Introduction
The number of patients with cartilage disorders has
been increasing globally (1). This
is a complex issue compounded by the lack of a consensus on
treatment; hence, the development of a conclusive therapy for
cartilage disorders is necessary.
The process of cartilage tissue growth or
longitudinal growth, also known as endochondral ossification,
occurs within the long bones at the growth plate located between
the epiphysis and the metaphysis (2,3). At
the growth plate, chondrogenic differentiation occurs from the
diaphyseal side toward the metaphyseal side and chondrocytes are
arranged longitudinally in a columnar shape, forming a layered
structure. These organized chondrocytes are divided into three
principal zones: The resting zone, the proliferative zone and the
hypertrophic zone. The resting zone consists of small and immature
chondrocytes, which differentiate into more mature chondrocytes in
the proliferating zone. Large chondrocytes are found in the
hypertrophic zone, where they exhibit a 5–10-fold increase in size
(3). In the proliferative zone,
chondrocytes produce numerous significant extracellular matrix
proteins (ECM), including type II collagen and aggrican, which are
structurally essential to the growth plate (4). In the hypertrophic zone, chondrocytes
produce type X collagen as they cease to proliferate (5). In the adjacent metaphyseal zone,
chondrocytes undergo apoptotic cell death, attract blood vessels
and lay down a true bone matrix within the cartilage matrix
(2). Thus, endochondral
ossification is characterized by the continual proliferation,
differentiation, and growth arrest of chondrocytes, and is
regulated by a number of factors and hormones (2).
Cyclin-dependent kinases (CDKs) are widely
recognized as regulators of cell cycle progression and CDK
activation is regulated by CDK inhibitors (CKIs) (6,7). p21
is a CKI which has been identified as a cell cycle regulator; its
induction by p53 during the DNA damage-induced G1-phase
checkpoint response inhibits CDK4 and CDK2 (8–10).
Asada et al (11) reported
that cytoplasmic p21 also acts as an inhibitor of apoptosis and
clinical research focusing on p21 has been conducted in the fields
of angiology and oncology (12,13).
Furthermore, the association between the p53/p21 pathway and
induced pluripotent stem cell generation has been established
(14,15). In the field of regenerative
medicine, Bedelbaeva et al (16) reported that a p21-knockout mouse
strain was able to close ear hole wounds and displayed increased
morphological and histological regenerative responses when compared
with the wild-type (WT) mouse strain, providing a firm link between
cell cycle checkpoint control and tissue regeneration. Several
studies have reported that the expression of p21 is essential for
chondrogenesis in vitro (17,18).
Negishi et al (4) reported
that the progression of chondrogenic differentiation requires the
downregulation of CDK2-associated kinase activity with an increase
in the levels of p21 protein, and the subsequent degradation of
this protein via a proteasomal pathway. Despite studies which
indicate the importance of p21, the original study of p21-knockout
mice in 1995 described that these mice may develop normally
(19). However, these results were
reported strictly in adult mice from histological findings in areas
such as muscles and vertebrae. Additionally, this study did not
contain any information regarding the roles of p21 in the
development of articular cartilage of limbs. The aim of the present
study was to clarify the function of p21 in the embryonic
endochondral ossification of articular cartilage in mice.
Materials and methods
Mouse breeding
All procedures were approved by the Animal Studies
Committee at Kobe University, Kobe, Japan. p21 knockout mice
(B6.129S6 (Cg)-Cdkn1atm1Led/J) were obtained from The Jackson
Laboratory (Bar Harbor, ME, USA). All mice were housed in cages
under pathogen-free conditions and were allowed unlimited access to
water and food.
The mice were bred in the animal facility at Kobe
University Graduate School of Medicine (Kobe, Japan). To generate
heterozygous mice, homozygous p21 knockout (KO) and WT mice
(C57BL/6J; CLEA Japan, Inc., Tokyo, Japan) were mated. Next,
heterozygous mice were mated to obtain embryos from the two groups
of mice: p21 KO and WT. A total of ten mice were used for each
experiment.
Tissue harvesting and
decalcification
Pregnant heterozygous mice were anesthetized by an
intraperitoneal injection of pentobarbital (50 mg/kg) and
sacrificed by cervical disolcation at embryonic days E13.5, E15.5
and E18.5 (n=10 for each time point). Following collection of the
embryos, the embryo forearms were dissected, fixed in 4%
paraformaldehyde buffered with phosphate-buffered saline (PBS),
decalcified with 10% formic acid and embedded in paraffin. Sagittal
histological sections were cut at a thickness of 6 μm using a
microtome and stained with Safranin O (Tokyo Chemical Industry Co.,
Ltd., Tokyo, Japan) and 5-bromo-2′-deoxyuridine (BrdU; BD
Biosciences, San Jose, CA, USA). Tissue sections were also
subjected to immunohistochemical and immunofluorescence
analyses.
BrdU labeling and staining
To confirm the cell cycle progression at the
G1/S phase, pregnant mice were injected
intraperitoneally with 200 μl BrdU and sacrificed by cervical
dislocation 2 h later to obtain embryonic tissues. Staining was
performed using a BrdU In-Situ Detection kit (BD
Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer’s instructions and the sections were examined using a
BZ-8100 confocal microscope (Keyence, Osaka, Japan).
Genotyping of mouse embryos
Genotypes were verified by polymerase chain reaction
(PCR) analysis of tail-derived DNA. Genomic DNA was extracted using
the DNeasy Blood & Tissue kit (Qiagen, Valencia, CA, USA). p21
deletion was confirmed by the presence of a 447-bp fragment unique
to the mutant genotype, which was amplified with a p21-specific
forward primer (5′-GTTGTCCTCGCCCTCATCTA-3′) and a mutant reverse
primer (5′-CTGTCCATCTGCACGAGACTA-3′) (sequences provided by The
Jackson Laboratory). WT alleles were confirmed by the presence of a
240 bp fragment amplified with the WT reverse primer
(5′-GCCTATGTTGGGAAACCAGA-3′) and the p21-specific forward primer.
DNA amplification was performed under the following PCR conditions:
94°C for 5 min, followed by 40 cycles of 94°C for 30 sec, 55°C for
30 sec and 72°C for 30 sec, and ending with 72°C for 2 min.
Immunohistochemistry
De-paraffinized sections were digested with
proteinase (Dako Retrieval Solution Ready-to-Use; Dako, Glostrup,
Denmark) for 20 min and treated with 3% hydrogen peroxide (Wako
Pure Chemical Industries, Osaka, Japan) to block endogenous
peroxidase activity. In addition to BrdU staining, the expression
of cyclin D1 was examined to determine cell cycle progression at
the G1/S phase, as cyclin D1 is a G1/S
phase-specific protein (20).
Furthermore, the expression levels of p16, type II collagen, type X
collagen, and Sox9 were examined. INK4 is a tumor suppressor
protein which causes G1 phase cell cycle arrest and p16
is a known representative of the INK4 family (21). Type II collagen is the foundation
for articular cartilage and hyaline cartilage and it has been
established that type X collagen is produced by hypertrophic
chondrocytes (4). Sox9 is
essential for chondrocyte differentiation and cartilage formation
(22). Tissue sections were
treated overnight at 4°C in Can Get Signal immunostain solution A
(Toyobo, Tokyo, Japan) and the following antibodies: rabbit
anti-mouse cyclin D1 polyclonal antibody (1:50 dilution; Cell
Signaling Technology, Inc., Danvers, MA, USA), rabbit anti-mouse
p16 polyclonal antibody (1:50 dilution; Abbiotec LLC, San Diego,
CA, USA), rabbit anti-mouse type II collagen polyclonal antibody
(1:100 dilution; Cosmo Bio Co., Ltd, Tokyo, Japan), rabbit
anti-mouse type II collagen polyclonal antibody (1:50 dilution;
Cosmo Bio Co., Ltd) and rabbit anti-mouse Sox9 polyclonal antibody
(1:100 dilution; Abcam, Cambridge, UK). Subsequently, the sections
were treated with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit immunoglobulin anitibody (N-Histofine® Simple Stain
Mouse MAX PO (R); Nichirei Bioscience, Tokyo, Japan) at room
temperature for 30 min. The signal was developed as a brown
reaction product using the peroxidase substrate
3,3′-diaminobenzidine (Histofine Simple Stain DAB solution;
Nichirei Bioscience), and the sections were examined using a
BZ-8000 Confocal microscope (Keyence, Osaka, Japan).
Immunofluorescence
Deparaffinized sections were digested with
proteinase (Dako Retrieval Solution Ready-to-Use) for 20 min and
treated overnight at 4°C with the following antibodies in Can Get
Signal immunostain solution A: Rabbit anti-mouse p27 polyclonal
antibody (1:50 dilution; Santa Cruz Biotechnology, Inc.). The
secondary antibodies used were goat anti-rabbit immunoglobulin
Alexa Fluor 488 (1:200 dilution; Life Technologies, Carlsbad, CA,
USA) for 30 min at room temperature. The nuclei were stained with
DAPI and images were captured using a BZ-8000 confocal microscope
(Keyence).
Statistical analysis
Statistical analysis was performed using the SPSS
version 16.0 software package (SPSS, Inc., Chicago, IL, USA). The
differences in the percentages of cyclin D1 or BrdU-positive cells
between the groups at each time point were analyzed using the Mann
Whitney U-test. P<0.05 was considered to indicate a
statistically significant difference. All data are expressed as the
mean ±standard deviation.
Results
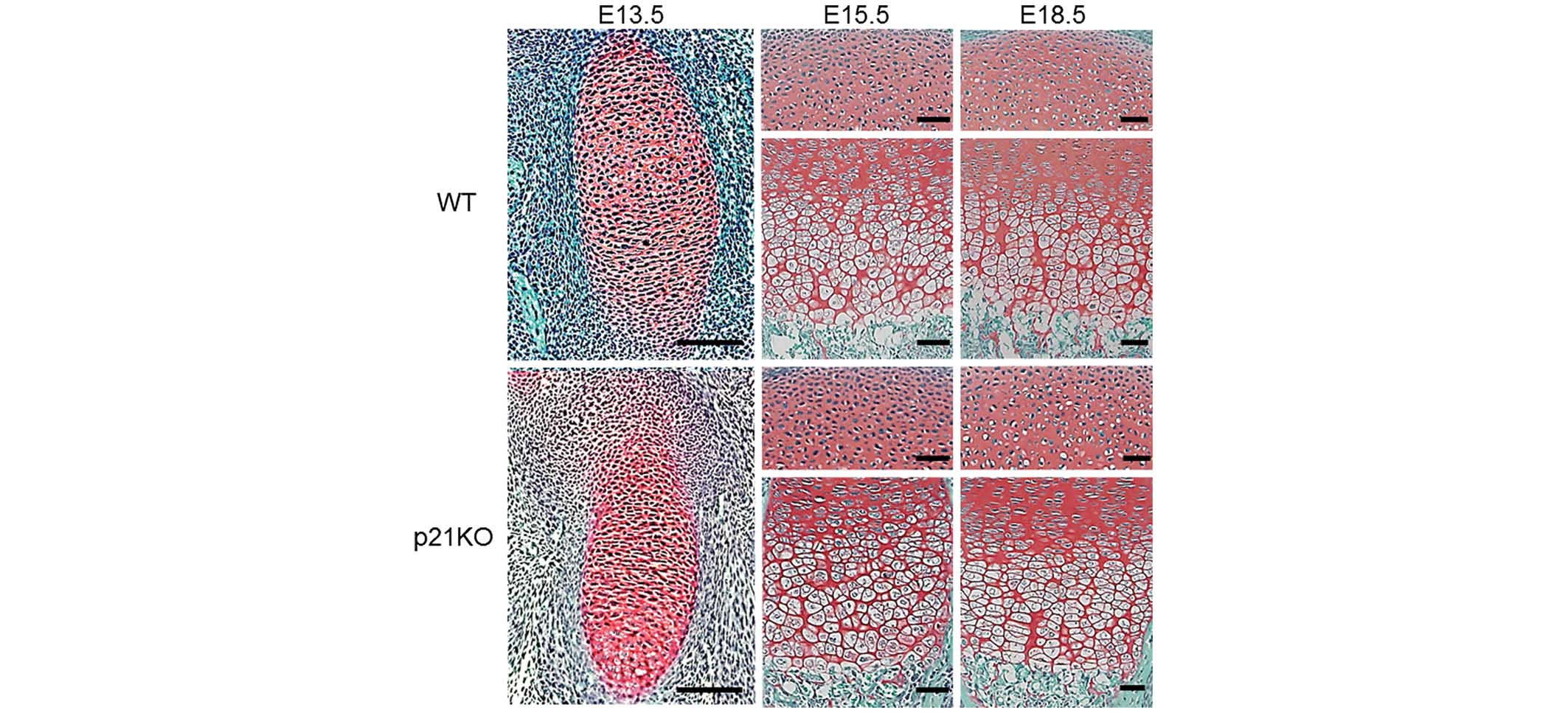
Cartilage tissue morphology in embryonic
mice is not altered by p21 deficiency
To investigate the in vivo function of p21 in
chondrogenesis, a histological analysis of cartilage tissues was
performed at E13.5, E15.5 and E18.5. Safranin O staining revealed
no structural changes at any time point between the embryonic
cartilage tissues of WT and p21KO mice (Fig. 1). These results indicate that p21
deficiency does not alter the morphology of embryonic cartilage
tissue in mice.
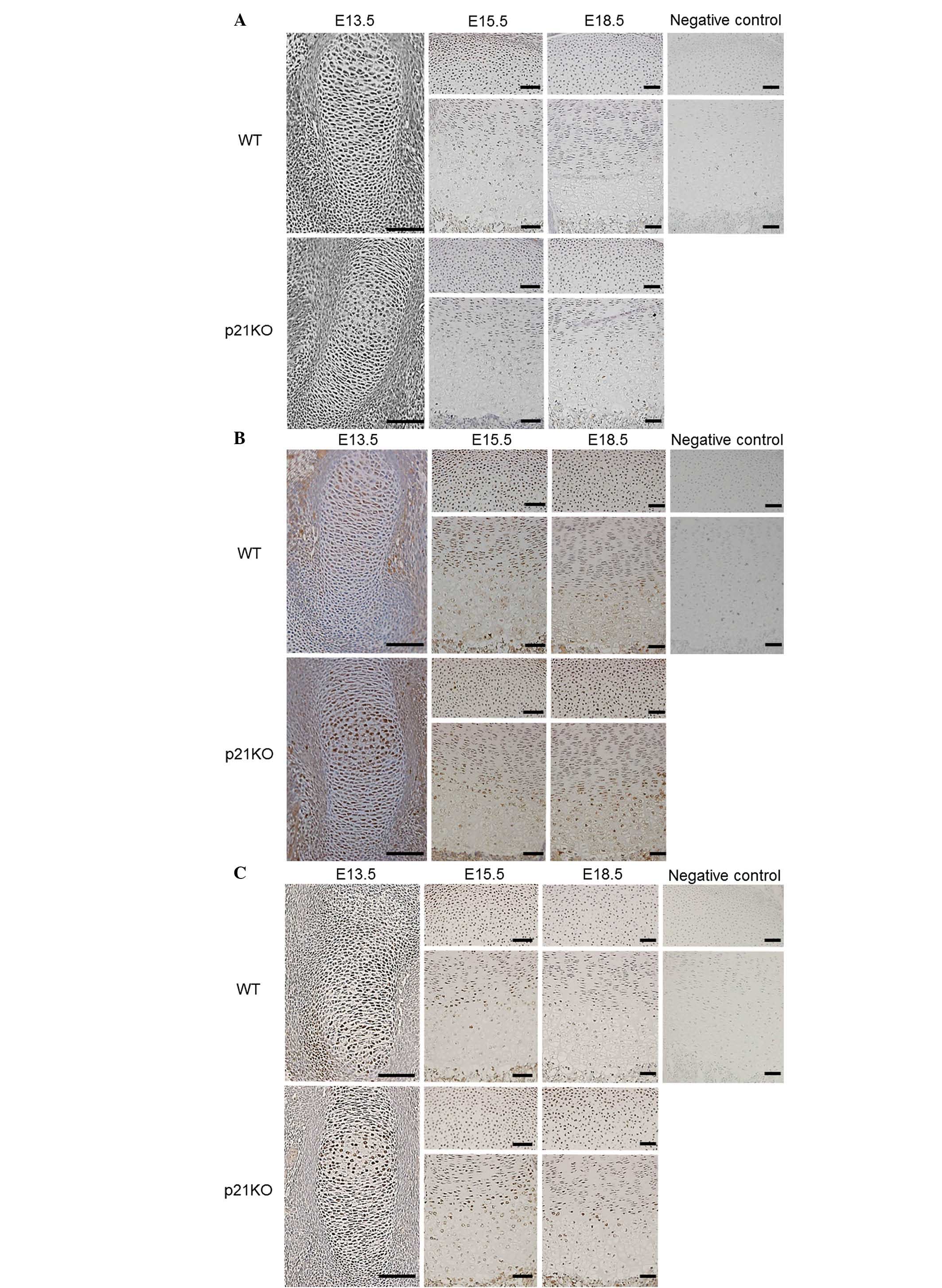
Expression of ECM proteins and Sox9 is
not altered by p21 deficiency
To investigate the in vivo function of p21 in
ECM production, immunohistochemical analysis of cartilage tissue
was performed at E13.5, E15.5, and E18.5. Type II collagen was
expressed ubiquitously in the cartilage tissues. However, no
differences were found between the embryonic cartilage tissues of
WT and p21KO mice at any time point (Fig. 2A). Type X collagen was expressed in
the hypertrophic zone. However, no differences were found between
the embryonic cartilage tissues of WT and p21KO mice at each time
point (Fig. 2B).
Immunohistochemical analysis revealed that the Sox9
expression levels of WT and p21KO mice did not differ at each time
point (Fig. 2C). These results
indicate that p21 deficiency does not affect ECM production in
endochondral ossification.
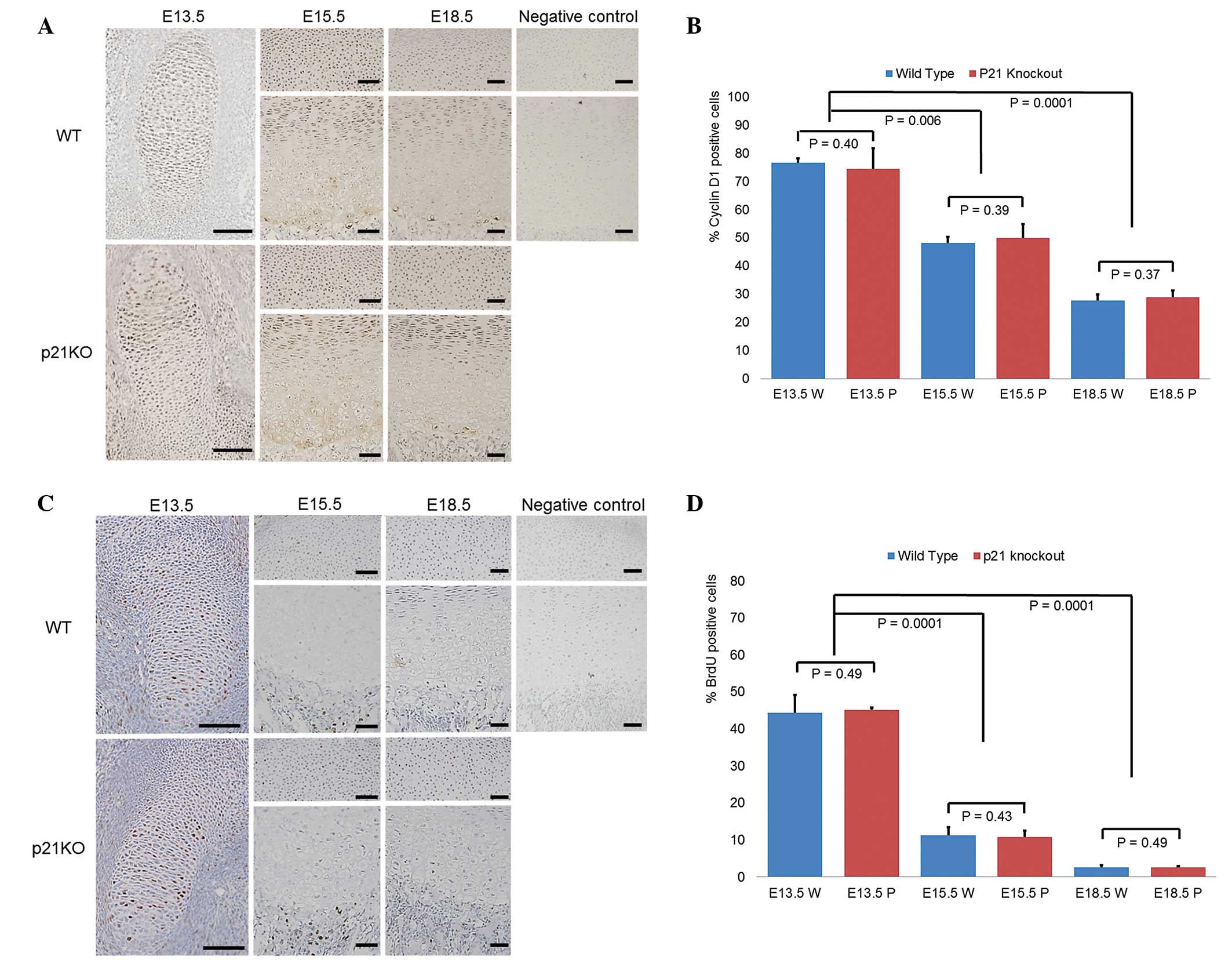
Chondrocyte proliferation is not altered
by p21 deficiency
The main function of p21 is as a negative regulator
of the G1/S transition, inducing ‘G1 arrest’
(23). Cyclin D1 staining was
performed to evaluate the cell cycle progression at the
G1 phase. Cyclin D1 forms complexes with CDK4 or CDK6,
whose activity is required for G1/S phase transition
(7). Although the expression
levels of cyclin D1 were higher in the E13.5 cartilage tissue
compared with those at subsequent time points, no differences were
found between the WT and p21KO mice (Fig. 3A). Enumeration of cyclin
D1-positive chondrocytes showed significant decreases at E15.5 and
E18.5 compared with that of E13.5 (P<0.05). However, no
significant inter-group differences were identified at any time
point (P>0.05) (Fig. 3B).
BrdU staining was performed as BrdU is incorporated
into proliferating cells (S phase) allowing it to be used to
evaluate DNA replication (24).
The uptake of BrdU appeared to be much higher in the E13.5
cartilage tissue compared with that at subsequent time points.
However, no differences in BrdU uptake were observed between the WT
and p21KO mice (Fig. 3C). The
number of BrdU-positive chondrocytes was significantly reduced at
E15.5 and E18.5 compared with the number at E13.5 (P<0.05).
However, no significant differences between the two groups were
observed at each time point (P>0.05) (Fig. 3D). These results indicate that p21
deficiency does not affect chondrocyte proliferation and that
chondrocyte proliferation is naturally more active during the early
embryonic period.
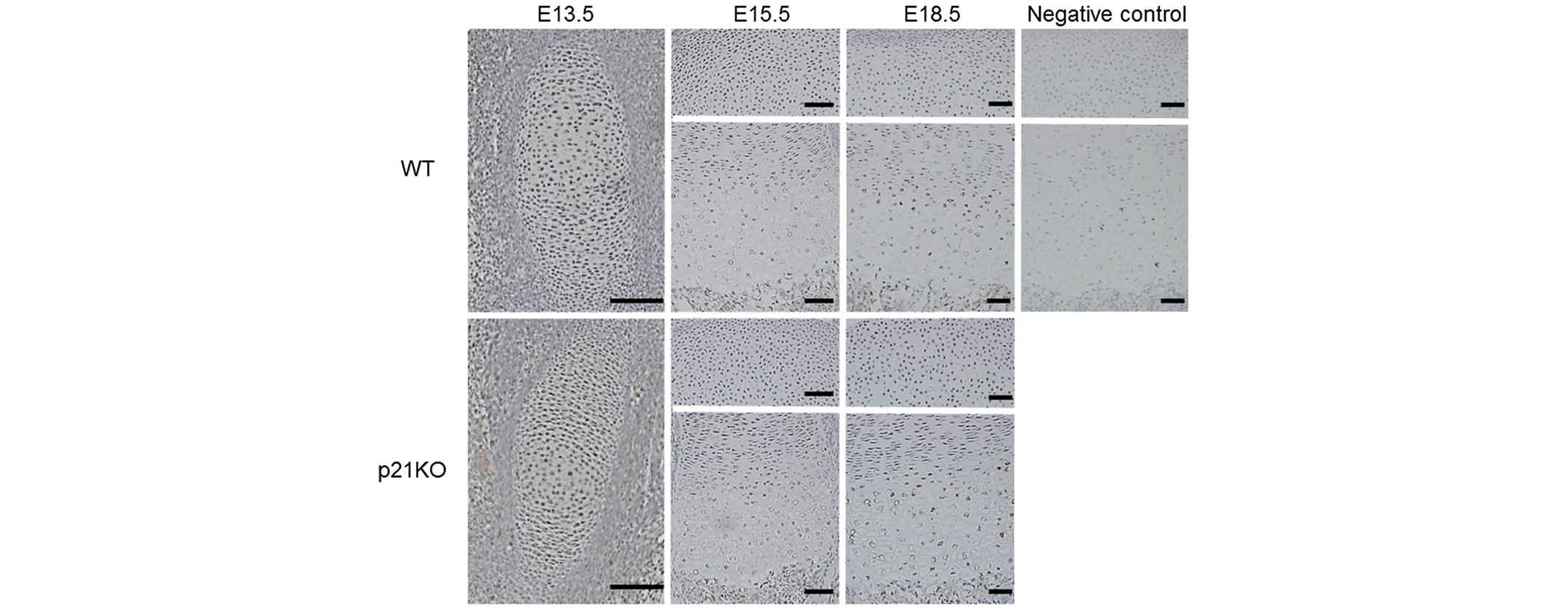
Evaluation of the expression levels of
p16
INK4 is a tumor suppressor and cell cycle regulatory
protein that acts during the G1 phase. p16 is a known
representative of the INK4 family which interacts with CDK4 and
CDK6, inhibiting their ability to interact with cyclin D (25). While p16 was expressed ubiquitously
throughout the tissue sections, no differences were observed
between the expression levels in the WT and p21KO mice (Fig. 4).
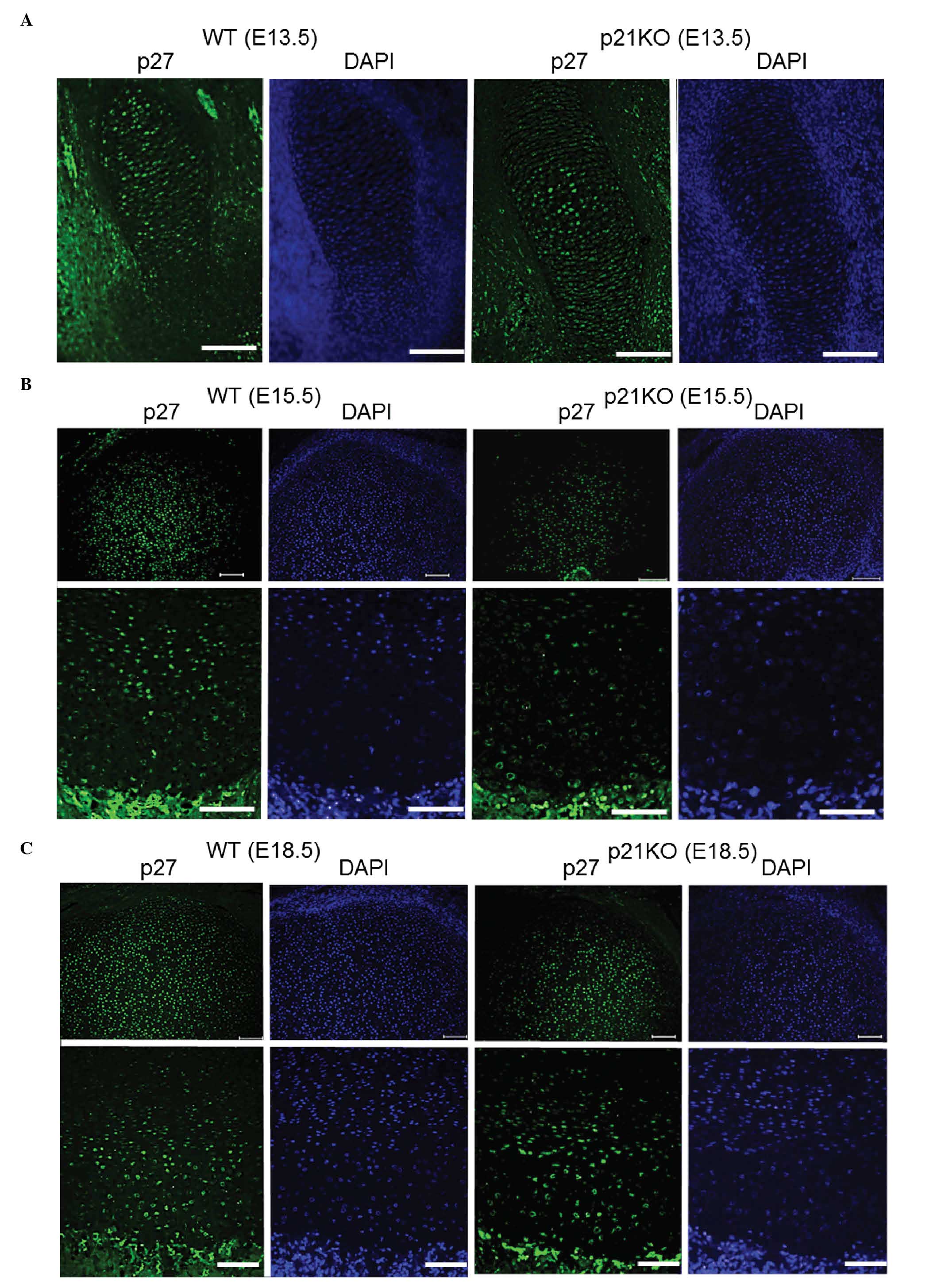
Immunofluorescence evaluation of the
expression of p27
The expression of p27, one of the Cip/Kip family
components, was evaluated by immunofluorescence and DAPI staining
(Fig. 5A–C). p27 was ubiquitously
expressed throughout the tissue sections, however, no differences
were observed between the expression levels in the WT and p21KO
mice (Fig. 5).
Discussion
Accurate control of the cell cycle is essential for
normal development, and CDKs are an integral part of cell cycle
regulation (26). CDKs are
specifically regulated by CKIs. Two distinct families of CKIs are
known: Cip/Kip and INK4. The INK4 family consists of
p15INK4b, p16INK4a, p18INK4c and
p19INK4d, which specifically inhibit the activity of
G1-phase cyclin D-CDK4 and CDK6 (7,25).
The Cip/Kip family, including p21CIP1,
p27KIP1 and p57KIP2, controls a broader
spectrum of cyclin-CDK complexes, including CDK2, CDK3, CDK4 and
CDK6 (7,27). Although members of the CIP/KIP
family possess a few similar functions, they also possess different
functions which are determined by the differences in expression
pattern and protein structure.
Previous studies have reported that p27 has an
important role in endochondral ossification. In p27KO mice,
multiple organ overgrowth has been observed (28) and Emons et al (29) reported that p27KO mice demonstrated
a modest increase in body length. Furthermore, the expression
levels of p27 mRNA were similar throughout the hypertrophic and
resting/proliferative zones in adult mice. In the present study,
p27 was ubiquitously expressed in the tissue sections assessed.
Previous studies have reported that p21 is expressed
in the majority of organs and tissues during murine embryonic and
postnatal development (30,31).
In myogenesis, the muscle-specific transcription factor MyoD
induces p21 expression in association with the terminal
differentiation of muscles (31),
suggesting that p21 has a crucial role in muscle development
(6). In the current study, it was
demonstrated that p21 deficiency did not alter the morphology of
embryonic cartilage tissue in mice, although p21 has been shown to
be expressed in the proliferative and hypertrophic zones in adult
WT mice (32). Furthermore, p21
deficiency did not affect ECM production or Sox9 expression.
Additionally, the expression of cyclin D1 and INK4
family members was evaluated. The expression levels of cyclin D1
and p16 did not differ between the WT and p21KO mice. The
similarity of the expression levels of cyclin D1 and p16 indicates
that there are no differences in the remaining activities of
G1-phase that the Cip/Kip family are involved in. The
uptake of BrdU was observed to be much higher at E13.5 compared
with E15.5 and E18.5. However, no differences in BrdU uptake were
found between WT and p21KO mice, revealing that the rate of
development was equivalent. Taking these results into
consideration, p21 deficiency did not affect chondrocyte
proliferation.
Therefore, the primary finding of the current study
is that p21 may not be essential for embryonic articular
chondrogenesis in mice. Negishi et al (26) reported that the reduction of
endogenous p21 caused inhibition of early chondrogenic
differentiation in ATDC5 cells, indicating that the p21 gene has an
important role in this cellular process in vitro (26). However, the current study did not
observe any marked changes in vivo, revealing a discrepancy
in these findings. While the impact of these results within the
general scientific community may not be great, the results obtained
from the KO mice provide important information for the researchers
in relevant fields. These results revealed that p21 deficiency does
not impact the morphology, ECM formation, chondrocytic marker
protein production, chondrocyte proliferation or cell cycle
regulatory proteins in the developing cartilage.
It has been hypothesized that various complicated
mechanisms control the expression and timing of the Cip/Kip family,
which appear to possess important roles in development and growth
regulation (6). Therefore, p21
deletion may be compensated by a complicated mechanism involving
other networks. Further studies are required to gain insight into
these phenomena. A clear understanding of these mechanisms may lead
to the development of novel therapeutic strategies for cartilage
disorders.
In conclusion, the current study revealed that p21
does not impact embryonic endochondral ossification in articular
cartilage of mice. Furthermore, compensation for the lack of p21
function does not appear to be mediated by components of the
Cip/Kip family, p27.
Acknowledgements
This study was presented at the Orthopaedic Research
Society Annual Meeting, 2014 (Poster no. 1322). The authors thank
Ms. Kyoko Tanaka, Ms. Minako Nagata, Ms. Maya Yasuda, Mr. Takeshi
Ueha for their technical assistance and Dr Mitsuru Morimoto for
technical assistance and giving critical suggestions.
References
|
1
|
Cross M, Smith E, Hoy D, et al: The global
burden of hip and knee osteoarthritis: estimates from the global
burden of disease 2010 study. Ann Rheum Dis. 3:1323–1330. 2014.
View Article : Google Scholar
|
|
2
|
Kronenberg HM: Developmental regulation of
the growth plate. Nature. 423:332–336. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Emons J, Chagin AS, Sävendahl L, Karperien
M and Wit JM: Mechanisms of growth plate maturation and epiphyseal
fusion. Hormone Res Paediatr. 75:383–391. 2011. View Article : Google Scholar
|
|
4
|
Negishi Y, Ui N, Nakajima M, et al:
p21Cip-1/SDI-1/WAF-1 gene is involved in chondrogenic
differentiation of ATDC5 cells in vitro. J Biol Chem.
276:33249–33256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmid TM and Linsenmayer TF:
Immunohistochemical localization of short chain cartilage collagen
(type X) in avian tissues. J Cell Biol. 100:598–605. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakayama K and Nakayama K: Cip/Kip
cyclin-dependent kinase inhibitors: brakes of the cell cycle engine
during development. BioEssays. 20:1020–1029. 1998. View Article : Google Scholar
|
|
7
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He G, Siddik ZH, Huang Z, et al: Induction
of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2
activities. Oncogene. 24:2929–2943. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seoane J, Le HV and Massagué J: Myc
suppression of the p21(Cip1) Cdk inhibitor influences the outcome
of the p53 response to DNA damage. Nature. 419:729–734. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asada M, Yamada T, Ichijo H, et al:
Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in
monocytic differentiation. EMBO J. 18:1223–1234. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Olive M, Mellad JA, Beltran LE, et al:
p21Cip1 modulates arterial wound repair through the
stromal cell-derived factor-1/CXCR4 axis in mice. J Clin Invest.
118:2050–2061. 2008.PubMed/NCBI
|
|
13
|
Gartel AL and Radhakrishnan SK: Lost in
transcription: p21 repression, mechanisms, and consequences. Cancer
Res. 65:3980–3985. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong H, Takahashi K, Ichisaka T, et al:
Suppression of induced pluripotent stem cell generation by the
p53-p21 pathway. Nature. 460:1132–1135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawamura T, Suzuki J, Wang YV, et al:
Linking the p53 tumour suppressor pathway to somatic cell
reprogramming. Nature. 460:1140–1144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bedelbaeva K, Snyder A, Gourevitch D, et
al: Lack of p21 expression links cell cycle control and appendage
regeneration in mice. Proc Natl Acad Sci USA. 107:5845–5850. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aikawa T, Segre GV and Lee K: Fibroblast
growth factor inhibits chondrocytic growth through induction of p21
and subsequent inactivation of cyclin E-Cdk2. J Biol Chem.
276:29347–29352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakajima M, Negishi Y, Tanaka H and
Kawashima K: p21(Cip-1/SDI-1/WAF-1) expression via the
mitogen-activated protein kinase signaling pathway in
insulin-induced chondrogenic differentiation of ATDC5 cells.
Biochem Biophys Res Commun. 320:1069–1075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng C, Zhang P, Harper JW, Elledge SJ and
Leder P: Mice lacking p21CIP1/WAF1 undergo normal
development, but are defective in G1 checkpoint control. Cell.
82:675–684. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Byeon IJ, Li J, Ericson K, et al: Tumor
suppressor p16INK4A: determination of solution structure
and analyses of its interaction with cyclin-dependent kinase 4. Mol
Cell. 1:421–431. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bi W, Deng JM, Zhang Z, Behringer RR and
de Crombrugghe B: Sox9 is required for cartilage formation. Nat
Genet. 22:85–89. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niculescu AB III, Chen X, Smeets M, Hengst
L, Prives C and Reed SI: Effects of p21(Cip1/Waf1) at both the G1/S
and the G2/M cell cycle transitions: pRb is a critical determinant
in blocking DNA replication and in preventing endoreduplication.
Mol Cell Biol. 18:629–643. 1998.PubMed/NCBI
|
|
24
|
Gratzner HG: Monoclonal antibody to
5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA
replication. Science. 218:474–475. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cánepa ET, Scassa ME, Ceruti JM, et al:
INK4 proteins, a family of mammalian CDK inhibitors with novel
biological functions. IUBMB Life. 59:419–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sherr CJ: G1 phase progression: cycling on
cue. Cell. 79:551–555. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
LaBaer J, Garrett MD, Stevenson LF, et al:
New functional activities for the p21 family of CDK inhibitors.
Genes Dev. 11:847–862. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kiyokawa H, Kineman RD, Manova-Todorova
KO, et al: Enhanced growth of mice lacking the cyclin-dependent
kinase inhibitor function of p27(Kip1). Cell. 85:721–732. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Emons JA, Marino R, Nilsson O, et al: The
role of p27Kip1 in the regulation of growth plate
chondrocyte proliferation in mice. Pediatr Res. 60:288–293. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Macleod KF, Sherry N, Hannon G, et al:
p53-dependent and independent expression of p21 during cell growth,
differentiation, and DNA damage. Genes Dev. 9:935–944. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Parker SB, Eichele G, Zhang P, et al:
p53-independent expression of p21Cip1 in muscle and
other terminally differentiating cells. Science. 267:1024–1027.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stewart MC, Farnum CE and MacLeod JN:
Expression of p21CIP1/WAF1 in chondrocytes. Calcif
Tissue Int. 61:199–204. 1997. View Article : Google Scholar : PubMed/NCBI
|