Introduction
Oral squamous cell carcinoma is the most common form
of head and neck cancer. It accounts for >90% of all such
cancers and has a poor prognosis that may be attributable to the
high frequency of lymph node metastasis and local invasion
(1). Tongue cancer is the most
common form of intraoral cancer. Its incidence is rising in
comparison with that of cancer in other head and neck sites
(2). Metastatic tongue carcinoma
is associated with poorer survival and a lower rate of local tumor
control than other sites of head and neck cancer and has a
five-year survival rate of just 50% (2). The development of oral tongue
squamous cell carcinoma (OTSCC) metastasis currently poses
significant clinical challenges due to the limited therapeutic
options that are available (3).
Twist-related protein 1 (TWIST), also known as
TWIST1, is a member of the basic helix-loop-helix transcription
factor family. During embryonic development, TWIST is essential in
the development of the mesoderm and differentiation of
mesoderm-derived tissues (4). A
high level of expression of TWIST has been detected in several
forms of cancer and has been associated with the initial phase of
metastatic progression (5). A
recent study has shown that overexpression of TWIST is associated
with a poor prognosis in patients with OTSCC and that knockdown of
TWIST inhibits OTSCC cell invasion (6).
β-catenin, originally identified as an essential
regulator of E-cadherin-mediated cell-cell interaction, is a key
component of the Wnt signaling pathway (7). In the majority of cells, β-catenin is
predominantly located at the plasma membrane in a complex with
cadherins and α-catenin. This forms the insoluble pool of
β-catenin. Under normal conditions, a small quantity of soluble
β-catenin, which is free from cadherin, is present in the cytoplasm
(8). Wnt signals are transduced
via specific cell surface receptors and activate a series of
biochemical reactions, involving a large protein complex consisting
of β-catenin and glycogen synthase kinase-3β (GSK-3β). This results
in stabilization of soluble β-catenin and therefore an increase in
the soluble pool of this molecule (9). Soluble β-catenin interacts with T
cell factor (Tcf) family transcription factors to activate a number
of downstream target genes, such as c-Myc and c-Jun, which are
important in the initiation and progression of carcinogenesis
(8,10,11).
Recent studies have provided in vitro evidence that
β-catenin signaling is pivotal in facilitating OTSCC cell invasion
(12,13).
A pilot study conducted by our group suggested that
TWIST may regulate β-catenin signaling in OTSCC cells. The current
study investigated the effect of TWIST on β-catenin signaling in
OTSCC cells and its impact on OSTCC cell invasion.
Materials and methods
Cells lines, plasmids and reagents
Human SCC-4 and TCA8113 OTSCC cell lines were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and Wuhan Boster Bio-Engineering Inc. (Wuhan, China),
respectively. Human Twist cDNA was subcloned into a pcDNA 3.1
expression vector (14). TOPflash
and FOPflash plasmids were obtained from Millipore (Billerica, MA,
USA). Twist (sc-38604-V) and β-catenin (sc-29209-V) short hairpin
(sh) RNA lentiviral particles, control shRNA lentiviral particles-A
(sc-108080), anti-TWIST (sc-81417) mouse monoclonal antibodies,
anti-β-catenin (sc-7963) mouse monoclonal antibodies and
anti-matrix metalloproteinase-2 (MMP-2, sc-53630) mouse monoclonal
antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa
Cruz, CA, USA). Anti-GSK-3β and Anti-phospho-GSK-3β (serine 9)
rabbit monoclonal antibodies were obtained from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Superfect™ transfection
reagent was purchased from Qiagen (Valencia, CA, USA). A
dual-luciferase reporter assay system was obtained from Promega
Corporation (Madison, WI, USA). G418, puromycin, LY294002 and all
chemicals of reagent grade were obtained from Sigma (St. Louis, MO,
USA).
Transfection and lentiviral
transduction
The TWIST expression construct was transfected into
cells using Superfect™ transfection reagent (Qiagen) according to
the manufacturer’s instructions. Pools of stable transfectants were
generated via selection with G418 (800 μg/ml) according to the
manufacturer’s instructions. Lentiviral transduction was performed
as previously described (15), and
pools of stable transductants were generated via selection with
puromycin (5 μg/ml).
Western blot analysis
Immunoblotting was performed with the appropriate
antibodies. Soluble cell lysate fractions were prepared as
previously described (15).
Briefly, cells were lysed in 0.1% Nonidet P-40 lysis buffer (0.1%
Nonidet P-40; 10 mM HEPES, pH 7.5; 142.5 mM KCl; 5 mM
MgCl2; and 1 mM ethylene glycol tetra acetic acid). The
lysates were centrifuged at 14,000 × g for 10 min and the
supernatants were saved as soluble cell lysate. To prepare the
whole cell lysate, cells were dissolved in 250 μl of 2X SDS loading
buffer (62.5 mm TrisHCl, pH 6.8; 2% SDS; 25% glycerol; 0.01%
bromphenol blue; and 5% 2-mercaptoethanol), and incubated at 95°C
for 10 min. Equal quantities of proteins for each sample were
separated by 10% SDS-polyacrylamide gel and blotted onto
polyvinylidene difluoride microporous membranes (Millipore).
Membranes were incubated for 1 h with a 1/1,000 dilution of primary
antibody, and then washed and revealed using secondary antibodies
conjugated to horseradish peroxidase (1/5,000, 1 h). Peroxidase was
visualized with a GE Healthcare enhanced chemiluminescence kit
(Beijing, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was prepared from cells using TRIzol reagent
(Life Technologies, Carlsbad, CA, USA) followed by purification
with a TURBO DNA-free system (Ambion, Austin, TX, USA). A total of
200 ng cDNA was synthesized using SuperScript II reverse
transcriptase (Invitrogen, Carlsbad, CA, USA). RT-qPCR was
performed on the LightCycler thermal cycler system (Roche
Diagnostics, Indianapolis, IN, USA) using SYBR Green I kit (Roche
Diagnostics) according to the manufacturer’s instructions. The PCR
amplification conditions were as follows: 20 sec at 95°C followed
by 40 cycles of 3 sec at 95°C and 30 sec at 60°C. The results were
normalized against those of the reference gene,
glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The following
primers were used: Forward: 5′-AGGGATTTTCTCAGTCCTTC-3′ and reverse:
5′-CATGCCCTCATCTAATGTCT-3′ for β-catenin; forward:
5′-GGACGACGAGACCTTCATCAA-3′ and reverse:
5′-CCAGCTTCTCTGAGACGAGCTT-3′ for human c-Myc; forward:
5′-CAAAGTTTGGATTGCATCAAGTG-3′ and reverse:
5′-TAACATTATAAATGGTCACAGCACATG-3′ for human c-Jun; and forward:
5′-GACTCATGACCA CAGTCCATGC-3′ and reverse: 5′-AGAGGCAGGGATG
ATGTTCTG-3′ for human GAPDH. Each experiment was repeated twice in
triplicate.
Luciferase assay
SCC-4 and TCA8113 cells were transfected with
TOPflash or FOPflash plasmids using Superfect transfection reagent
(Qiagen). PRL-CMV plasmid encoding Renilla reniformis
luciferase (at a concentration of one fifth molar ratio relative to
the test plasmids) was co-transfected as an internal control. The
luciferase assays were performed following 24 h transfection with a
dual-luciferase reporter assay system (Promega Corporation)
according to the manufacturer’s instructions. Each experiment was
repeated three times in duplicate.
In vitro cell invasion assay
Transwell® cell-culture chambers with
8-μm pores (BD Biosciences, Bedford, MA, USA) and 24 wells per
plate were coated with 50 μl Matrigel (10 mg/ml; BD Biosciences;
diluted 1:3). SCC-4 and TCA8113 cells were seeded in the upper
chamber at 5×105 cells per well, in Dulbecco’s modified
Eagle’s medium and RPMI-1640 serum-free medium, respectively.
Complete medium (600 μl) with 10% fetal bovine serum was added to
the lower chamber. After 24 h of incubation, cells were fixed and
stained with crystal violet. Invading cells were counted in five
random fields per chamber under an inverted microscope (IX83;
Olympus, Bejing, China). Each experiment was repeated three times
in duplicate.
Statistical analysis
Statistical analyses were performed using SPSS for
Windows 10.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard deviation. Comparisons of means between groups
were performed with one-way analysis of variance followed by post
hoc pairwise comparisons using Tukey’s tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
TWIST expression is increased by
transfection with a TWIST expression vector and decreased by
transduction of TWIST shRNA
To investigate the function of TWIST in OTSCC cells,
SCC-4 and TCA8113 human OTSCC cells were stably transfected with a
TWIST expression vector to induce overexpression of TWIST. In
addition, a separate group of cells was stably transduced with
TWIST-shRNA in order to knock down the expression of this gene. As
shown in Fig. 1, TWIST is
constitutively expressed in SCC-4 and TCA8113 cells. Its expression
was reduced by ~80% by stable transduction of TWIST-shRNA. Compared
with controls, TWIST expression was increased three-fold in SCC-4
and TCA8113 cells that had been stably transfected with TWIST.
These results were not altered by transduction of β-catenin-shRNA
(Fig. 1).
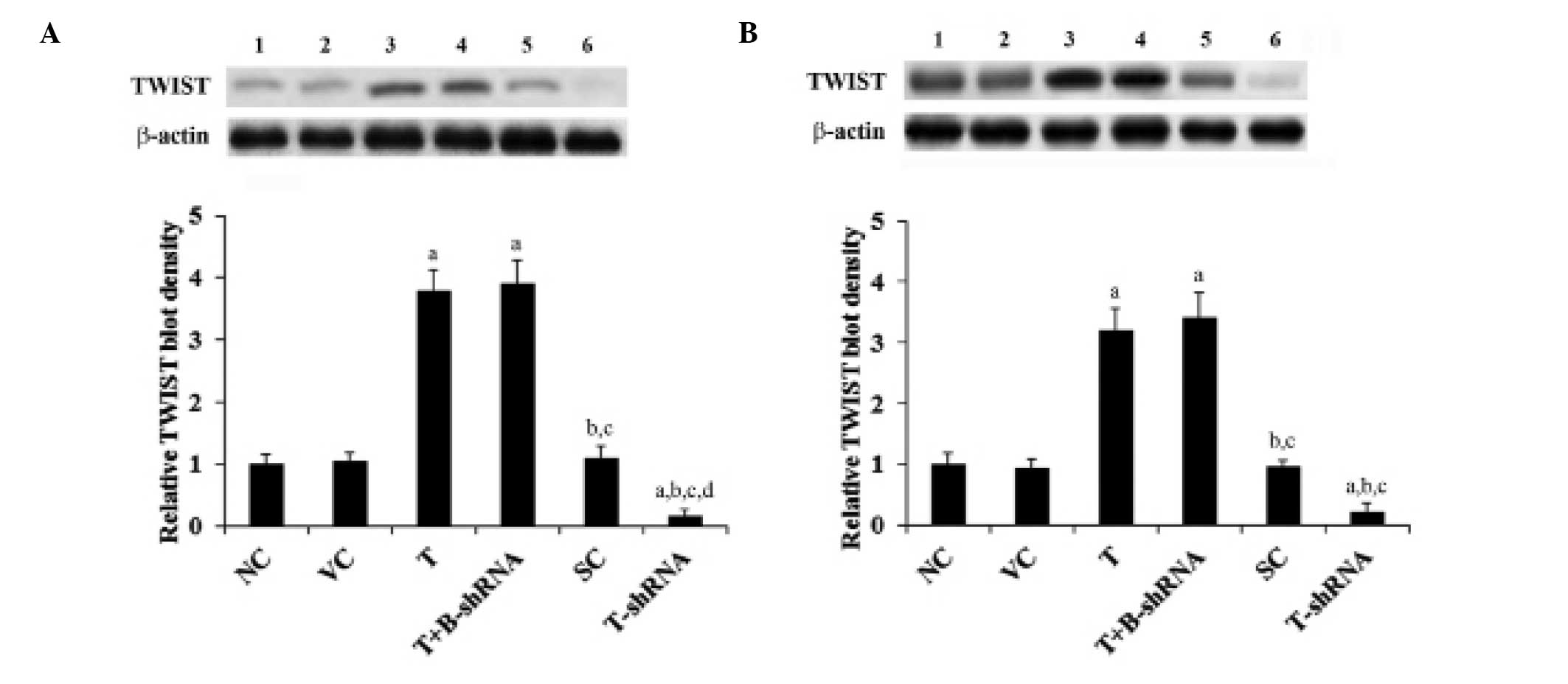 | Figure 1TWIST expression in OTSCC cells with
overexpression and knockdown of TWIST. (A) SCC-4 and (B) TCA8113
human OTSCC cells. Lane 1, NC; lane 2, VC; lane 3, T; lane 4,
T+B-shRNA; lane 5, SC; lane 6, T-shRNA. Expression was analyzed
with western blot analysis. β-actin was used as a loading control.
The density of the TWIST blots were normalized against that of
β-actin blots to obtain a relative blot density. This was expressed
as a fold change of the relative TWIST blot density compared with
the NC group (designated as 1). aP<0.05, compared
with NC or VC, bP<0.05, compared with T,
cP<0.05, compared with T+B-shRNA and
dP<0.05, compared with SC. OTSCC, oral tongue
squamous cell carcinoma; NC, normal control; VC, cells transfected
with empty pcDNA3 vector; T, cells transfected with pcDNA3-TWIST
expression vector; T+B-shRNA, cells transfected with pcDNA-TWIST
expression vector and β-catenin short hairpin RNA; SC, cells
transfected with scrambled control shRNA; T-shRNA, cells
transfected with TWIST shRNA; TWIST, twist-related protein 1. |
SCC-4 and TCA8113 cells overexpressing
TWIST show an increase in β-catenin transcriptional activity
As shown in Fig. 2,
the transcriptional activity of β-catenin signaling in SCC-4 and
TCA8113 cells was measured with TOPflash, a synthetic
β-catenin/Tcf-dependent luciferase reporter (11). Compared with controls, the
luciferase activity of TOPflash was increased seven-fold in SCC-4
and TCA8113 cells overexpressing TWIST. This effect was eliminated
in cells stably transduced with β-catenin-shRNA. By contrast,
knockdown of TWIST decreased the luciferase activity of TOPflash by
~70% (Fig. 2). However, little
change was observed with FOPflash, a negative control reporter with
a mutation in the Tcf binding elements (11) (Fig.
2). These results suggest that TWIST may regulate β-catenin
signaling in OTSCC cells.
 | Figure 2Effect of TWIST on β-catenin
luciferase reporter activity in OTSCC cells. (A) SCC-4 and (B)
TCA8113 human OTSCC cells were transfected with TOPflash, a
synthetic β-catenin luciferase reporter, or FOPflash, a negative
control reporter for TOPflash. After 24 h, the luciferase activity
in each group was analyzed. The luciferase activity was expressed
as a fold change relative to that of the NC group (designated as
1). aP<0.05, compared with NC or VC,
bP<0.05, compared with T, cP<0.05,
compared with B-shRNA, dP<0.05, compared with
T+B-shRNA and eP<0.05, compared with SC. OTSCC, oral
tongue squamous cell carcinoma; NC, normal control; VC, cells
transfected with empty pcDNA3 vector; T, cells transfected with
pcDNA3-TWIST expression vector; T+B-shRNA, cells transfected with
pcDNAs-TWIST expression vector and β-catenin short hairpin RNA; SC,
cells transfected with scrambled control shRNA; T-shRNA, cells
transfected with TWIST shRNA; TWIST, twist-related protein 1. |
TWIST increases mRNA levels of target
genes of β-catenin signaling
As shown in Fig. 3,
RT-qPCR demonstrated that overexpression or knockdown of TWIST had
no significant effect on β-catenin mRNA levels in SCC-4 and TCA8113
cells. However, the mRNA levels of target genes (c-Myc and c-Jun)
of β-catenin signaling were increased 4.5-fold in cells
overexpressing TWIST. Again, this effect was abrogated by
transduction of β-catenin-shRNA and by the phosphatidylinositol
3-kinase (PI3K) inhibitor, LY294002. Knockdown of TWIST decreased
the mRNA levels of c-Myc and c-Jun by ~60%, compared with the
control (Fig. 3).
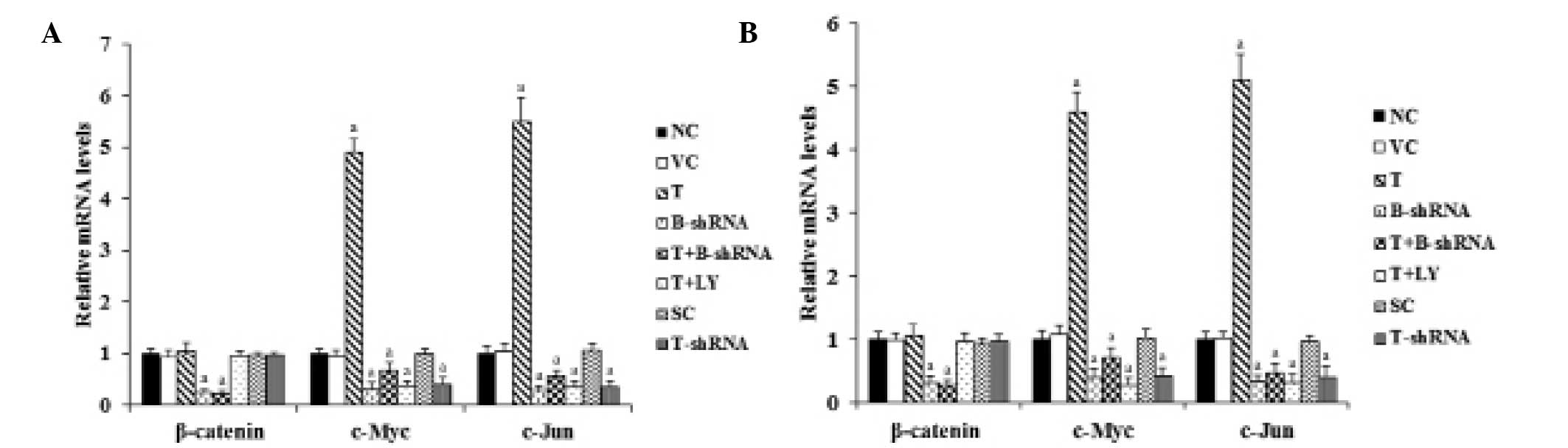 | Figure 3Effect of TWIST on mRNA levels of
β-catenin, C-Myc and C-Jun in OTSCC cells. (A) SCC-4 and (B)
TCA8113 human OTSCC. Reverse transcription-qunatitative polymerase
chain reaction was performed in each group. The mRNA levels were
expressed as a fold change relative to that of the NC group
(designated as 1). aP<0.05, compared with NC or VC.
OTSCC, oral tongue squamous cell carcinoma; NC, normal control; VC,
cells transfected with empty pcDNA3 vector; T, cells transfected
with pcDNA3-TWIST expression vector; T+B-shRNA, cells transfected
with pcDNAs-TWIST expression vector and β-catenin short hairpin
RNA; T+Ly, cells transfected with pcDNAs-WIST expression vector and
treated with 50 μm LY294002; SC, cells transfected with scrambled
control shRNA; T-shRNA, cells transfected with TWIST shRNA; TWIST,
twist-related protein 1. |
TWIST increases levels of soluble, but
not of total, β-catenin protein levels
As shown in Fig. 4,
total β-catenin protein levels in SCC-4 and TCA8113 cells were not
altered by overexpression or knockdown of TWIST. By contrast,
overexpression of TWIST increased the levels of soluble β-catenin
by 5.5-fold. This effect was eliminated by transduction of
β-catenin-shRNA and by administration of LY294002. Knockdown of
TWIST decreased the soluble β-catenin level by over 60% (Fig. 4).
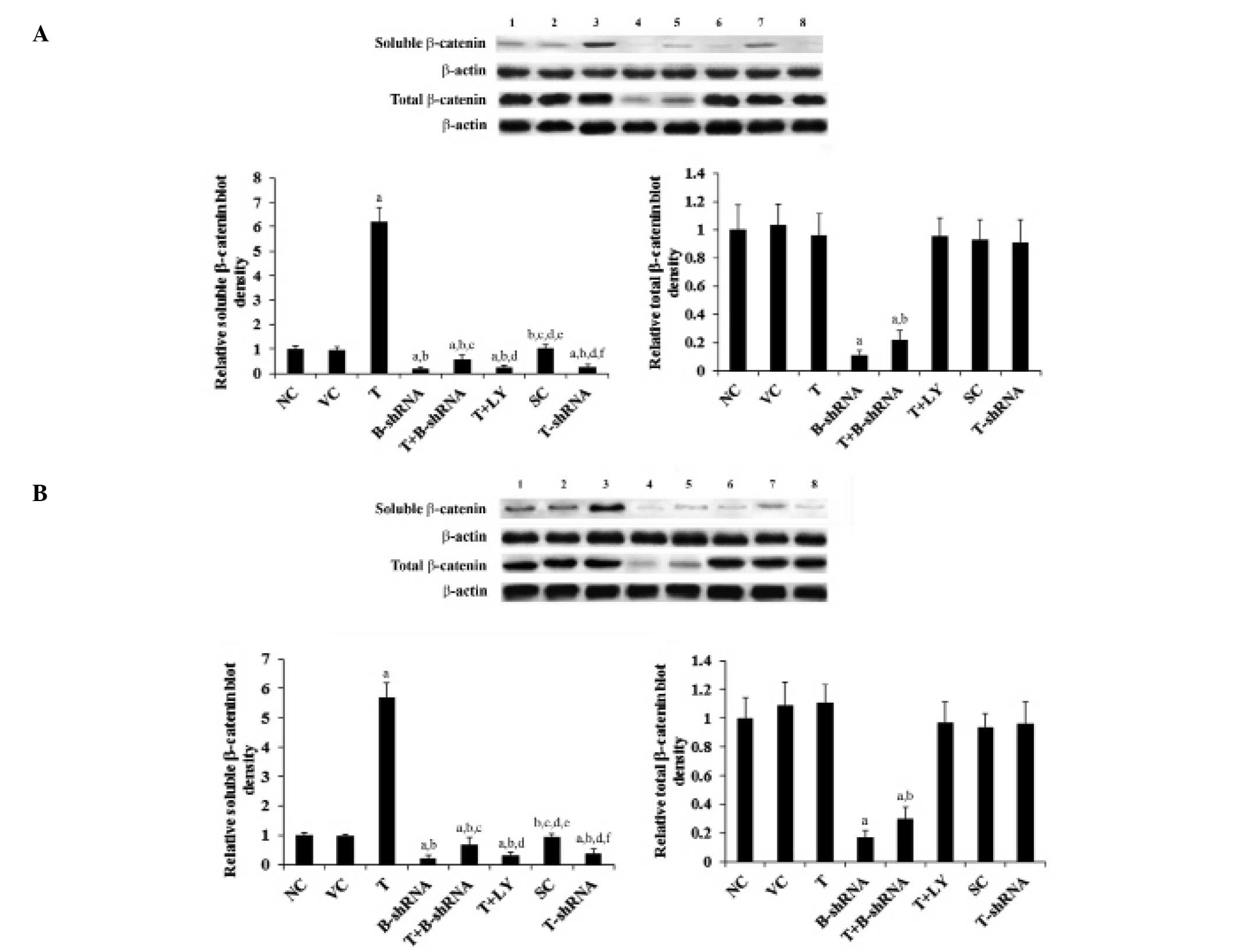 | Figure 4Effect of TWIST on levels of β-catenin
protein in OTSCC cells. (A) SCC-4 and (B) TCA8113 human OTSCC
cells. Lane 1, NC; lane 2, VC; lane 3, T; lane 4, B-shRNA; lane 5,
T+B-shRNA; lane 6, T+LY; lane 7, SC; lane 8, T-shRNA. The soluble
and total β-catenin protein levels were analyzed with western blot
analysis. β-actin was used as a loading control. The density of the
β-catenin blot was normalized against that of β-actin to obtain a
relative blot density, which was expressed as a fold change to the
relative β-catenin blot density in the NC group (designated as 1).
aP<0.05, compared with NC or VC,
bP<0.05, compared with T, cP<0.05,
compared with B-shRNA, dP<0.05, compared with
T+B-shRNA, eP<0.05, compared with T+LY and
fP<0.05, compared with SC. OTSCC, oral tongue
squamous cell carcinoma; NC, normal control; VC, cells transfected
with empty pcDNA3 vector; T, cells transfected with pcDNA3-TWIST
expression vector; T+B-shRNA, cells transfected with pcDNAs-TWIST
expression vector and β-catenin short hairpin RNA; T+Ly, cells
transfected with pcDNAs-WIST expression vector and treated with 50
μm LY294002; SC, cells transfected with scrambled control shRNA;
T-shRNA, cells transfected with TWIST shRNA; TWIST, twist-related
protein 1. |
TWIST increases levels of soluble
β-catenin by increasing the phosphorylation of GSK-3β
GSK-3β is a major downstream target of the PI3K/Akt
pathway. It is inactivated by phosphorylation at serine 9 by
PI3K/Akt. This results in the stabilization and accumulation of
soluble β-catenin (16). As shown
in Fig. 5, the total GSK-3β
protein level in SCC-4 and TCA8113 cells was not altered by
overexpression or knockdown of TWIST. Overexpression of TWIST
increased phosphorylation of GSK-3β at serine 9 by 3.5-fold. This
effect was eradicated by administration of LY294002, although not
by transduction of β-catenin-shRNA. Knockdown of TWIST decreased
serine 9 phosphorylation by ~70% (Fig.
5). These results indicate that TWIST is able to regulate
levels of soluble β-catenin via induction of phosphorylation of
GSK-3β by PI3K/Akt in OTSCC cells.
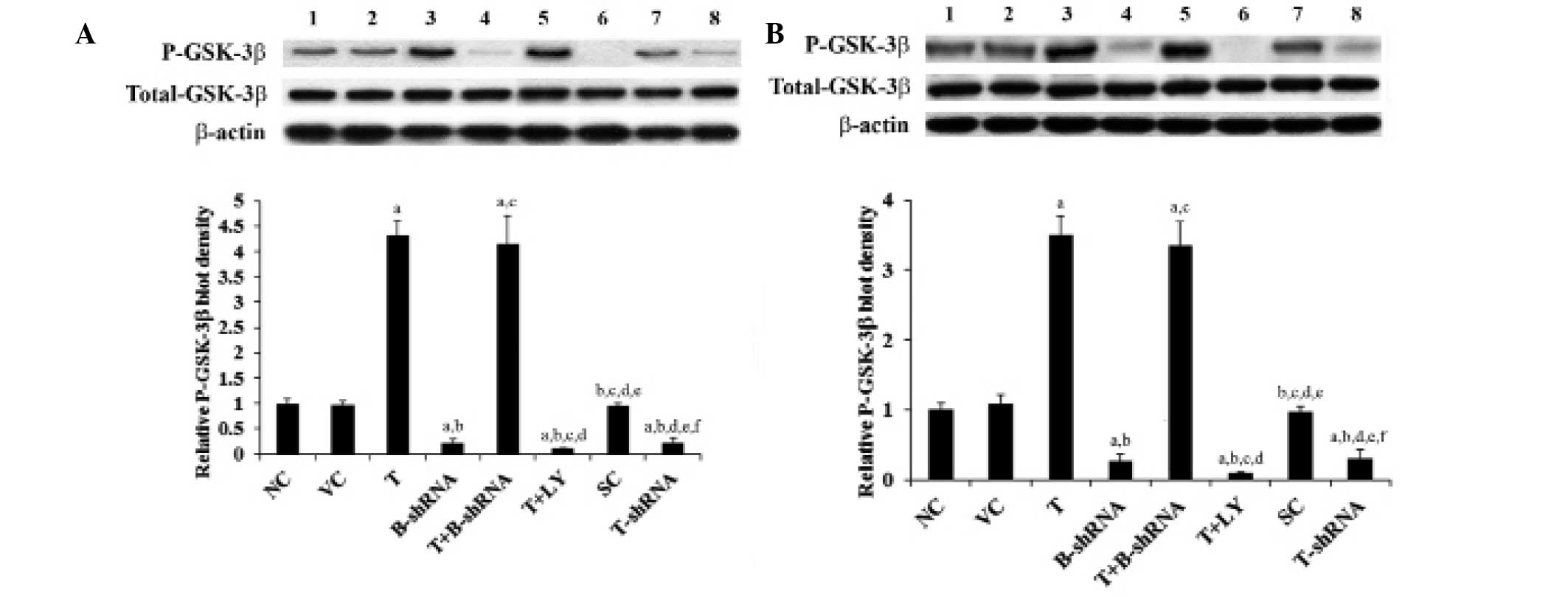 | Figure 5Effect of TWIST on phosphorylated
GSK-3β levels in OTSCC cells. (A) SCC-4 and (B) TCA8113 human OTSCC
cells. Lane 1, NC; lane 2, VC; lane 3, T; lane 4, B-shRNA; lane 5,
T+B-shRNA; lane 6, T+LY; lane 7, SC; lane 8, T-shRNA.
Phosphorylation of GSK-3β at serine 9 in each group was analyzed
with western blotting. β-actin was used as a loading control. The
density of the P-GSK-3β blot was normalized against that of total
GSK-3β and β-actin to obtain a relative blot density, which was
expressed as a fold change of the relative P-GSK-3β blot density in
the NC group (designated as 1). aP<0.05, compared
with NC or VC, bP<0.05, compared with T,
cP<0.05, compared with B-shRNA,
dP<0.05, compared with T+B-shRNA,
eP<0.05, compared with T+LY and
fP<0.05, compared with SC. P-GSK-3β, phosphorylated
glycogen synthase-3β; OTSCC, oral tongue squamous cell carcinoma;
NC, normal control; VC, cells transfected with empty pcDNA3 vector;
T, cells transfected with pcDNA3-TWIST expression vector;
T+B-shRNA, cells transfected with pcDNAs-TWIST expression vector
and β-catenin short hairpin RNA; T+Ly, cells transfected with
pcDNAs-WIST expression vector and treated with 50 μm LY294002; SC,
cells transfected with scrambled control shRNA; T-shRNA, cells
transfected with TWIST shRNA; TWIST, twist-related protein 1. |
TWIST overexpression increases OTSCC cell
invasion and the expression of MMP-2
TWIST and β-catenin are important for OTSCC cell
invasion (6,12,13).
MMPs are also known to be involved in cancer cell invasion
(17,18). The effect of TWIST and β-catenin on
OTSCC cell invasion and MMP expression was investigated. As shown
in Fig. 6, overexpression of TWIST
markedly increased SCC-4 and TCA8113 cell invasiveness. This effect
was abrogated by transduction with β-catenin-shRNA and by
administration of LY294002. By contrast, knockdown of TWIST
markedly decreased cell invasion (Fig.
6). In accordance with these findings, overexpression of TWIST
led to an increase in MMP-2 expression, whilst knockdown of TWIST
led to a reduction in MMP-2 expression compared with controls
(Fig. 7).
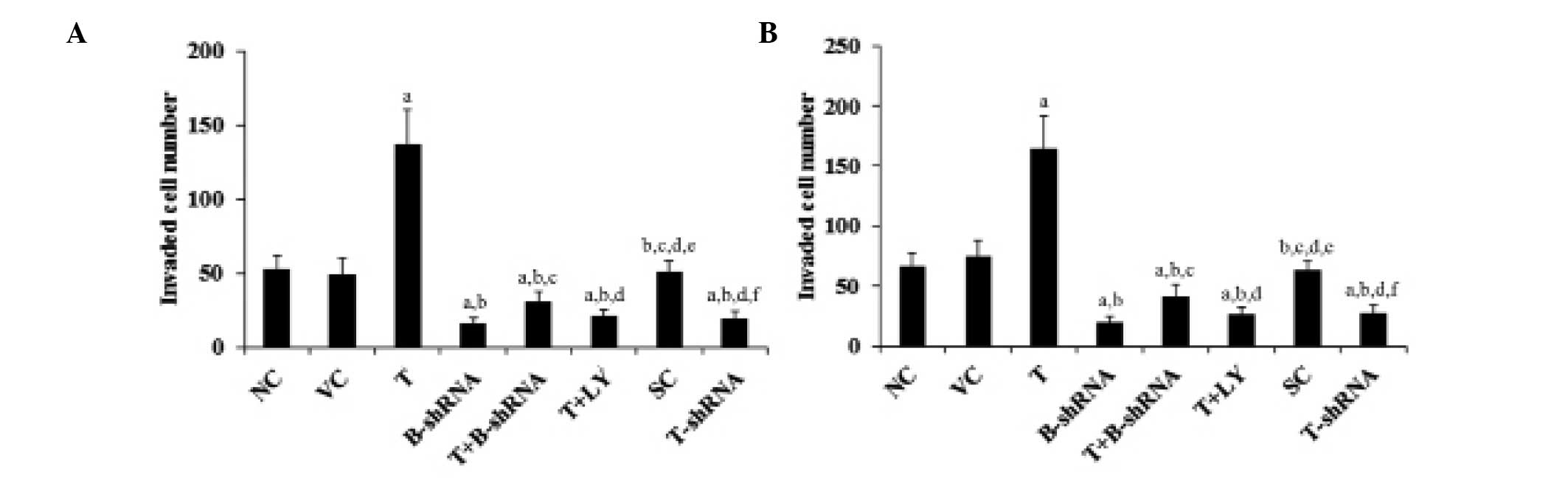 | Figure 6Effect of TWIST on OTSCC cells. (A)
SCC-4 and (B) TCA8113 human OTSCC cells. Transwell invasion assays
were performed in each group and the number of cells that had
invaded were counted. aP<0.05, cpmpared with NC or
VC, bP<0.05, compared with T, cP<0.05,
compared with B-shRNA, dP<0.05, compared with
T+B-shRNA, eP<0.05, compared with T+LY and
fP<0.05, compared with SC. OTSCC, oral tongue
squamous cell carcinoma; NC, normal control; VC, cells transfected
with empty pcDNA3 vector; T, cells transfected with pcDNA3-TWIST
expression vector; T+B-shRNA, cells transfected with pcDNAs-TWIST
expression vector and β-catenin short hairpin RNA; T+Ly, cells
transfected with pcDNAs-WIST expression vector and treated with 50
μm LY294002; SC, cells transfected with scrambled control shRNA;
T-shRNA, cells transfected with TWIST shRNA; TWIST, twist-related
protein 1. |
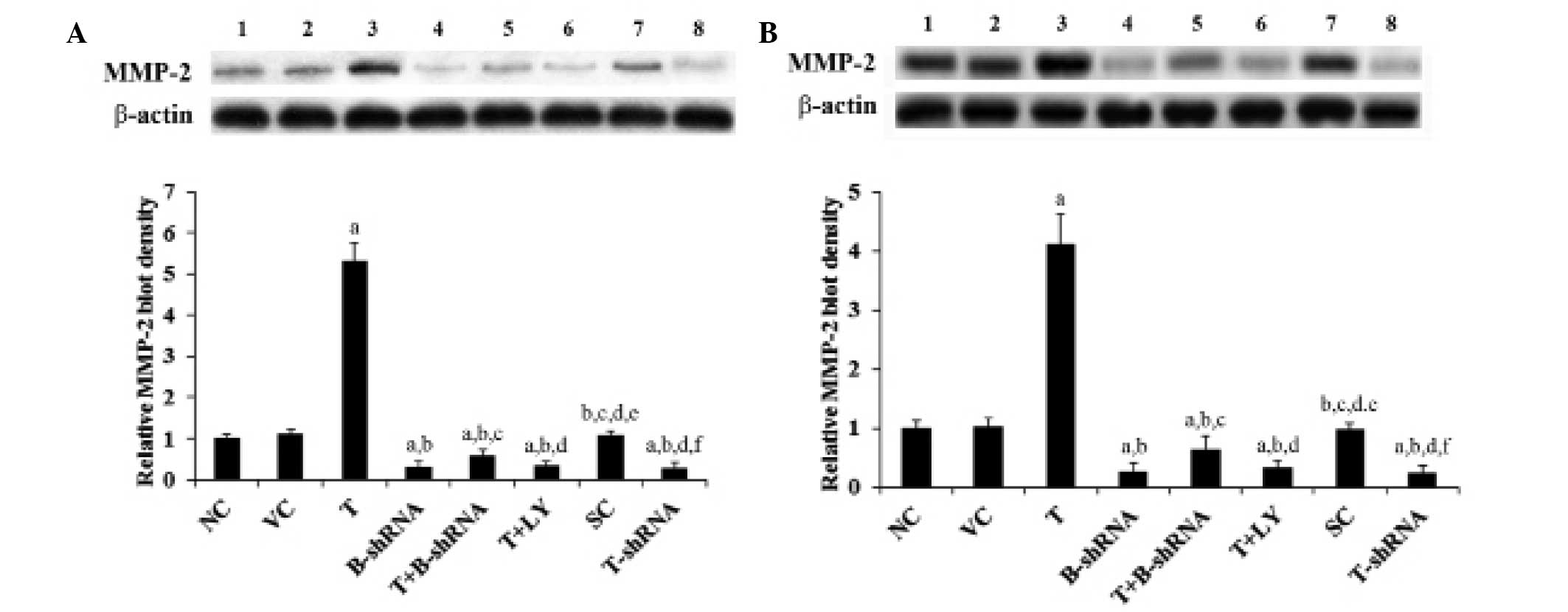 | Figure 7Effect of TWIST on expression of MMP-2
in OTSCC cells. (A) SCC-4 and (B) TCA8113 human OTSCC cells. Lane
1, NC; lane 2, VC; lane 3, T; lane 4, B-shRNA; lane 5, T+B-shRNA;
lane 6, T+LY; lane 7, SC; lane 8, T-shRNA. The expression of MMP-2
in each group was measured using western blot analysis. β-actin was
used as a loading control. The density of the MMP-2 blot was
normalized against that of β-actin to obtain a relative blot
density, which was expressed as a fold change of the relative MMP-2
blot density in the NC group (designated as 1).
aP<0.05, compared with NC or VC,
bP<0.05, compared with T, cP<0.05,
compared with B-shRNA, dP<0.05, compared with
T+B-shRNA, eP<0.05, compared with T+LY and
fP<0.05, compared with SC. MMP-2, matrix
metalloproteinase-2, OTSCC, oral tongue squamous cell carcinoma;
NC, normal control; VC, cells transfected with empty pcDNA3 vector;
T, cells transfected with pcDNA3-TWIST expression vector;
T+B-shRNA, cells transfected with pcDNAs-TWIST expression vector
and β-catenin short hairpin RNA; T+Ly, cells transfected with
pcDNAs-WIST expression vector and treated with 50 μm LY294002; SC,
cells transfected with scrambled control shRNA; T-shRNA, cells
transfected with TWIST shRNA; TWIST, twist-related protein 1. |
Discussion
Accumulating in vitro evidence suggests that
β-catenin signaling is important in OTSCC cell invasion (12,13).
Abnormal activation of TWIST has been implicated in several types
of human cancer (19). A recent
study has reported that overexpression of TWIST is associated with
a poor prognosis in patients with OTSCC, and may enhance OTSCC cell
invasion (6). To the best of our
knowledge, this study provides the first evidence that TWIST
enhances OTSCC cell invasion through regulation of β-catenin
signaling. The data were highly consistent in the two OTSCC cell
lines.
In this study, overexpression and knockdown of TWIST
in OTSCC cells increased and decreased the levels of soluble
β-catenin, respectively. β-catenin interacts with Tcf transcription
factors to activate a number of downstream target genes, including
c-Myc and c-Jun (8,10,11).
Overexpression and knockdown of TWIST increased and decreased,
respectively, the TOPflash β-catenin signaling reporter activity,
as well as the mRNA levels of c-Myc and c-Jun. Notably, TWIST did
not alter the total levels of the β-catenin protein, indicating
that TWIST may regulate the level of soluble β-catenin via a
post-transcriptional mechanism. This is in accordance with the
observed increase in serine-9 phosphorylation of GSK-3β, which
ultimately results in stabilization and accumulation of soluble
β-catenin (16). GSK-3β is a major
downstream target of the PI3K/Akt pathway (16). Since the PI3K inhibitor, LY294002,
eliminated the increase in serine 9 phosphorylation of GSK-3β and
the increase in soluble β-catenin induced by overexpression of
TWIST, it is likely that TWIST regulates the soluble β-catenin
level in OTSCC cells through the PI3K/Akt/GSK-3β pathway.
Overexpression of TWIST markedly enhanced cell
invasion and MMP-2 expression in OTSCC cells. This result was
corroborated by a significant reduction in cell invasion and MMP-2
expression in OTSCC cells with the knockdown of TWIST. The
enhancing effect of TWIST on OTSCC cell invasion and MMP-2
expression was almost completely eradicated by knocking down
β-catenin with shRNA, suggesting that β-catenin signaling is an
essential mediator of the effect of TWIST on OSTCC cell
invasion.
Abnormal activation of β-catenin signaling is
critical in the progression of a variety of cancers, including
OTSCC (11–13). Yin et al (15) showed that TWIST negatively
regulated β-catenin signaling via a PI3K-dependent mechanism in
osteosarcoma cells. Their findings are in accordance with the fact
that in a homogeneous cohort of osteosarcoma patients, the TWIST
gene is frequently found to be deleted in the tumors at diagnosis,
and haploinsufficiency of this gene is significantly correlated
with a poorer patient outcome (15). The present study, however, found
that TWIST was a positive regulator of β-catenin signaling by a
PI3K-dependent mechanism. This finding is in agreement with a
recent study demonstrating that overexpression of TWIST is
associated with a poor prognosis in patients with OTSCC (6). This discrepancy suggests that the
regulatory effect of TWIST on β-catenin signaling may be dependent
on the type of tissue or cancer involved.
MMPs are critical for cancer cell invasion (17,18).
Recent studies have suggested that MMP-2 is important for OTSCC
lymph node metastasis in vivo and OTSCC cell invasion in
vitro (6,20). This study found that TWIST markedly
increased MMP-2 expression through β-catenin signaling, suggesting
that the TWIST/β-catenin signaling axis is important for OTSCC
progression. In addition, as TWIST and β-catenin signaling are
abnormally activated in a variety of cancers, the TWIST/β-catenin
signaling axis may be important in cancers other than OTSCC. This
hypothesis requires further investigation in future studies.
In conclusion, the current study demonstrated that
TWIST enhances cell invasion and MMP-2 expression in OTSCC cells
through its effects on β-catenin signaling, which are likely to be
mediated via a PI3K-dependent mechanism. This study provides novel
insights into the molecular mechanisms underlying OTSCC
progression.
References
|
1
|
Choi KK, Kim MJ, Yun PY, Lee JH, Moon HS,
Lee TR and Myoung H: Independent prognostic factors of 861 cases of
oral squamous cell carcinoma in Korean adults. Oral Oncol.
42:208–217. 2006. View Article : Google Scholar
|
|
2
|
Xing Y, Qi J, Deng S, Wang C, Zhang L and
Chen J: Small interfering RNA targeting ILK inhibits metastasis in
human tongue cancer cells through repression of
epithelial-to-mesenchymal transition. Exp Cell Res. 319:2058–2072.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Su HH, Chu ST, Hou YY, Chang KP and Chen
CJ: Spindle cell carcinoma of the oral cavity and oropharynx:
factors affecting outcome. J Chin Med Assoc. 69:478–483. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Entz-Werlé N, Lavaux T, Metzger N, et al:
Involvement of MET/TWIST/APC combination or the potential role of
ossification factors in pediatric high-grade osteosarcoma
oncogenesis. Neoplasia. 9:678–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Entz-Werlé N, Choquet P, Neuville A, et
al: Targeted apc;twist double-mutant mice: a new model of
spontaneous osteosarcoma that mimics the human disease. Transl
Oncol. 3:344–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
da Silva SD, Alaoui-Jamali MA, Soares FA,
et al: TWIST1 is a molecular marker for a poor prognosis in oral
cancer and represents a potential therapeutic target. Cancer.
120:352–362. 2014. View Article : Google Scholar
|
|
7
|
Chesire DR and Isaacs WB: Beta-catenin
signaling in prostate cancer: an early perspective. Endocr Relat
Cancer. 10:537–560. 2003. View Article : Google Scholar
|
|
8
|
Cawthorn WP, Heyd F, Hegyi K and Sethi JK:
Tumour necrosis factor-alpha inhibits adipogenesis via a
beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ.
14:1361–1373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nusse R: WNT targets. Repression and
activation. Trends Genet. 15:1–3. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gan XQ, Wang JY, Xi Y, Wu ZL, Li YP and Li
L: Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading
to stabilization of beta-catenin-TCF interaction. J Cell Biol.
180:1087–1100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun P, Xiong H, Kim TH, Ren B and Zhang Z:
Positive inter-regulation between beta-catenin/T cell factor-4
signaling and endothelin-1 signaling potentiates proliferation and
survival of prostate cancer cells. Mol Pharmacol. 69:520–531. 2006.
View Article : Google Scholar
|
|
12
|
Wang LP, Chen SW, Zhuang SM, Li H and Song
M: Galectin-3 accelerates the progression of oral tongue squamous
cell carcinoma via a Wnt/β-catenin-dependent pathway. Pathol Oncol
Res. 19:461–474. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawakita A, Yanamoto S, Yamada SI, Naruse
T, Takahashi H, Kawasaki G and Umeda M: MicroRNA-21 promotes oral
cancer invasion via the Wnt/β-catenin pathway by targeting DKK2.
Pathol Oncol Res. Sep 3–2013.(Epub ahead of print).
|
|
14
|
Matsuo N, Shiraha H, Fujikawa T, et al:
Twist expression promotes migration and invasion in hepatocellular
carcinoma. BMC Cancer. 9:2402009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu J, Liao Q, He H, Zhong D and Yin K:
TWIST interacts with β-catenin signaling on osteosarcoma cell
survival against cisplatin. Mol Carcinog. 53:440–446. 2014.
View Article : Google Scholar
|
|
16
|
Sharma M, Chuang WW and Sun Z:
Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway
through GSK3beta inhibition and nuclear beta-catenin accumulation.
J Biol Chem. 277:30935–30941. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan HX, Li HX, Chen D, Gao ZX and Zheng
JH: Changes in the expression of MMP2, MMP9, and ColIV in stromal
cells in oral squamous tongue cell carcinoma: relationships and
prognostic implications. J Exp Clin Cancer Res. 31:902012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Song H, Luo J, Liang J, Zhao S and
Su R: Knockdown of glucose-regulated protein 78 decreases the
invasion, metalloproteinase expression and ECM degradation in
hepatocellular carcinoma cells. J Exp Clin Cancer Res. 31:392012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Entz-Werlé N, Stoetzel C, Berard-Marec P,
et al: Frequent genomic abnormalities at TWIST in human pediatric
osteosarcomas. Int J Cancer. 117:349–355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao W, Jiang M, Li H, Li C, Su R and
Huang K: Knockdown of FAK inhibits the invasion and metastasis of
Tca-8113 cells in vitro. Mol Med Rep. 8:703–707. 2013.PubMed/NCBI
|





















