Introduction
Dihydromyricetin (DHM,
C15H12O8, PubChem CID: 161557,
Fig. 1A) is an active component in
extracts of Ampelopsis grossedentata and a biologically
active flavonoid compound (1). DHM
possesses potent antitumor activity both in vivo and in
vitro (2). It has been
reported that DHM has numerous pharmacological functions, including
anti-inflammatory, antibacterial, cough-relief, antioxidant,
antihypertensive, hepatoprotective and anti-cancer effects
(3,4). It exerts an antioxidative effect by
chelating Fe2+ (5). In
addition, it was demonstrated that DHM was able to decrease
accumulation of reactive oxygen species (ROS) (6,7).
Previous studies have reported significant inhibitory activity of
DHM against breast cancer MCF-7 (8) and MDA-MB-231 (9) cells, nasopharyngeal carcinoma HK-1
cells, liver cancer Bel-7402 cells (10), leukemia HL-60 and K-562 cells and
lung cancer H1299 cells (11).
Based on evidence from previous studies, the present study aimed to
elucidate the association between transforming growth factor-β
(TGF-β) and nicotinamide adenine dinucleotide phosphate oxidase 4
(NOX4) during DHM-induced apoptosis in mouse hepatocellular
carcinoma Hepal-6 cells.
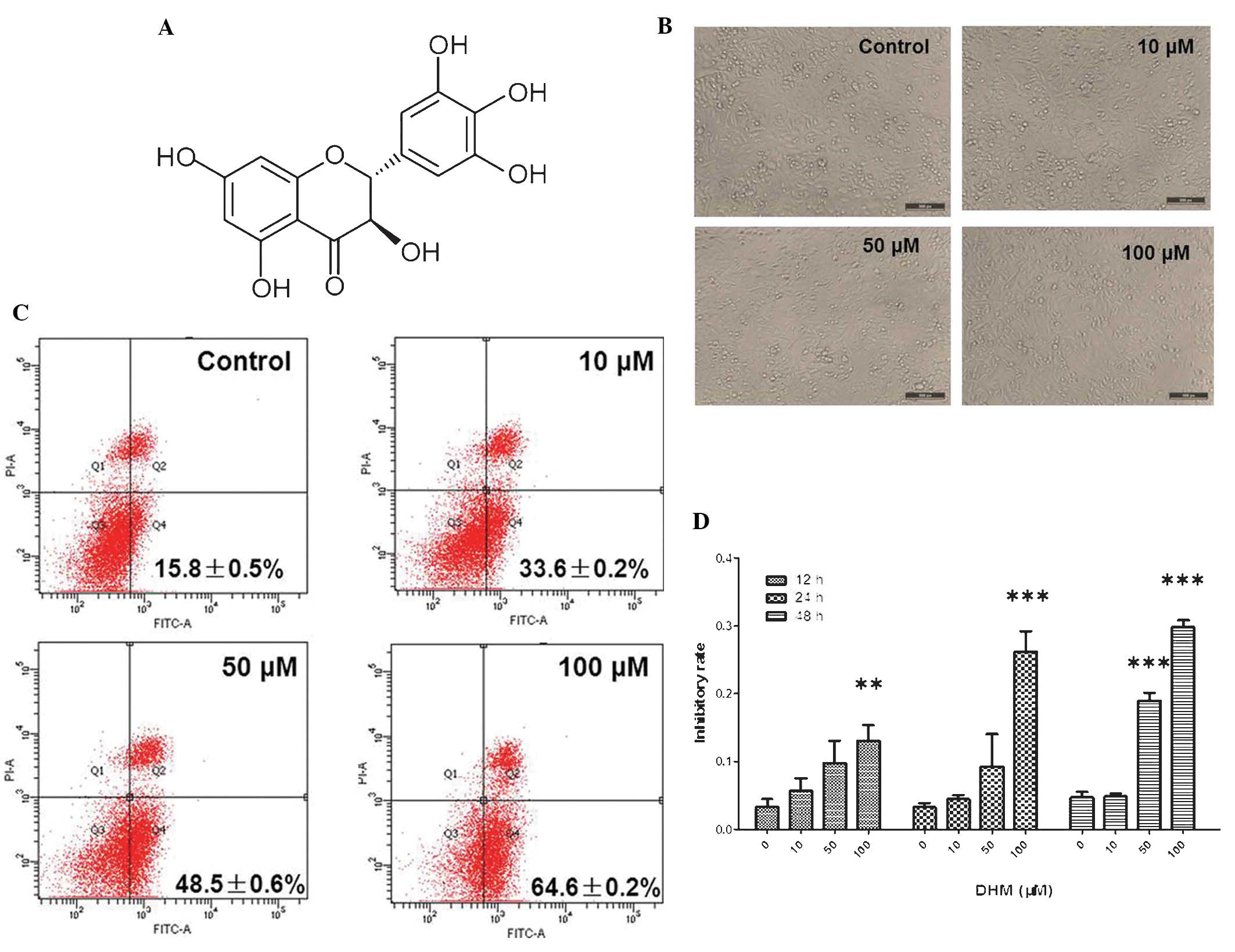 | Figure 1DHM induces cell growth inhibition and
apoptosis in Hepal-6 cells. (A) Chemical structure of DHM. (B) DHM
induced cell proliferation in Hepal-6 at various concentrations
(10, 50 and 100 μM) for 48 h, visualized by microscopy
(magnification, ×100). (C) Hepal-6 cells were treated with various
concentrations (10, 50, or 100 μM ) of DHM for 48 h and the results
were analyzed by flow cytometry. Each sample was measured in
duplicate, and the figure is a representative of three independent
assays. (D) MTT assay analyzed cell growth inhibition rates in
cells treated with different concentrations (10, 50 and 100 μM) of
DHM for 12, 24, 48 h. Values are expressed as the mean ± standard
deviation of three independent experiments. **P<0.01,
***P<0.001 vs. 0 μM DHM. DHM, dihydromyricetin; FITC,
fluorescein isothiocyanate; PI, propidium iodide; A, area. |
Though TGF-β was initially suggested to be involved
in a tumor supressor pathway due to its cytostatic activity in
epithelial cells, further studies have identified TGF-β as a
pro-tumorigenic factor. The majority of human tumors, including
melanoma, secrete significant amounts of TGF-β, which directly
influences the tumor microenvironment, promoting peritumoral
angiogenesis as well as tumor cell migration and invasiveness,
immune evasion and dissemination to metastatic sites (12,13).
TGF-β signaling is mediated by TGF-type II (TβRII) and type I
(TβRI) receptors. TGF-β binding induces the formation of
heteromeric complexes which promote the phosphorylation, and
therefore activation, of TβRI by TβRII. Activated TβRI
phosphorylates receptor (R)-Smads, including Smad2 and -3 (14). These activated R-Smads form
heteromeric complexes with Smad4, which accumulate in the nucleus
and regulate target-gene transcription (15). TGF-β has been shown to increase
NOX4 expression in various cell types; however, the localization of
NOX4 remains to be elucidated (16). Tobar et al (17) reported that TGF-β upregulated NOX4
expression via a factor-induced apoptotic pathway in fetal rat
hepatocytes. Furthermore, ROS production in human hepatocyte cell
lines previously infected with the hepatitis C virus depends on
NOX4 activity whose expression is stimulated by TGF-β (18). Several studies have reported that
TGF-β promotes NOX4 production of intracellular ROS (19,20).
ATP production and biosynthesis of building blocks are required to
sustain cellular function and cell viability is functionally
coordinated by interlocking regulatory mechanisms that control
electron transport in the respiratory chain (21). The present study therefore aimed to
investigate whether DHM was able to reduce ATP levels and ROS
production via the TGF-β signaling pathway in mouse hepatoma
Hepal-6 cells.
Materials and methods
Reagents
DHM was purchased from Sigma (St. Louis, MO, USA)
and was dissolved to a concentration of 50 mM in dimethylsulfoxide
(DMSO) as a stock solution and stored at −20°C. The final DMSO
concentration did not exceed 0.1% DMSO throughout the study. Rabbit
antibodies to TGF-β, TGF-βRII, Smad3, phosphorylated (p)-Smad2/3
and GAPDH were obtained from Cell Signaling Technology (Beverly,
MA, USA). Goat anti-rabbit immunoglobulin G-horseradish peroxidase
(IgG-HRP; EarthOx, Millbrae, CA, USA) was used as the secondary
antibody.
Cell culture and DHM treatment
The mouse Hepal-6 cell line was provided by the
Maternal and Child Health Hospital of Shanghai (Shanghai, China).
Cells were cultured in RPMI-1640 medium supplemented with 10% (v/v)
fetal bovine serum (Gibco-BRL, Invitrogen Life Technologies,
Carlsbad, CA, USA), penicillin 100 U/ml and streptomycin 100 U/ml
(Hyclone, Logan, UT, USA), and maintained in a humidified
atmosphere of 95% air and 5% CO2 at 37°C. Hepal-6 cells
were grown in standard media and when the confluency reached
50–60%, cells were treated with DHM (10, 50 or 100 μM) for 48
h.
Measurement of intracellular ROS
levels
To detect the accumulation of intracellular ROS in
Hepal-6 cells, a ROS assay kit was purchased from BioVision Inc.
(Milpitas, CA, USA). Briefly, following treatment of cells with
different concentrations of DHM (10, 50 and 100 μM) for 48 h in a
96-well plate at a cell density of 2500 cells/well, 100 μl
2′,7′-dichlorofluorescin diacetate (DCFDA) mix was added and
incubated for 45 min at 37°C in the dark, including blank wells
(with non-stained cells). The fluorescence intensity was measured
using a fluorescence plate reader (EnSpire™ 2300 Multilabel Reader;
Perkin Elmer, Inc., Waltham, MA, USA) at
excitation/emission=488/525 nm.
Measurement of adenosine triphosphate
(ATP) production
Intracellular ATP levels were measured using the
ApoSENSOR cell viability assay kit (BioVision) according to the
manufacturer’s instructions. Briefly, cells were treated with DHM
(10, 50 and 100 μM) for 48 h, then incubated with 100 μl nuclear
releasing reagent for 5 min at room temperature with gentle
shaking, followed by further incubation with 5 μl ATP monitoring
enzyme. Detection was performed using a luminometer (Sirius L;
Titertek-Berthold, Pforzheim, Germany).
Annexin V/propidium iodide (PI) double
staining assay
Apoptotic cells were quantified using an Annexin
V-fluorescein isothiocyanate (FITC)/PI kit (BioVision) and detected
by flow cytometry (FACSCalibur; Becton-Dickinson, BD Biosciences,
Franklin Lakes, NJ, USA), and analyzed by Modfit and CellQuest Vida
6.1 software (BD Biosciences). Briefly, cells were pretreated with
10, 50 or 100 μM DHM for 48 h and washed with phosphate-buffered
saline (PBS). Cells were subsequently collected and resuspended in
binding buffer [pH 7.5, 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 2.5 mM
CaCl2 and 140 mM NaCl]. Cells were incubated with
Annexin V-FITC and PI for 10 min in the dark, prior to flow
cytometric analysis. In the early stages of apoptosis, cells were
Annexin V-positive, whereas Annexin V and PI-positive cells were
considered to be in the late stage of apoptosis.
MTT assay
Cell densities were adjusted to 2×104
cells/100 μl. Cells were seeded into a 96-well plate, which was
placed in an incubator overnight to allow for attachment and
recovery. Briefly, cells were pretreated with 10, 50 or 100 μM DHM
for 48 h. MTT was dissolved at 5 mg/ml in warm assay medium and 20
μl MTT solution was transferred to each well to yield a final
volume of 120 μl/well. Plates were incubated for 4 h at 37°C and 5%
CO2. Following incubation, supernatants were removed,
and 150 μl DMSO was added. The plate was placed on an orbital
shaker for 5 min and subsequently, the absorbance at 595 nm was
recorded with an EnSpire™ 2300 Multilabel Reader (Perkin Elmer,
Inc.).
DHM-regulated protein analysis
Cells were collected following DHM treatment and
lysed in lysis buffer [100 mM Tris-HCl, pH 6.8, 4% (m/v) SDS, 20%
(v/v) glycerol, 200 mM 2-mercaptoethanol, 1 mM phenylmethyl
sulfonylfluoride, and 1 g/ml aprotinin] for 30 min on ice. The
total protein concentrations in the supernatants were detected
using a bicinchoninic acid (BCA) assay with the BCA Protein Assay
kit purchased from Beyotime Institute of Biotechnology (Haimen,
Jiangsu, China). SDS-PAGE was performed using an 8–15% gradient on
standard polyacrylamide gels. Proteins were subsequently
transferred to nitrocellulose membranes saturated with 5% milk in
Tris-buffered saline and 1% Tween-20 (TBST) and incubated with
primary antibodies in diluent overnight at 4°C. The membranes were
washed three times with TBST, incubated with anti-rabbit IgG-HRP
for 1 h and washed a further four times with TBST. Detection was
performed using the Odyssey Infrared Imaging System (LI-COR
Biosciences Inc., Lincoln, NE, USA).
Quantitative PCR (qPCR): Quantification
of messenger RNA (mRNA) expression
mRNA expression levels were determined by qPCR using
SYBR green. mRNA was reverse-transcribed to cDNA using the
PrimeScript RT Reagent kit with the gDNA Eraser kit (Takara Bio,
Inc., Otsu, Japan) The following primer sequences were used: 18S
forward, 5′-CGGCGACGACCCATTCGAAC-3′ and reverse,
5′-GAATCGAACCCTGATTCCCCGTC-3′; TGF-β forward,
5′-GGACTACTATGCTAAAGAGGTCAC-3′ and reverse,
5′-CTGTATTCCGTCTCCTTGGTTCAGC-3′; NOX4 forward,
5′-GTTCGGCACATGGGTAAAAG-3′ and reverse, 5′-ACCAAGGGCCAGAGTATCAC-3′.
Total RNA was prepared using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). qPCR was performed with the MJ
chromo 4 RT-PCR detection system (Bio-Rad Laboratories, Hercules,
CA, USA). The expression levels of the housekeeping gene 18S were
measured as an internal control.
Statistical analysis
All values are presented as the mean ± standard
deviation from triplicate experiments performed in a parallel
manner unless otherwise indicated. Statistical differences were
evaluated using Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference between values. All
figures exhibited in the present study are representative of ≥three
independent experiments.
Results
DHM inhibits proliferation and promotes
apoptosis of Hepal-6 cells
Untreated Hepal-6 cells grew normally with clear
skeletons, whereas the morphology of cells treated with DHM was
distorted, some became round and the number of sloughed cells
increased in a dose-dependent manner (Fig. 1B). The rate of cell apoptosis also
increased in a concentration-dependent manner (Fig. 1C). The results of the MTT assay
demonstrated that DHM inhibited cell growth in a time- and
concentration-dependent manner in Hepal-6 cells following 12, 24
and 48 h treatment (Fig. 1D).
These data revealed that DHM exerted a significant inhibitory
effect on the viability of Hepal-6 cells, which may contribute to
its anti-tumor potency. In cells treated with 50 μM DHM cell growth
was inhibited and the majority of Hepal-6 cells underwent apotosis
(IC50 of DHM on Hepal-6 cells was 190 μM for 48 h
treatment). These results demonstrated that DHM inhibited
proliferation and promoted apoptosis in Hepal-6 cells in a time-
and concentration-dependent manner.
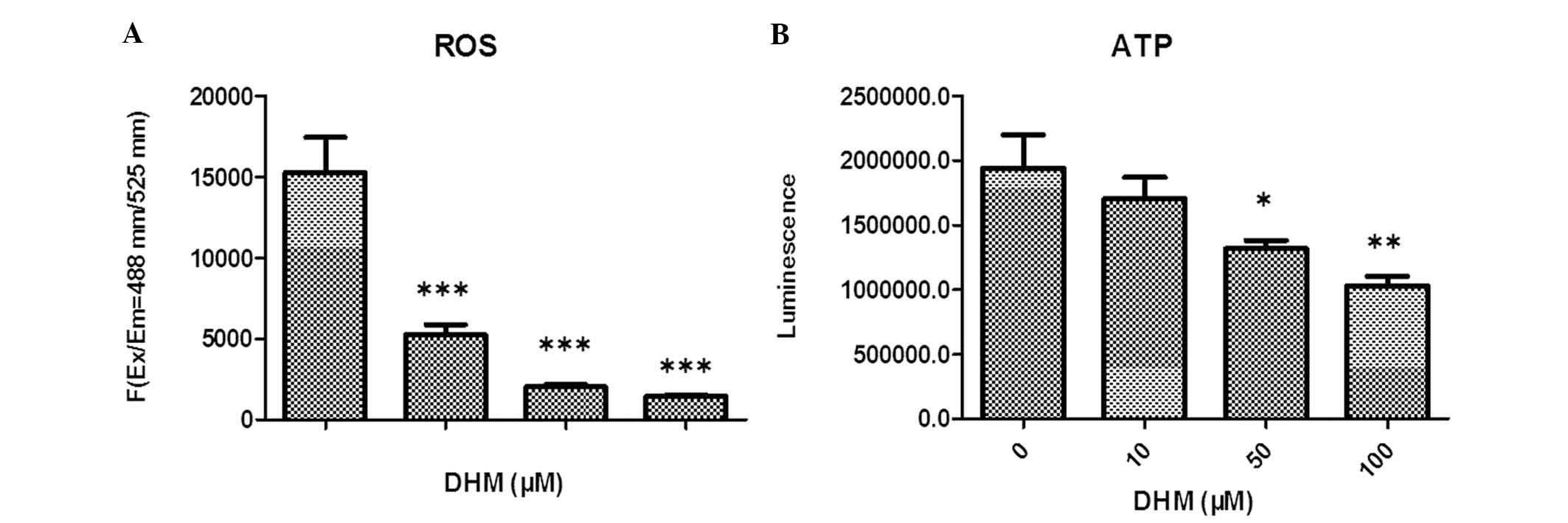
DHM reduces ROS production in Hepal-6
cells
The levels of ROS in Hepal-6 cells treated with
various concentrations of DHM for 48 h were evaluated. The
cell-permeant DCFDA, which is oxidized to green fluorescent
2′,7′-dichlorofluorescein by various peroxide-like ROS and nitric
oxide-derived reactive intermediates, was used as a probe. These
data demonstrated that DHM significantly decreased ROS production
in Hepal-6 cells, and that this ROS imbalance may promote
mitochondrial dysfunction and trigger mitochondria-mediated
apoptosis. Intracellular levels of ROS in cells treated with 10, 50
and 100 μM DHM decreased in a concentration-dependent manner,
compared with those in vehicle-treated cells (Fig. 2A).
DHM decreases intracellular ATP
expression levels in Hepal-6 cells
In order to examine whether DHM caused a dysfunction
of mitochondrial energy, intracellular levels of ATP in DHM-treated
cells were investigated. Cells were treated with various
concentrations of DHM for 48 h and the results indicated that the
intracellular levels of ATP were markedly decreased in a
concentration-dependent manner (Fig.
2B).
DHM downregulates TGF-β and NOX4
In the present study, cells were treated with 10, 50
or 100 μM DHM for 24 h and mRNA expression levels of TGF-β and NOX4
were evaluated by qPCR. Cells were also treated with 10, 50 or 100
μM DHM for 48 h and TGF-β, TGF-β II, Smad3, p-Smad2/3 and NOX4
protein expression levels were evaluated by western blot analysis.
The results indicated that TGF-β and NOX4 mRNA expression levels
decreased, and that protein expression levels of TGF-β, TGF-β II,
Smad3, p-Smad2/3 and NOX4 were reduced in a concentration-dependent
manner (Fig. 3A–C).
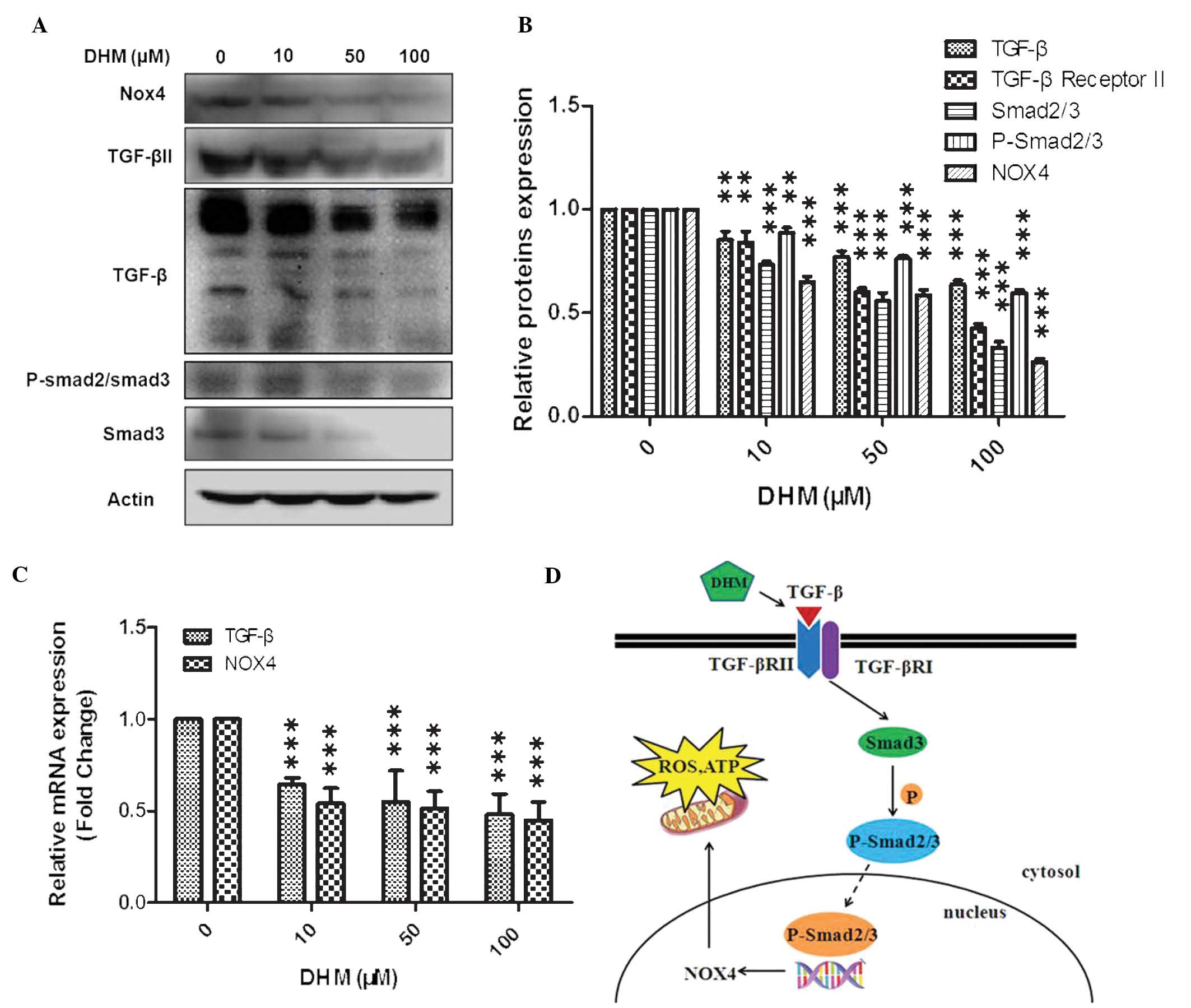 | Figure 3Western blot analysis of the effects
of DHM on mRNA and protein expression levels in Hepal-6 cells. (A)
Western blot analysis of cells treated with various concentrations
(10, 50 and 100 μM) of DHM for 48 h (representative of three
independent experiments). (B) Column diagram for A. (C) Cells were
treated with DHM (10, 50 and 100 μM) for 24 h and TGF-β and NOX4
mRNA expression were measured by quantitative polymerase chain
reaction. The experiment was performed in triplicate.
**P<0.01, ***P<0.001 vs. 0 μM DHM.
Values are expressed as the mean ± standard deviation (D) Schematic
of the suggested mechanism of action of DHM. DHM, dihydromyricetin;
mRNA, messenger RNA; NOX4, NADPH oxidase 4; TGF-β, transforming
growth factor-β; ROS, reactive oxygen species; ATP, adenosine
triphosphate; TGF-βR, transforming growth factor-β receptor; p,
phosphorylated. |
Discussion
Western blot analysis was performed in order to
measure TGF-β, TGF-βRII, Smad3, p-Smad2/3 and NOX4 protein
expression levels, while qPCR analysis was used to measure TGF-β
and NOX4 mRNA expression levels. The results demonstrated that DHM
decreased TGF-β and NOX4 mRNA expression levels in cells treated
with DHM for 24 h and furthermore, induced a reduction in TGF-β,
TGF-βRII, Smad3, p-Smad2/3 and NOX4 protein expression levels in a
concentration-dependent manner. It was further demonstrated that
DHM induced a decrease in ROS and ATP production in a
concentration-dependent manner. The TGF-β signaling pathway is
involved in multiple cellular processes, including cell growth,
differentiation, adhesion, migration and apoptosis. TβRI, TβRII and
intracellular mediators, including Smad proteins, mediate TGF-β
signaling (22,23). The binding of TGF-β to TβRII
induces phosphorylation of TβRI at glycine-serine repeats in the
cytoplasmic tail domain by TβRII, leading to TβRI activation
(24). At present, it is
hypothesized that the Smad complex remains associated and is
actively involved in transcriptional regulation (25,26).
A previous study demonstrated that Smad3 and -4 had important roles
in TGF-β-induced epithelial to mesenchymal transition and breast
cancer metastasis (27). It was
reported that abrogation of the Smad pathway in M4 cells by using a
dominant negative Smad3 mutant or via overexpression of a
Smad-binding defective TβRI mutant suppressed metastasis (28,29).
Cancer progression has been associated with
oxidative stress (30). Loss of
TGF-β signaling in mammary carcinoma cells increased the abundance
of smooth muscle actin-positive stroma and enhanced tumor cell
survival and heterogeneity (31–33).
A study by Giannelli et al (34) indicated that the inhibition of
TGF-β signalling resulted in numerous downstream effects, which may
improve clinical outcomes in hepatocellular carcinoma treatment.
Furthermore, it has been demonstrated that ROS production in human
hepatocyte cell lines previously infected with the hepatitis C
virus depends on NOX4 activity, whose expression is stimulated by
TGF-β (18). Superoxide and
hydrogen peroxide, which are redox signaling molecules involved in
various cellular functions, are major producers of ROS (35). Redox imbalance occurs due to
excessive or insufficient ROS production and is a
pathophysiological induction factor for numerous pathological
conditions, including cancer development and progression. It has
previously been demonstrated that, apart from mitochondria, the
nicotinamide adenine dinucleotide phosphate oxidase complex is the
most significant intracellular source of ROS (36). NOX4 expression has been
demonstrated to be regulated by differentiating factors including
TGF-β, as observed in the present study (37). It was also reported that
TGF-β1-stimulated expression of NOX4 resulted in the oxidation of
mitogen-activated protein kinase phosphatase-1, which led to the
factor-dependent modification of gene expression in murine
fibroblasts (38). Several studies
have reported that TGF-β induces NOX4 to generate intracellular ROS
(19,39). Smad3-mediated gene transcription
has an important role in the induction of NOX4 expression following
TGF-β stimulation (40).
In conclusion, the present study revealed that DHM
induced a reduction in TGF-β, TGF-βRII, Smad3, p-Smad2/3 and NOX4
protein expression levels, as well as a reduction in ROS and ATP
production in Hepal-6 cells. Conventional anti-cancer drugs induce
cancer cell apoptosis by elevating ROS; however this results in
significant damage to normal cells. DHM enhanced the rate of
apoptosis in Hepal-6 cells, whilst reducing ROS levels. This means
that DHM may be capable of exerting anti-cancer effects whilst
causing minimal damage to normal cells. Further studies are
required to elucidate the potential of DHM to be used as an
anti-cancer drug.
Acknowledgements
This work was supported in part by the following
grants: Guangdong Province Natural Science Funds (no.
S2011010003750), Zhanjiang 2012 Annual Financial Capital
Competitive Project Science and Technology Project (no.
2012C0302-52) and Guangdong Medical College Scientific Research
Fund project (no. M2013012).
References
|
1
|
Wu S, Liu B, Zhang Q, et al:
Dihydromyricetin reduced Bcl-2 expression via p53 in human hepatoma
hepg2 cells. PLoS One. 8:e768862013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li H, Li Y, Zhang Y, Shi H, Hu W and Zhang
Z: Comparison of refluxing, ultrasonic-and microwave-assisted
extraction of dihydromyricetin from Ampelopsis grossedentata. J
AOAC Int. 91:1278–1283. 2008.
|
|
3
|
Ye J, Guan Y, Zeng S and Liu D: Ampelopsin
prevents apoptosis induced by H2O2 in MT-4 lymphocytes. Planta Med.
74:252–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kundaković K, Stanojković T, Milenković M,
Grubin J, Juranić Z, Stevanović B and Kovačević N: Cytotoxic,
antioxidant, and antimicrobial activities of Ampelopsis
brevipedunculata and Parthenocissus tricuspidata (Vitaceae). Arch
Biol Sci. 60:641–647. 2008. View Article : Google Scholar
|
|
5
|
Zhou Y, Shu F, Liang X, et al: Ampelopsin
induces cell growth inhibition and apoptosis in breast cancer cells
through ROS generation and endoplasmic reticulum stress pathway.
PLoS One. 9:e890212014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kou X, Shen K, An Y, Qi S, Dai WX and Yin
Z: Ampelopsin inhibits H2O2-induced apoptosis
by ERK and Akt signaling pathways and up-regulation of heme
oxygenase-1. Phytother Res. 26:988–994. 2012. View Article : Google Scholar
|
|
7
|
Qi S, Xin Y, Guo Y, Diao Y, Kou X, Luo L
and Yin Z: Ampelopsin reduces endotoxic inflammation via repressing
ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int
Immunopharmacol. 12:278–287. 2012. View Article : Google Scholar
|
|
8
|
Jeon SH, Chun W, Choi YJ and Kwon YS:
Cytotoxic constituents from the bark of Salix hulteni. Arch Pharm
Res. 31:978–982. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou FZ, Zhang XY and Guo Y:
Anti-proliferation effect of combining dihydromyricetin and
adriamycin on MDA-MB-231 cell in vitro. Journal of Hubei University
for Nationalities (Medical Edition). 4:0012010.
|
|
10
|
Guo X, Zhu K, Zhang H and Yao H:
Anti-tumor activity of a novel protein obtained from tartary
buckwheat. Int J Mol Sci. 11:5201–5211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang QY, Li R, Zeng GF, et al:
Dihydromyricetin inhibits migration and invasion of hepatoma cells
through regulation of MMP-9 expression. World J Gastroenterol.
20:10082–10093. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Busse A and Keilholz U: Role of TGF-β in
melanoma. Curr Pharm Biotechnol. 12:2165–2175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perrot CY, Javelaud D and Mauviel A:
Insights into the transforming growth factor-β signaling pathway in
cutaneous melanoma. Ann Dermatol. 25:135–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moustakas A, Souchelnytskyi S and Heldin
CH: Smad regulation in TGF-beta signal transduction. J Cell Sci.
114(Pt 24): 4359–4369. 2001.
|
|
15
|
Varelas X, Samavarchi-Tehrani P, Narimatsu
M, et al: The Crumbs complex couples cell density sensing to
Hippo-dependent control of the TGF-β-SMAD pathway. Developmental
cell. 19:831–844. 2010. View Article : Google Scholar
|
|
16
|
Brown DI and Griendling KK: Nox proteins
in signal transduction. Free Radic Biol and Med. 47:1239–1253.
2009. View Article : Google Scholar
|
|
17
|
Tobar N, Guerrero J, Smith PC and Martínez
J: NOX4-dependent ROS production by stromal mammary cells modulates
epithelial MCF-7 cell migration. Br J Cancer. 103:1040–1047. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boudreau HE, Emerson SU, Korzeniowska A,
Jendrysik MA and Leto TL: Hepatitis C virus (HCV) proteins induce
NADPH oxidase 4 expression in a transforming growth factor
β-dependent manner: a new contributor to HCV-induced oxidative
stress. J Virol. 83:12934–12946. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barnes JL and Gorin Y: Myofibroblast
differentiation during fibrosis: role of NAD(P)H oxidases. Kidney
Int. 79:944–956. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu R-M and Gaston Pravia K: Oxidative
stress and glutathione in TGF-β-mediated fibrogenesis. Free Radical
Biology and Medicine. 48:1–15. 2010. View Article : Google Scholar
|
|
21
|
Pike LS, Smift AL, Croteau NJ, Ferrick DA
and Wu M: Inhibition of fatty acid oxidation by etomoxir impairs
NADPH production and increases reactive oxygen species resulting in
ATP depletion and cell death in human glioblastoma cells. Biochim
Biophys Acta. 1807.726–734. 2011.
|
|
22
|
Kisseleva T and Brenner DA: Mechanisms of
fibrogenesis. Exp Biol Med (Maywood). 233:109–122. 2008. View Article : Google Scholar
|
|
23
|
Pérez-Gómez E, Del Castillo G, Santibáñez
JF, Lopez-Novoa JM, Bernabéu C and Quintanilla M: The role of the
TGF-β coreceptor endoglin in cancer. Scientific World Journal.
10:2367–2384. 2010. View Article : Google Scholar
|
|
24
|
Baek HJ, Pishvaian MJ, Tang Y, Kim TH,
Yang S, Zouhairi ME, Mendelson J, Shetty K, Kallakury B, Berry DL,
et al: Transforming growth factor-β adaptor, β2-spectrin, modulates
cyclin dependent kinase 4 to reduce development of hepatocellular
cancer. Hepatology. 53:1676–1684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Taatjes DJ: The human Mediator complex: a
versatile, genome-wide regulator of transcription. Trends Biochem
Sci. 35:315–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moses H and Barcellos-Hoff MH: TGF-beta
biology in mammary development and breast cancer. Cold Spring
Harbor Perspect Biol. 3:a0032772011. View Article : Google Scholar
|
|
27
|
Wiercinska E, Naber HP, Pardali E, van der
Pluijm G, van Dam H and ten Dijke P: The TGF-β/Smad pathway induces
breast cancer cell invasion through the up-regulation of matrix
metalloproteinase 2 and 9 in a spheroid invasion model system.
Breast Cancer Res Treat. 128:657–666. 2011. View Article : Google Scholar
|
|
28
|
Petersen M, Pardali E, Van Der Horst G,
Cheung H, van den Hoogen C, van der Pluijm G and Ten Dijke P: Smad2
and Smad3 have opposing roles in breast cancer bone metastasis by
differentially affecting tumor angiogenesis. Oncogene.
29:1351–1361. 2010. View Article : Google Scholar
|
|
29
|
Dzwonek J, Preobrazhenska O, Cazzola S,
Conidi A, Schellens A, van Dinther M, Stubbs A, Klippel A,
Huylebroeck D, ten Dijke P and Verschueren K: Smad3 is a key
nonredundant mediator of transforming growth factor beta signaling
in Nme mouse mammary epithelial cells. Mol Cancer Res. 7:1342–1353.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Szatrowski TP and Nathan CF: Production of
large amounts of hydrogen peroxide by human tumor cells. Cancer
Res. 51:794–798. 1991.PubMed/NCBI
|
|
31
|
Bierie B and Moses HL: Gain or loss of
TGFbeta signaling in mammary carcinoma cells can promote
metastasis. Cell Cycle. 8:3319–3327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bierie B, Chung CH, Parker JS, Stover DG,
Cheng N, Chytil A, Aakre M, Shyr Y and Moses HL: Abrogation of
TGF-beta signaling enhances chemokine production and correlates
with prognosis in human breast cancer. J Clin Invest.
119:1571–1582. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang L, Huang J, Ren X, Gorska AE, Chytil
A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC and Moses
HL: Abrogation of TGF beta signaling in mammary carcinomas recruits
Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell.
13:23–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giannelli G, Mazzocca A, Fransvea E, Lahn
M and Antonaci S: Inhibiting TGF-β signaling in hepatocellular
carcinoma. Biochim Biophys Acta. 1815.214–223. 2011.
|
|
35
|
Aon MA, Cortassa S and O’Rourke B:
Redox-optimized ROS balance: a unifying hypothesis. Biochim Biophys
Acta. 1797:865–877. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Parga J, Rodríguez-Pallares J, Joglar B,
Diaz-Ruiz C, Guerra M and Labandeira-Garcia JL: Effect of
inhibitors of NADPH oxidase complex and mitochondrial ATP-sensitive
potassium channels on generation of dopaminergic neurons from
neurospheres of mesencephalic precursors. Dev Dyn. 239:3247–3259.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li S, Tabar SS, Malec V, Eul BG, Klepetko
W, Weissmann N, Grimminger F, Seeger W, Rose F and Hänze J: NOX4
regulates ROS levels under normoxic and hypoxic conditions,
triggers proliferation, and inhibits apoptosis in pulmonary artery
adventitial fibroblasts. Antioxid Redox Signal. 10:1687–1698. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu RM, Choi J, Wu JH, Gaston Pravia KA,
Lewis KM, Brand JD, Mochel NS, Krzywanski DM, Lambeth JD, Hagood
JS, et al: Oxidative modification of nuclear mitogen-activated
protein kinase phosphatase 1 is involved in transforming growth
factor beta1-induced expression of plasminogen activator inhibitor
1 in fibroblasts. J Biol Chem. 285:16239–16247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu R-M and Gaston Pravia K: Oxidative
stress and glutathione in TGF-β-mediated fibrogenesis. Free Radic
Biol Med. 48:1–15. 2010. View Article : Google Scholar
|
|
40
|
Hecker L, Vittal R, Jones T, Jagirdar R,
Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ and Thannickal
VJ: NADPH oxidase-4 mediates myofibroblast activation and
fibrogenic responses to lung injury. Nat Med. 15:1077–1081. 2009.
View Article : Google Scholar : PubMed/NCBI
|