Introduction
Diabetic peripheral neuropathy is usually
characterized by spontaneous pain, hyperalgesia and allodynia,
typical features of neuropathic pain (NPP) which are an important
problem, influencing the quality of life and lifespan of patients.
Currently, the therapeutic efficacy of treatments for diabetic
neuropathic pain (DNP) is still poor. Thus, it is imperative to
investigate the pathogenesis of DNP and develop novel strategies
for its treatment (1,2).
Previous studies have shown that an abnormal
activation of complements in the spinal dorsal horn has an
important role in the occurrence and development of NPP secondary
to peripheral nerve compression (3,4). In
type II diabetic patients with NPP, a biopsy and an
immunohistochemical examination of the sural nerve revealed
immunoglobulin G (IgG), IgM, C3 and C4 stored in the perineurium
and endoneurium. The expression levels of immunocompetent
complements in the microvascular wall of the endoneurium were
significantly higher than those in type II diabetic patients
without NPP (5,6).
Generally, complement regulatory proteins (CRPs)
function to inactivate complements and stop cascades, thus, the
functional status of the CRP determines the intensity of the
complement cascade. Of the CRPs, decay-accelerating factor (DAF),
or cluster of differentiation 55 (CD55), is widely expressed and
has evident functions. CD55 can compete with C2 to bind to C4b,
which inhibits the assembly of the C3/C5 convertase and the
formation of membrane attack complex (MAC), promoting the decay of
C3/C5 convertase and blocking the activation of the classical and
alternative complement pathways (7,8).
On the basis of above-mentioned findings, we
speculated that the downregulation of the expression and activity
of CD55 are crucial for the occurrence and development of DNP, and
that it may be an initiator for the abnormal activation of CRPs. To
confirm this hypothesis, quantitative polymerase chain reaction
(qPCR) and an immunohistochemical examination were performed in
order to detect the mRNA and protein expression levels of C3 and
CD55 in the spinal cords of mice with DNP. In addition, the
behaviors of these mice were observed. This study aimed to
investigate the correlation between CD55 expression and the
pathogenesis of DNP.
Materials and methods
Preparation of the animals
Healthy adult male C57BL/6J mice (specific pathogen
free; n=120) weighing 18–20 g were employed in this study, provided
by the Animal Experiment Center of the Third Military Medical
University, Chongqing, China [Animal Medical Certificate no. SCXK
(Military) 2002008]. The mice were housed (n=5 per cage) in the
Experimental Animal Center of Southwest Hospital (Chongqing,
China). The cage floor was covered with sawdust and maintained at a
temperature of 20 ± 2°C. The animals were exposed to a strict
alternating light-dark illumination pattern, each for 12 h, and
were supplied with adequate water and food. The animals were
treated according to the Guide for the Care and Use of Laboratory
Animals drawn up by the World Society for the Protection of
Animals, and the number of animals used, as well as pain, was
minimized in this study. The study was approved by the Laboratory
Animal Welfare and Ethics Committee of the Third Military Medical
University, PLA, (Chingqing, China). The committee conducts the
work according to Chinese legislation on the protection of animals
and the National Institutes of Health Guide for the Care and Use of
Laboratory animals. The committee examined and approved this
research project on June 27, 2012
Animal groupings and experimental
procedures
Establishment of a DNP model
A total of 60 mice were randomly selected for the
control group, while 60 mice were selected for the experimental
group. For accommodation, the experimental group mice were housed
for 3 days. Their baseline body weight, peripheral blood glucose,
mechanical pain threshold and thermal pain threshold were
determined. The animals fasted for 16 h. Following sterilization of
the abdomen, the mice were intraperitoneally injected with 120
mg/kg streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO, USA) in a
1% citrate buffer (pH 4.5; Guoguang Biochemistry Co., Ltd.,
Zhejiang, China). In the control group, the mice were
intraperitoneally treated with a citrate buffer of an equal volume
(9,10).
Detection
Prior to STZ injection, and at 3, 7, 14 and 21 days
following, the peripheral blood glucose level was measured, and the
mechanical pain threshold and thermal pain threshold were measured
and compared between the two groups.
Detection of the C3 and CD55 protein
and mRNA expression levels in the spinal cord
Three mice from each group were sacrificed at each
time point 3, 7, 14, 21 and 28 days following the STZ injection,
and an immunohistochemical examination was performed in order to
detect the C3 and C55 expression levels in the spinal dorsal horn.
A further six mice from each group were sacrificed at each time
point, and the mRNA expression of C3 and C5 in the spinal cord was
detected by qPCR.
Observation of behavioral changes
General behavioral observation
Following the STZ injection, the hair, water intake,
urine, spontaneous activity and body weight of the mice were
monitored.
Detection of the mechanical pain
threshold
The mice were placed in a transparent plexiglass
chamber with an audio amplifier, and their tails were placed out of
the chamber. A marker was placed 3 cm away from the root of the
tail. The mice were allowed to accommodate to the environment for
10 min prior to their tails being placed in an electronic
tenderness instrument (YLS-3E; Huaibei Zhenghua Bio Equipment Co.,
Ltd., Anhui, China). Compression was administered at the marker
site (at increments of 10 g/sec). If responses (screaming and
struggling) to compression were observed, the compression was
stopped and the pressure (g) recorded. Detection was performed
three times with an interval of ~10 min. The mean pressure was
calculated as the mechanical pain threshold (11).
Detection of the thermal pain
threshold
The mice were placed on a thick glass plate (3 mm in
thickness) and allowed to accommodate to the environment for 10
min. The foot of the right hindlimb was placed in a thermal
radiation source (PL-200; Chengdu Taimeng Science and Technology
Co., Ltd., Chengdu, China). The instrument was powered on. If foot
elevation occurred, the radiation was automatically stopped. The
time from radiation initiation to radiation discontinuation was
recorded. Detection was done thrice with an interval of ~10 min.
The mean time was calculated as the thermal pain threshold
(12).
Biochemical measurements
Detection of peripheral blood
glucose
The tails of the mice were sterilized, the tail tip
was cut and blood was collected into a blood glucose test strip.
Detection of blood glucose was done with a blood glucose meter
(BGM501; Isotech Co., Ltd., Seoul, Korea). Following sterilization
of the injured site, the mice were housed in cages (13).
Detection of expression of C3 and CD55
proteins in the spinal dorsal horn by immunohistochemistry
i) Preparation of the spinal cord sections. The mice
were intraperitoneally anesthetized with chloral hydrate (Moving
Your Chemistry Forward, Co., Ltd., Shanghai, China). Following a
thoracotomy, the heart was exposed. The mice were transcardially
perfused with heparinized normal saline until the liver became
white. Perfusion was done with 4% paraformaldehyde (50 ml) at 4°C.
The spinal cord segment, L4-6, was separated and fixed in 4%
paraformaldehyde (Zhejiang Guoguang Biochemistry Co., Ltd.) at 4°C
for 24 h. The tissues were embedded in paraffin and sectioned
(thickness, 8 μm).
Immunohistochemistry
The sections were heated at 60°C for 2 h,
deparaffinized with xylene, hydrated with ethanol, and washed in
phosphate-buffered saline (PBS; Guoguang Biochemistry Co., Ltd.).
Antigen retrieval was performed in a citrate buffer in a microwave
oven. Once the sections had cooled to room temperature, they were
washed in PBS and treated with 50 μl of a blocking buffer at room
temperature for 10 min, in order to block endogenous peroxidase,
and washed in PBS. Following the addition of goat serum (50 μl;
Haoranbio Co., Ltd., Shanghai, China), incubation was performed at
room temperature for 10 min. The sections were incubated with a
primary antibody [50 μl C3 monoclonal rat anti-mouse antibody
(sc-58926), dilution, 1:200; 50 μl CD55 monoclonal goat anti-mouse
antibody (sc-31208), dilution, 1:150; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA] at 4°C overnight in a humidified
chamber.
Visualization
The sections were washed in PBS and treated with
biotin-conjugated goat anti-rat secondary antibody (50 μl; Santa
Cruz Biotechnology, Inc.) at room temperature for 10 min. The
sections were washed in PBS and incubated with horseradish
peroxidase-conjugated streptavidin-biotin (50 μl) at room
temperature for 10 min. Following washing in PBS, the sections were
treated with diaminobenzidine for visualization (100 μl/section).
The sections were washed in water and counterstaining was performed
with hematoxylin for 40–60 sec. These sections were treated with
hydrochloric acid in ethanol, dehydrated in ethanol,
transparentized in xylene and air-dried and mounted with neutral
gum (Zhejiang Guoguang Biochemistry Co., Ltd.). Observation was
performed under a light microscope (Phoenix Optical Group Co.,
Ltd., Jiangxi, China) (14,15).
Detection of the mRNA expression of C3
and CD55 in the spinal cord by qPCR
i) Extraction of total RNA. The mice were weighed
and intraperitoneally anesthetized with 5% chloral hydrate. The
L4-6 spinal cord was exposed and stored in liquid nitrogen at
−80°C. For detection, 20–40 mg of the spinal cord was ground in
liquid nitrogen and mixed with 0.7 ml TRIzol (Beijing Kangwei
Biotech Co., Ltd., Beijing, China) in the presence of chloroform
for lysis. Following centrifugation at a low temperature, the
supernatant was collected and mixed with isopropanol of an equal
volume, followed by further centrifugation at 15,984 × g and 4°C.
The supernatant was removed and mixed with 0.7 ml of 75% ethanol,
centrifuged at a low temperature, the supernatant was removed and
the pellets were maintained at room temperature for 2–3 min and
dissolved in 30 μl of distilled water. The resulting RNA solution
was stored at −80°C.
Detection of the RNA concentration
using an ultraviolet spectrophotometer
In brief, 1 μl of RNA was dissolved in enzyme-free
water (100 μl) and transferred into a cuvette; 100 μl of the
enzyme-free water served as a control. The optical density (OD) was
measured at 260 nm with an ultraviolet visible spectrophotometer
(Ultrospec 4300 pro; Hitachi, Ltd., Tokyo, Japan). The RNA
concentration (μg/μl) was calculated as follows: RNA concentration
= OD260 × folds of dilution × 40/1,000.
qPCR
The reaction mixture contained 2.5 mM dNTP mix (4
μl), primer mix (2 μl), a RNA template (2 μg), 5X RT buffer (4 μl),
0.1 M DTT (2 μl), 200 U/μl HiFi-MMLV (1 μl) and RNase-free water
(final, 20 μl). Following centrifugation, PCR was performed in a
thermal cycler at 42°C for 50 min and then at 70°C for 15 min.
Following transient centrifugation, the products were stored at
−20°C for later use. PCR amplification was performed in the
following manner: the mRNA sequence of mouse C3 was obtained from
GenBank, and Prime premier was employed to design primers, which
were synthesized by Shanghai Shengbo Biotech Co., Ltd., Shanghai,
China. The primers used for C3 amplification were as follows:
forward, 5′-AGCAGGTCATCAAGTCAGGC-3′ (167 bp) and reverse,
5′-GATGTAGCTGGTGTTGGGCT-3′ for C3; forward,
5′-GAGTCCTTCAACACCCCAGC-3′ (263 bp) and reverse,
5′-ATGTCACGCACGATTTCCC-3′ for β-actin. PCR of C3 was performed in a
Sprint Thermal Cycler (Thermo Electron Corporation, Milford, MA,
USA). The reaction conditions were as follows: Preconditioning at
94°C for 2 min, followed by a total of 35 cycles of denaturation at
94°C for 30 sec, annealing at 53.4°C (C3) or 58°C (β-actin) for 30
sec, extension at 72°C for 30 sec and a final extension at 73°C for
2 min. The primers used for CD55 amplification were as follows:
forward, 5′-CTCTGTTGCTGCTGTCCC-3′ (477 bp) and reverse,
5′-CGAATAATATGCCGGTTG-3′ for CD55; forward,
5′-GAGTCCTTCAACACCCCAGC-3′ (263 bp) and reverse,
5′-ATGTCACGCACGATTTCCC-3′ for β-actin. The reaction conditions were
as follows: predenaturation at 94°C for 2 min, followed by a total
of 35 cycles of denaturation at 94°C for 30 sec, annealing at 52°C
(CD55) or 58°C (β-actin) for 30 sec, extension at 72°C for 30 sec
and a final extension at 73°C for 2 min.
Identification of the PCR products by
agarose gel electrophoresis
A 1.2% agarose gel was melted by heating to 60°C.
Then, 10 μl of ethidium bromide was added. The liquid agarose was
added to a tray and cooled to room temperature. The gel was placed
in an electrophoresis chamber, and 0.5X TBE was added until the gel
was immersed in the solution. The samples were added to the gel (5
μl), and a DNA marker (5 μl) was added to the left well.
Electrophoresis was performed at 100 V until a blue band reached
two-thirds of way to the lower edge. Images were captured and
analyzed with a Gel Doc 2000 gel image analysis (16,17).
Statistical analysis
Statistical analysis was performed with SPSS 13.0
software (SPSS., Inc., Chicago, IL, USA). The data are expressed as
the mean ± standard deviation (SD). The measurement data and
numeration data were statistically analyzed with a t-test
and χ2 test, respectively. P<0.05 was considered to
indicate a statistically significant difference.
Results
Behavioral changes
In the control group, the mice had smooth, soft
hair, their water intake and urine volume remained unchanged and
their blood glucose was stable. In the experimental group, the mice
had matted hair, their water intake and urine volume increased
significantly, their level of spontaneous activity was reduced, and
their body weight was decreased. Their peripheral blood glucose was
markedly increased 3, 7, 14 and 21 days following the STZ
injection. In the control group, the mechanical pain threshold and
thermal pain threshold remained unchanged. In the experimental
group, the mechanical pain threshold and thermal pain threshold
began to decrease 14 days following the STZ injection. The
mechanical pain threshold and thermal pain threshold were
dramatically decreased 21 and 28 days following the STZ injection
(Table I).
 | Table IBlood glucose levels and pain
thresholds of the mice at different time points. |
Table I
Blood glucose levels and pain
thresholds of the mice at different time points.
| Item | Group | Before STZ injection
(n) | After STZ
injection |
|---|
|
|---|
| 3 days (n) | 7 days (n) | 14 days (n) | 21 days (n) | 28 days (n) |
|---|
| Blood glucose
(mmol/l) | Control | 5.02±0.39 (10) | 5.38±0.82 (10) | 5.66±0.88 (10) | 5.13±0.92 (10) | 5.37±0.92 (10) | 5.02±0.39 (10) |
| STZ | 5.03±0.80 (10) | 24.9±3.77a,c
(10) | 24.9±3.92a,c
(10) | 25.6±2.82a,c
(10) | 25.2±4.82a,c
(10) | 24.3±3.25a,c
(10) |
| MPT (g) | Control | 219±20.7 (40) | 210±24.7 (40) | 227±29.3 (40) | 220±24.0 (40) | 219±28.8 (40) | 207±26.1 (40) |
| STZ | 216±14.2 (40) | 199±22.0 (40) | 189±30.8b (40) | 199±27.5 (40) | 126±28.6a,c
(40) | 128±34.7a,c
(40) |
| TPT (s) | Control | 9.9±0.43 (40) | 9.4±0.83 (40) | 9.8±0.53 (40) | 9.7±0.87 (40) | 9.7±0.95 (40) | 9.7±0.89 (40) |
| STZ | 9.7±0.41 (40) | 9.4±0.89 (40) | 9.3±0.73 (40) | 8.9±1.68 (40) | 6.2±1.61a,c
(40) | 5.7±2.14a,c
(40) |
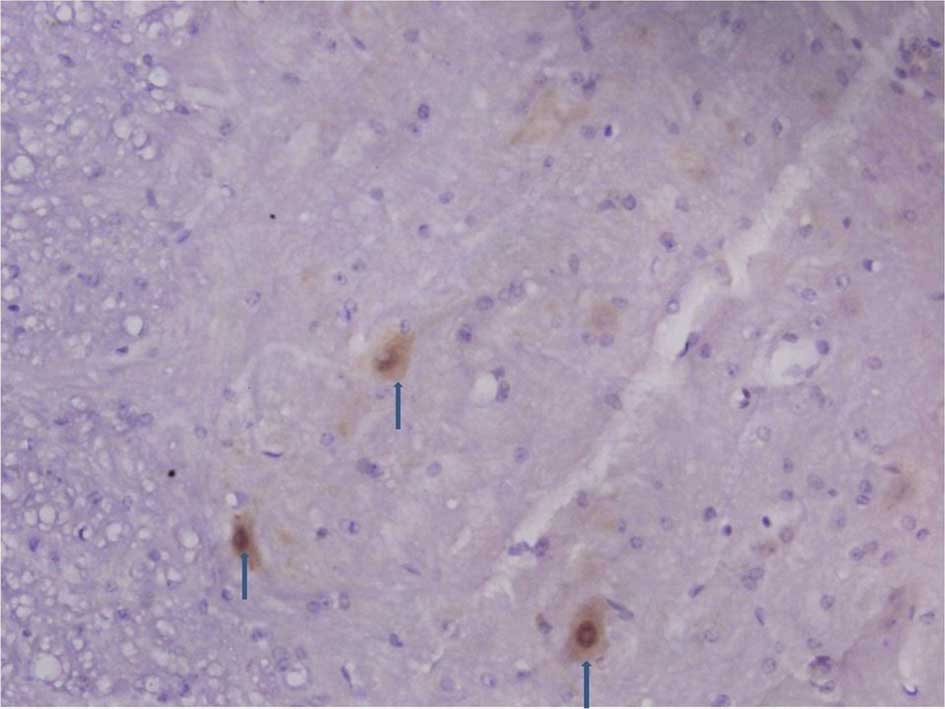
Immunohistochemistry of the spinal dorsal
horn
The number of C3 positive cells began to increase in
the spinal dorsal horn 3, 7 and 14 days following an
intraperitoneal injection of STZ. The number of C3 positive cells
including astrocytes and microglia markedly increased 21 and 28
days after the STZ injection (Figs.
1 and 2).
Agarose gel electrophoresis of the qPCR
products
A band was noted at 263 bp (Fig. 3) that was consistent with the
length of C3. Quantity One analysis software version 4.0 (Bio-Rad,
Hercules, CA, USA) was employed in order to detect the OD of the C3
and the β-actin bands. Fourteen days after the STZ injection, the
mRNA expression increased dramatically, as compared with that prior
to the STZ injection (P<0.05), and this increase continued until
28 days after the STZ injection (Table II).
 | Table IImRNA expression of C3 in the spinal
cord of mice. |
Table II
mRNA expression of C3 in the spinal
cord of mice.
| | Days post-STZ
injection |
|---|
| |
|
|---|
| Group | Before STZ | 3 | 7 | 14 | 21 | 28 |
|---|
| Control | 0.17±0.021 | 0.16±0.013 | 0.19±0.017 | 0.17±0.023 | 0.16±0.018 | 0.17±0.024 |
| STZ | 0.15±0.015 | 0.18±0.018 | 0.20±0.011 | 0.45±0.089a,b | 0.53±0.094a,b | 0.70±0.088a,b |
In the experimental group, 3 days after an
intraperitoneal injection of STZ, the number of CD55 positive cells
in the spinal dorsal horn began to decline. Seven days after the
STZ injection, the number of CD55 positive cells in the spinal
dorsal horn was markedly reduced and remained at a low level
(Figs. 4 and 5).
mRNA expression of CD55 in the spinal
cord
Following 1.2% agarose gel electrophoresis, a band
was noted at 477 bp that was consistent with the length of CD55
(Fig. 6). Quantity One version 4.0
was employed in order to detect the OD of CD55 and β-actin
(Table III). When compared with
the control group, the mRNA expression of CD55 was markedly reduced
7 days post-STZ injection (P<0.05), and this reduction continued
for 28 days following the STZ injection.
 | Table IIICluster of differentiation 55 mRNA
expression in the spinal cord of mice at different time points. |
Table III
Cluster of differentiation 55 mRNA
expression in the spinal cord of mice at different time points.
| | Days post-STZ
injection |
|---|
| |
|
|---|
| Group | Before STZ
injection | 3 | 7 | 14 | 21 | 28 |
|---|
| Control | 0.89±0.24 | 0.79±0.21 | 0.78±0.17 | 0.82±0.27 | 0.80±0.19 | 0.85±0.18 |
| STZ | 0.85±0.13 | 0.78±0.18 | 0.61±0.14a | 0.54±0.21b,c | 0.41±0.12b,c | 0.23±0.12b,c |
Discussion
Previous studies have shown that the occurrence and
development of NPP secondary to peripheral nerve injury is closely
associated with the abnormal activation of complements (3,4).
Neurons have fewer CRPs. When complements act on neurons, activated
complements cannot be inactivated in a timely ma and thus are
unable to block the attack of neurons by MAC. The attack of neurons
by MACs results in an increase in the permeability of the neural
membrane, and a large amount of Ca2+ enters the neurons.
The excitability of the neurons elevates, and a large amount of
reactive oxygen species are released, which in turn increase the
sensitivity the of neurons to complements. This cycle finally leads
to the excitotoxicity of the neurons, resulting in hyperalgesia
(18,19). Neurons with increased
excitotoxicity may release a large amount of glutamate and ATP,
which act on glial cells, leading to the release of a large amount
of pro-inflammatory cytokines. This further activates more
complements, resulting in a vicious cycle and subsequent immune
injury to the nerves (20).
In an animal model of DNP, there were prerequisites
for the activation of alternative and classic pathways. Thus, a DNP
model has been used as the optimal tool for the investigation of
abnormal complement activation in NPP. Whether the occurrence of
DNP is associated with abnormal complement activation, as in NPP
secondary to peripheral nerve injury, remains unclear.
Currently, in diabetic animal models, an
intraperitoneal injection of STZ is widely used to induce diabetes.
STZ is a compound with nitroso groups that causes diabetes in one
of the following ways: i) It may directly damage the islet β cells,
STZ enters the islet β cells via the glucose transporter 2 and then
reduces coenzyme I (nicotinamide adenine dinucleotide) in the islet
β cells, resulting in β-cell death; ii) it may induce the
production of nitric oxide (NO) and free radicals, resulting in
β-cell death; or iii) STZ may induce autoimmune reactions,
resulting in damage to the β cells (21,22).
In the present study, a diabetes mellitus (DM) mouse model was
successfully established, and the clinical manifestations of DM
were clear (polyuria, polydipsia, polyphagia and emaciation). In
addition, following an intraperitoneal injection of STZ, the
mechanical and thermal pain thresholds were significantly reduced
in the C57BL/6J mice. These findings were consistent with those
reported by Courteix et al (23). The occurrence of DNP is closely
associated with the course of DM. The incidence of NPP increases
over the course of DM (24).
In the present study, the results showed that 14
days after an STZ injection, the mRNA expression of C3
significantly increased in the spinal cord of mice and continued to
increase, which was consistent with the hyperalgesic time course in
these mice. Twenty-one days after an STZ injection, the number of
C3 positive cells had markedly increased in the spinal dorsal horn,
and continued to increase until the end of the study (28 days). The
results revealed that the number of mice with hyperalgesia was
small 14 days following the STZ injection, and no increase in the
C3 protein expression was evident. Protein production involves
transcription and translation, and thus the increase in the mRNA
expression of C3 was observed earlier than that of the protein
expression of C3 (25,26). The findings of the current study at
the protein and mRNA levels confirmed that the occurrence and
development of DNP is closely associated with an abnormal
activation of complements in the spinal dorsal horn, as in NPP
following peripheral nerve injury (27,28).
Activation of the complement system is strictly
controlled by CRPs. Abnormalities in the expression and function of
CRP may cause an abnormal activation of the complement system,
resulting in tissue damage. There is evidence that the expression
of certain membrane-bound CRPs (mCRPs) is influenced by blood
glucose and insulin. Of these mCRPs, CD46, CD55 and CD59 are widely
expressed, have a variety of functions and are closely associated
with the pathogenesis of DM (29,30).
CD55 is also known as a DAF and is widely expressed in tissues. In
the central nervous system, the astrocytes, microglia and neurons
can synthesize and express CD55 and its receptor. CD55 primarily
competes with C2 in order to bind to C4b, inhibits the formation of
C3 convertase and promotes the decay of C3/C5 convertase, which
avoids the overdeposition and activation of C3 and C5 and inhibits
the activation of the classic and alternative pathways (31,32).
Previous studies have shown that in mice with CD55 knock out and
myasthenia gravis (MG) induction, the MG symptoms were more severe,
there was a large amount of C3 at the neuromuscular junctions and
the pathological changes were more obvious compared with mice that
had MG alone (33). In rats with
STZ-induced diabetic retinopathy, an increase in complement
activation was accompanied by a reduction in the expression of CD55
and CD59 (34). Thus, we
hypothesize that the downregulated expression and activation of
CD55 in the spinal cord may be one of the mechanisms that initiate
abnormal complement activation and an increase in C3 in NPP.
In the present study, qPCR and an
immunohistochemical examination were employed in order to detect
CD55 expression in the spinal dorsal horn of mice with STZ-induced
DNP at different time points. The results demonstrated that the
mRNA and protein expression of CD55 in the spinal dorsal horn was
significantly reduced 3 days after an STZ injection; this reduction
continued for 28 days. The reduction in CD55 expression was earlier
than the increase in C3 expression, which is consistent with
expectations. The results demonstrated that the downregulated CD55
expression occurred prior to an abnormal C3 activation, and had an
important role in the occurrence and development of DNP, which may
be an initiator of abnormal complement activation that is involved
in the occurrence and development of NPP.
Acknowledgements
The authors extend their gratitude to Professor
Jiangkai Lin in the Department of Neurosurgery, Southwest Hospital,
who made several suggestions for improving the technical details of
the experiment. This study was supported by grants from the
National Natural Science Foundation of China (NSFC-30772077).
References
|
1
|
Kessler NJ and Hong J: Whole body
vibration therapy for painful diabetic peripheral neuropathy: a
pilot study. J Bodyw Mov Ther. 17:518–522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalra B, Kalra S and Bajaj S: Vulvodynia:
An unrecognized diabetic neuropathic syndrome. Indian J Endocrinol
Metab. 17:787–789. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nie F, Wang J, Su D, Shi Y, Chen J, Wang
H, Qin W and Shi L: Abnormal activation of complement C3 in the
spinal dorsal horn is closely associated with progression of
neuropathic pain. Int J Mol Med. 31:1333–1342. 2013.PubMed/NCBI
|
|
4
|
Levin ME, Jin JG, Ji RR, Tong J, Pomonis
JD, Lavery DJ, Miller SW and Chiang LW: Complement activation in
the peripheral nervous system following the spinal nerve ligation
model of neuropathic pain. Pain. 137:182–201. 2008. View Article : Google Scholar
|
|
5
|
Rosoklija GB, Dwork AJ, Younger DS,
Karlikaya G, Latov N and Hays AP: Local activation of the
complement system in endoneurial microvessels of diabetic
neuropathy. Acta Neuropathol. 99:55–62. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lalive PH, Truffert A, Magistris MR,
Landis T and Dosso A: Peripheral autoimmune neuropathy assessed
using corneal in vivo confocal microscopy. Arch Neurol. 66:403–405.
2009.PubMed/NCBI
|
|
7
|
Mamidi S, Cinci M, Hasmann M, Fehring V
and Kirschfink M: Lipoplex mediated silencing of membrane
regulators (CD46, CD55 and CD59) enhances complement-dependent
anti-tumor activity of trastuzumab and pertuzumab. Mol Oncol.
7:580–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bani-Ahmad M, El-Amouri IS, Ko CM, Lin F,
Tang-Feldman Y and Oakley OR: The role of decay accelerating factor
in the immunopathogenesis of cytomegalovirus infection. Clin Exp
Immunol. 163:199–206. 2011. View Article : Google Scholar :
|
|
9
|
Galeotti N, Maidecchi A, Mattoli L, Burico
M and Ghelardini C: St. John’s Wort seed and feverfew flower
extracts relieve painful diabetic neuropathy in a rat model of
diabetes. Fitoterapia. 92:23–33. 2014. View Article : Google Scholar
|
|
10
|
Ikeda H, Ikegami M, Kai M, Ohsawa M and
Kamei J: Activation of spinal cannabinoid CB2 receptors inhibits
neuropathic pain in streptozotocin-induced diabetic mice.
Neuroscience. 250:446–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shukla M, Quirion R and Ma W: Reduced
expression of pain mediators and pain sensitivity in amyloid
precursor protein over-expressing CRND8 transgenic mice.
Neuroscience. 250:92–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murakami T, Kanchiku T, Suzuki H, Imajo Y,
Yoshida Y, Nomura H, Cui D, Ishikawa T, Ikeda E and Taguchi T:
Anti-interleukin-6 receptor antibody reduces neuropathic pain
following spinal cord injury in mice. Exp Ther Med. 6:1194–1198.
2013.PubMed/NCBI
|
|
13
|
Bak EJ, Kim J, Jang S, Woo GH, Yoon HG,
Yoo YJ and Cha JH: Gallic acid improves glucose tolerance and
triglyceride concentration in diet-induced obesity mice. Scand J
Clin Lab Invest. 73:607–614. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmitt J, Roderfeld M, Sabrane K, Zhang
P, Tian Y, Mertens JC, Frei P, Stieger B, Weber A, Müllhaupt B,
Roeb E and Geier A: Complement factor C5 deficiency significantly
delays the progression of biliary fibrosis in bile duct-ligated
mice. Biochem Biophys Res Commun. 418:445–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kato C, Kato A, Adachi K, Fujii E, Isobe
K, Watanabe T, Ito T and Suzuki M: Expression of membrane
complement regulatory proteins Crry and CD55 in normal rats. J
Toxicol Pathol. 26:223–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mishra J, Sahoo PK, Mohanty BR and Das A:
Sequence information, ontogeny and tissue-specific expression of
complement component C3 in Indian major carp, Labeo rohita
(Hamilton). Indian J Exp Biol. 47:672–678. 2009.PubMed/NCBI
|
|
17
|
Zell S, Geis N, Rutz R, Schultz S, Giese T
and Kirschfink M: Down-regulation of CD55 and CD46 expression by
anti-sense phosphorothioate oligonucleotides (S-ODNs) sensitizes
tumour cells to complement attack. Clin Exp Immunol. 150:576–584.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Loo LS and McNamara JO: Impaired volume
regulation is the mechanism of excitotoxic sensitization to
complement. J Neurosci. 26:10177–10187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tegla CA, Cudrici C, Rus V, Ito T, Vlaicu
S, Singh A and Rus H: Neuroprotective effects of the complement
terminal pathway during demyelination: implications for
oligodendrocyte survival. J Neuroimmunol. 213:3–11. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tender GC, Li YY and Cui JG: The role of
nerve growth factor in neuropathic pain inhibition produced by
resiniferatoxin treatment in the dorsal root ganglia. Neurosurgery.
73:158–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sundaram B, Singhal K and Sandhir R:
Ameliorating effect of chromium administration on hepatic glucose
metabolism in streptozotocin-induced experimental diabetes.
Biofactors. 38:59–68. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pandey AK, Gupta PP and Lal VK:
Preclinical evaluation of hypoglycemic activity of Ipomoea digitata
tuber in streptozotocin-induced diabetic rats. J Basic Clin Physiol
Pharmacol. 24:35–39. 2013. View Article : Google Scholar
|
|
23
|
Courteix C, Eschalier A and Lavarenne J:
Streptozocin-induced diabetic rats: behavioural evidence for a
model of chronic pain. Pain. 53:81–88. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suehiro K, Funao T, Fujimoto Y, Yamada T,
Mori T and Nishikawa K: Relationship between noradrenaline release
in the locus coeruleus and antiallodynic efficacy of analgesics in
rats with painful diabetic neuropathy. Life Sci. 92:1138–1144.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsieh CC, Chou HS, Yang HR, Lin F, Bhatt
S, Qin J, Wang L, Fung JJ, Qian S and Lu L: The role of complement
component 3 (C3) in differentiation of myeloid-derived suppressor
cells. Blood. 121:1760–1768. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berg A, Zelano J, Stephan A, Thams S,
Barres BA, Pekny M, Pekna M and Cullheim S: Reduced removal of
synaptic terminals from axotomized spinal motoneurons in the
absence of complement C3. Exp Neurol. 237:8–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mika J, Zychowska M, Popiolek-Barczyk K,
Rojewska E and Przewlocka B: Importance of glial activation in
neuropathic pain. Eur J Pharmacol. 716:106–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Doehring A, Geisslinger G and Lötsch J:
Epigenetics in pain and analgesia: an imminent research field. Eur
J Pain. 15:11–16. 2011. View Article : Google Scholar
|
|
29
|
Yamamoto H, Fara AF, Dasgupta P and Kemper
C: CD46: the ‘multitasker’ of complement proteins. Int J Biochem
Cell Biol. 45:2808–2820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nevo Y, Ben-Zeev B, Tabib A, Straussberg
R, Anikster Y, Shorer Z, Fattal-Valevski A, Ta-Shma A, Aharoni S,
Rabie M, Zenvirt S, Goldshmidt H, Fellig Y, Shaag A, Mevorach D and
Elpeleg O: CD59 deficiency is associated with chronic hemolysis and
childhood relapsing immune-mediated polyneuropathy. Blood.
121:129–135. 2013. View Article : Google Scholar
|
|
31
|
Margolles-Clark E, Jacques-Silva MC,
Ganesan L, Umland O, Kenyon NS, Ricordi C, Berggren PO and Buchwald
P: Suramin inhibits the CD40-CD154 costimulatory interaction: a
possible mechanism for immunosuppressive effects. Biochem
Pharmacol. 77:1236–1245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nowicki B and Nowicki S: DAF as a
therapeutic target for steroid hormones: implications for
host-pathogen interactions. Adv Exp Med Biol. 735:83–96. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin F, Kaminski HJ, Conti-Fine BM, et al:
Markedly enhanced susceptibility to experimental autoimmune
myasthenia gravis in the absence of decay-accelerating factor
protection. J Clin Invest. 110:1269–1274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang J, Gerhardinger C and Lorenzi M:
Early complement activation and decreased levels of
glycosylphosphatidylinositol-anchored complement inhibitors in
human and experimental diabetic retinopathy. Diabetes.
51:3499–3504. 2002. View Article : Google Scholar : PubMed/NCBI
|