-1,
migration of human skin fibroblasts, and wound healing in vitro and
in vivo. Int J Mol Med. 26:901–906. 2010.PubMed/NCBI
|
|
5
|
Lv XJ, Zhou GD, Liu Y, et al: In vitro
proliferation and differentiation of adipose-derived stem cells
isolated using anti-CD105 magnetic beads. Int J Mol Med.
30:826–834. 2012.PubMed/NCBI
|
|
6
|
Yao J, Li X, Bao C, et al: Ectopic bone
formation in adipose-derived stromal cell-seeded osteoinductive
calcium phosphate scaffolds. J Biomater Appl. 24:607–624. 2010.
View Article : Google Scholar
|
|
7
|
Yoon HH, Bhang SH, Shin JY, Shin J and Kim
BS: Enhanced cartilage formation via three-dimensional cell
engineering of human adipose-derived stem cells. Tissue Eng Part A.
18:1949–1956. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fallouh L, Nakagawa K, Sasho T, et al:
Effects of autologous platelet-rich plasma on cell viability and
collagen synthesis in injured human anterior cruciate ligament. J
Bone Joint Surg Am. 92:2909–2916. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang YH, Jeon SH, Park JY, et al:
Platelet-rich fibrin is a Bioscaffold and reservoir of growth
factors for tissue regeneration. Tissue Eng Part A. 17:349–359.
2011. View Article : Google Scholar
|
|
10
|
Vadalà G, Di Martino A, Tirindelli MC,
Denaro L and Denaro V: Use of autologous bone marrow cells
concentrate enriched with platelet-rich fibrin on corticocancellous
bone allograft for posterolateral multilevel cervical fusion. J
Tissue Eng Regen Med. 2:515–520. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cho JW, Kim SA and Lee KS: Platelet-rich
plasma induces increased expression of G1 cell cycle regulators,
type I collagen, and matrix metalloproteinase-1 in human skin
fibroblasts. Int J Mol Med. 29:32–36. 2012.
|
|
12
|
Marx RE, Kline SN, Johnson RP, et al: The
use of freeze-dried allogeneic bone in oral and maxillofacial
surgery. J Oral Surg. 39:264–274. 1981.PubMed/NCBI
|
|
13
|
Coviello V, Peluso F, Dehkhargani SZ, et
al: Platelet-rich plasma improves wound healing in multiple myeloma
bisphosphonate-associated osteonecrosis of the jaw patients. J Biol
Regul Homeost Agents. 26:151–155. 2012.PubMed/NCBI
|
|
14
|
Driver VR, Hanft J, Fylling CP and Beriou
JM: Autologel Diabetic Foot Ulcer Study Group: A prospective,
randomized, controlled trial of autologous platelet-rich plasma gel
for the treatment of diabetic foot ulcers. Ostomy Wound Manage.
52:68–74. 2006.
|
|
15
|
Lucarelli E, Beccheroni A, Donati D, et
al: Platelet-derived growth factors enhance proliferation of human
stromal stem cells. Biomaterials. 24:3095–3100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akeda K, An HS, Okuma M, et al:
Platelet-rich plasma stimulates porcine articular chondrocyte
proliferation and matrix biosynthesis. Osteoarthritis Cartilage.
14:1272–1280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moon MH, Jeong JK, Lee YJ, Seol JW and
Park SY: Sphingosine-1-phosphate inhibits interleukin-1β-induced
inflammation in human articular chondrocytes. Int J Mol Med.
30:1451–1458. 2012.PubMed/NCBI
|
|
18
|
Kim YH, Furuya H and Tabata Y: Enhancement
of bone regeneration by dual release of a macrophage recruitment
agent and platelet-rich plasma from gelatin hydrogels.
Biomaterials. 35:214–224. 2014. View Article : Google Scholar
|
|
19
|
Van Pham P, Bui KH, Ngo DQ, et al:
Activated platelet-rich plasma improves adipose-derived stem cell
transplantation efficiency in injured articular cartilage. Stem
Cell Res Ther. 4:912013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xue K, Qi L, Zhou G and Liu K: A two-step
method of constructing mature cartilage using bone marrow-derived
mesenchymal stem cells. Cells Tissues Organs. 197:484–495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xue K, Zhu Y, Zhang Y, Chiang C, Zhou G
and Liu K: Xenogeneic chondrocytes promote stable subcutaneous
chondrogenesis of bone marrow-derived stromal cells. Int J Mol Med.
29:146–152. 2012.
|
|
22
|
Wu J, Xue K, Li H, Sun J and Liu K:
Improvement of PHBV scaffolds with bioglass for cartilage tissue
engineering. PloS One. 8:e715632013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cima LG, Vacanti JP, Vacanti C, Ingber D,
Mooney D and Langer R: Tissue engineering by cell transplantation
using degradable polymer substrates. J Biomech Eng. 113:143–151.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vinatier C, Bouffi C, Merceron C, et al:
Cartilage tissue engineering: towards a biomaterial-assisted
mesenchymal stem cell therapy. Curr Stem Cell Res Ther. 4:318–329.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dorotka R, Windberger U, Macfelda K,
Bindreiter U, Toma C and Nehrer S: Repair of articular cartilage
defects treated by microfracture and a three-dimensional collagen
matrix. Biomaterials. 26:3617–3629. 2005. View Article : Google Scholar
|
|
26
|
Liu K, Zhou GD, Liu W, et al: The
dependence of in vivo stable ectopic chondrogenesis by human
mesenchymal stem cells on chondrogenic differentiation in vitro.
Biomaterials. 29:2183–2192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qing W, Guang-Xing C, Lin G and Liu Y: The
osteogenic study of tissue engineering bone with BMP2 and BMP7
gene-modified rat adipose-derived stem cell. J Biomed Biotechnol.
2012:4108792012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Declercq HA, De Caluwé T, Krysko O,
Bachert C and Cornelissen MJ: Bone grafts engineered from human
adipose-derived stem cells in dynamic 3D-environments.
Biomaterials. 34:1004–1017. 2013. View Article : Google Scholar
|
|
29
|
Guasti L, Vagaska B, Bulstrode NW,
Seifalian AM and Ferretti P: Chondrogenic differentiation of
adipose tissue-derived stem cells within nanocaged POSS-PCU
scaffolds: a new tool for nanomedicine. Nanomedicine. 10:279–289.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kakudo N, Minakata T, Mitsui T, Kushida S,
Notodihardjo FZ and Kusumoto K: Proliferation-promoting effect of
platelet-rich plasma on human adipose-derived stem cells and human
dermal fibroblasts. Plast Reconstr Surg. 4:1352–1360. 2008.
View Article : Google Scholar
|