Introduction
The interneurons in the dorsal cochlear nucleus
(DCN), cartwheel cells (CWCs), are a group of neurons that have
crucial roles in processing auditory and non-auditory signals in
the brainstem complex (1–3). While other DCN neurons only respond
to external stimuli with a simple discharge pattern of single
action potentials (SAPs), CWCs respond with complex action
potentials (CAPs), consisting of grouped SAPs superimposed on a
slow depolarization (4,5).
As CWCs relay inhibitory signals to fusiform cells
(FCs), the principle neurons in the DCN forming major projections
to higher central auditory pathways, the capacity of CWCs to
exhibit a complex discharge pattern has a profound modulatory
impact on normal or abnormal sound and sensory transmission in the
brain (3,6,7).
However, the exact cellular or molecular mechanisms underlying the
intrinsic properties of the complex discharge pattern in CWCs are
largely unknown.
Nav1.9 was initially identified by a polymerase
chain reaction assay, and was subsequently sequenced and identified
as a tetrodotoxin-resistant (TTX-R), slow-inactivating persistent
sodium channel (8,9). In spinal cord dorsal root ganglion
(DRG) neurons, Nav1.9 channels are highly expressed in nociceptive
units, and contribute to non-inactivating and persistent
depolarization of membrane potentials of the neurons, therefore
regulating signaling transduction between central and peripheral
nociceptive pathways (9–11). In the present study, western
blotting and immunohistochemistry were utilized to examine the
expression of Nav1.9 in dorsal cochlear CWCs. Furthermore,
electrophysiology along with gene silencing were applied to examine
the cellular mechanisms of Nav1.9 in contributing to the intrinsic
action potential firing patterns of CWCs in the central auditory
pathway.
Materials and methods
Brain slices
The present study was approved by the Ethics
Committee of the Department of Otolaryngology, Beijing Military
General Hospital (Beijing, China). C57BL/6 mice (postnatal 12–14
days; The Jackson Laboratory, Bar Harbor, ME, USA) were
anesthetized with subcutaneous injections of a mixture of ketamine
(50 mg/kg) and xylazine (50 mg/kg) administered in the abdominal
area. The mice were decapitated, and the cortex and cerebellum were
removed to expose the brainstem. The brainstem was then quickly
separated and placed in a glass petri dish filled with ice-cold
artificial cerebrospinal fluid (ACSF), containing 130 mM NaCl, 3 mM
KCl, 1.25 mM KH2PO4, 20 mM NaHCO3,
10 mM glucose, 2.5 mM CaCl2 and 1.3 mM MgSO4.
A vibratory microtome (Vibratome; Oxford Instruments Microspec, San
Mateo, CA, USA) was used to cut 200-μm trans-strial slices, which
were maintained in ACSF supplemented with 5% CO2 at
30°C.
Electrophysiology
The in vitro whole-cell current clamp
electrophysiology was conducted on a recording chamber mounted on
an Olympus BX51W1 upright microscope (Olympus Corporation, Tokyo,
Japan) with a 63X, 0.8 N.A. water immersion objective. The
recording was conducted by a Multiclamp 700A amplifier (Axon
Instruments, Foster City, CA, USA), filtered at 5 KHz and digitized
at 50 KHz with a 12-bit A/D digitizer (National Instruments
Corporation, Austin, TX, USA). The brain slices were maintained in
the recording chamber with continuous incubation with ACSF (2–4
ml/min, 95% O2 and 5% CO2 to maintain a pH
level of 7.1–7.5). The recording pipette solution contained 120 mm
potassium gluconate, 20 mm KCl, 2 mm sodium phosphocreatine, 4 mm
MgATP, 10 mm HEPES, 0.3 mm NaGTP and 1.1 mm EGTA, maintained at pH
7.2 with 10 mm KOH. For the pharmacological ion-channel
antagonists, 100 μM CdCl2 was used to block the
voltage-gated Ca2+ channels and 100 nm TTX to block the
fast-inactivating sodium channels.
Western blotting
For western blotting analysis, the brain slices of
cochlea nucleus were further micro-dissected under the microscope
to separate the superficial layer, mainly consisting of CWCs, from
the molecular layer, mainly constituting pyramidal neurons. After
being quickly frozen on dry ice, RIPA buffer, containing 150 mm
NaCl, 50 mm Tris, 1% Triton X-100, 0.1% sodium dodecyl sulfate and
1% Nadeoxycholate (pH 7.4) was used to collect brain tissue. A
Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA) was
then applied to equal the protein concentrations between the two
types of samples. The protein lysates were resolved by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis, followed by
transfer onto nitrocellulose membranes (Amersham Biosciences). The
blocking medium was phosphate-buffered saline (PBS) containing 0.2%
Tween-20 and 5% non-fat dry milk. The lysates were then incubated
with rabbit anti-Nav1.9/NaN primary antibody (1:1,000; Alomone
Laboratories Ltd., Jerusalem, Israel) and goat anti-rabbit
horseradish peroxidase-labeled secondary antibody, as detected by
X-ray film.
Immunohistochemistry
The superficial layer of the cochlear nucleus slices
were dissociated and plated in 6-well plates. After 1 h, the
dissociated cells were fixed with 4% paraformaldehyde in 10 mm
phosphate buffer (PB) at room temperature for 1 h. Next, the cells
were incubated for 1 h in 0.05% Triton X-100 and 10% goat serum in
PBS at room temperature, followed by incubation with primary
antibody (rabbit anti-Nav1.9/NaN; 1:100) at 4°C overnight.
Following three washes with PBS, the cells were incubated with
polyclonal goat-anti-rabbit IgG secondary antibody (Alexa 488;
1:500; Invitrogen Life Technologies, Carlsbad, CA, USA) for 2 h at
room temperature. The bottoms of the plates were then mounted with
glass cover slips to prevent detachment, then imaged with a
confocal microscopy (Olympus Corporation).
Nav1.9 gene silencing
Mouse Nav1.9 (SCN11A) small interfering (si)RNA and
scrambled siRNA (negative control) were purchased from Integrated
DNA Technologies, Inc. (Coralville, IL, USA). Cochlear nucleus
slices were transfected with SCN11A-siRNA (100 nm) or the scrambled
siRNA using GeneSilencer (Genlantis, San Diego, CA, USA) according
to the manufacturer’s instructions. Following transfection, the
slices were incubated in a petri dish containing Dulbecco’s
modified Eagle’s medium, 10% fetal bovine serum, N2 and B27
supplements (Invitrogen Life Technologies) at 37°C in 5%
CO2, overnight prior to electrophysiology.
Statistical analysis
Student’s t test was performed to determine
significance, using SPSS version 11.0 software (SPSS Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
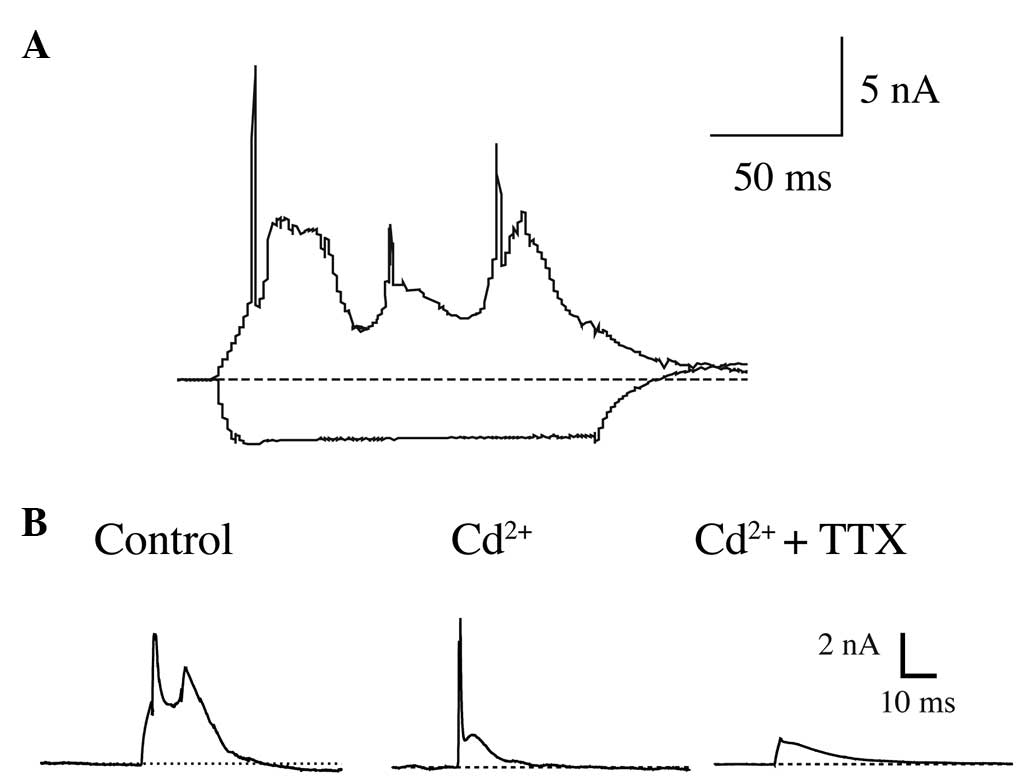
Slow depolarization of CAPs in CWCs is
resistant to Ca2+/Na+ antagonists
In vitro whole-cell current-clamp recordings
were conducted on 122 neurons on the superficial layer of the
cochlear nucleus slides. The cells were initially held with holding
potentials of ~−70 mV and 150 msec of current injections were used
to generate suprathreshold responses from the cells. Among them, 84
of the recorded neurons demonstrated characteristic suprathreshold
responses of CWCs, which were CAPs, consisting of trains of SAPs
superimposed on slow depolarizations (Fig. 1A).
In order to identify the intrinsic sub-types of the
ion channels contributing to CAPs in CWCs, short-stimuli current
clamp recordings (5 msec current injections) were then utilized,
along with pharmacological reagents, to block specific ion
channels. The results demonstrated that under control conditions,
the suprathreshold current injection elicited CAPs consisting of
two SAPs on a slow-rising depolarization (Fig. 1B, left). Notably, while the
voltage-gated calcium channel antagonist Cd2+ (100 μM)
was applied in the bath medium of ACSF, the blockage of
Ca2+ currents did not eliminate the slow depolarization.
Instead it resulted in a decreased slow depolarization with a SAP
superimposed on it (Fig. 1B,
middle). Further application of TTX (100 nm), the antagonist of
fast-inactivating sodium channels, blocked SAPs but did not block
the slow depolarization.
Therefore, the results demonstrated that ionic
currents, other than calcium currents and TTX-sensitive
fast-inactivating sodium currents, contributed to the slow
depolarizations of CAPs in CWCs.
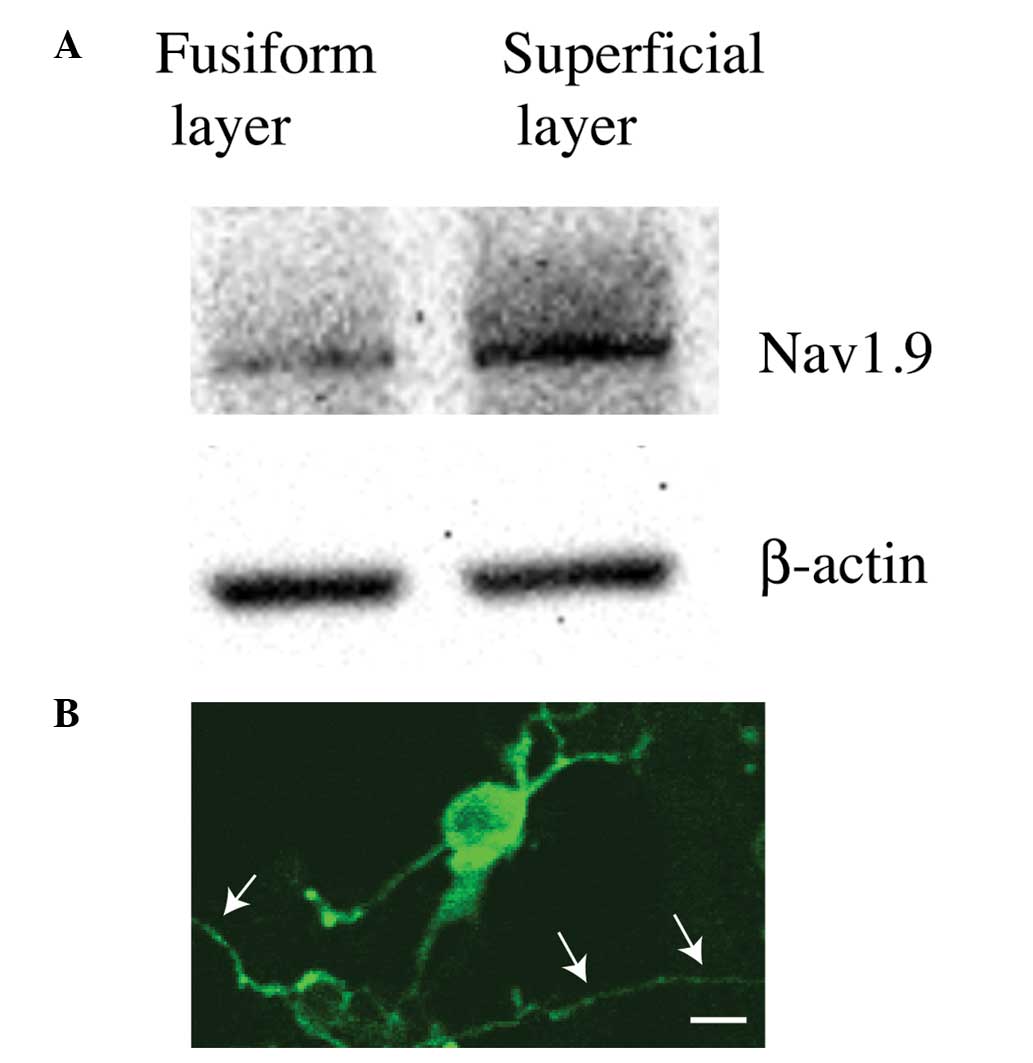
Expression of Nav1.9 in CWCs
Other experimental methods were then utilized to
determine the identity of the ion channels contributing to the
signature firing pattern of CAP in CWCs.
Following micro-array screening of ion channel
expression in the cochlear nucleus, it was noted that Nav1.9, a
TTX-resistant slow-inactivating sodium channel was likely to be
expressed in the dorsal cochlear nucleus. Then, western blot
analysis was used to confirm whether Nav1.9 was expressed in the
dorsal cochlear nucleus or even CWCs. Following micro-dissection,
the superficial layer, which mainly consisted of CWCs from the
dorsal cochlear nucleus, was further separated from the fusiform
layer (which mainly consisted of principal pyramidal neurons) on
the cochlear nucleus brainstem slides. The two types of tissues
then underwent western blot analysis using an antibody against
Nav1.9. The results demonstrated that, as compared with the
fusiform layer, the superficial layer had significantly stronger
reaction to the Nav1.9 antibody (Fig.
2A), suggesting that the TTX-resistant slow-inactivating Nav1.9
Na+ channel was highly likely to be expressed in CWCs,
the major cellular population in the superficial layer of the
dorsal cochlear nucleus.
The western blot analysis result was also confirmed
by immunohistochemistry. The superficial layer was dissociated with
trituration and the dissociated cells were plated on 6-well plates.
After the application of a Nav1.9 primary antibody followed by a
fluorescent secondary antibody, positive staining was observed in a
number of the cells (Fig. 2B).
Based on morphological characterizations, those Nav1.9-positive
cells had cell body sizes of ~20 μM and their dendrites branched at
wide angles or even curved back toward the somas, and were
therefore positively identified as CWCs from the dorsal cochlear
nucleus (Fig. 2B) (12).
Nav1.9 functionally contributes to the
firing of CAPs in CWCs
Finally, the functional role of Nav1.9 in
contributing to the ionic properties in CWCs was examined by a
loss-of-function siRNA genetic silencing assay. Brainstem slices
containing dorsal cochlear nucleus were treated with either Nav1.9
siRNA (SCN11A-siRNA, 100 nm), or a non-specific control siRNA
(scramble-siRNA, 100 nm). Twenty-four hours later, whole-cell
current-clamp recordings were conducted on the neurons in the
superficial layer of the dorsal cochlear nucleus with the addition
of Cd2+ in the bath medium to block voltage-gated
Ca2+ channels.
Subesquent to genetically silencing Nav1.9 in the
cochlear nucleus, it was identified that the majority of the
neurons from superficial layer, presumably CWCs, responded to
suprathreshold current stimuli with SAPs, not CAPs (Fig. 3A). The statistical analysis
demonstrated that 87±4% of the recorded CWCs fired complex spikes
in the brainstem slices treated with non-specific scramble-siRNA,
whereas only 11±6% of the neurons fired complex spikes in the
slices treated with Nav1.9 siRNA (P<0.05; Fig. 3B). Therefore, the present results
strongly suggest that Nav1.9 functionally contributed to the
generation of CAPs in CWCs in the dorsal cochlear nucleus.
Discussion
In the brainstem cochlear nucleus, CWCs are the only
group of neurons that fire CAPs in response to suprathreshold
stimuli. This unique electrophysiological property of CWCs
undoubtedly has profound effects on the signaling processing in
auditory and non-auditory pathways in the brain. Until now, little
has been determined regarding the intrinsic ionic mechanisms
contributing to the generation of CAPs in CWCs. In the present
study, it was demonstrated that a persistent, slow-inactivating and
TTX-resistant Na+ channel subtype, Nav1.9, was present
in the CWCs and was functionally responsible for the firing pattern
of CAPs.
In other neural populations, persistent
Na+ currents are associated with various patterns of
action potentials, including spontaneous action potentials
(13–16), activation of after-depolarization
(17–19) or even subthreshold membrane
potential oscillations (20–22).
In dorsal cochlear CWCs, the neurons do not fire subthreshold
oscillations or spontaneous action potentials, although a low
frequency of spontaneous activity has previously been recorded from
the cochlear nucleus of decerebrate cats under pathological
conditions (23). Therefore, it
appears that a persistent Na+ current in CWCs
functionally contributes to the generation of after-depolarizations
or subthreshold slow depolarizations, as demonstrated by the
evidence that genetic silencing of Nav1.9 eliminated
after-depolarizations, therefore converted CAPs into SAPs (Fig. 3). Notably, other ionic currents,
including TTX-sensitive fast-inactivating Na+ current
and voltage-gated Ca2+ current also contributed to the
generation of CAPs in CWCs, as demonstrated in our recordings with
pharmacological antagonists to those ion channels. Therefore, it is
highly likely that the delicate ionic homeostasis or the balance of
ion concentrations of various cations, including Na+ and
Ca2+or K+, may be decisive in generating
complex or simple firing patterns in CWCs.
In conclusion, to the best of our knowledge, the
present study is the first report the presence and functional role
of persistent Na+ channels in the dorsal cochlear
nucleus. The results undoubtedly further the understanding of the
underlying cellular and molecular mechanisms of the neural circuits
in the auditory central nervous system.
References
|
1
|
Kuo SP and Trussell LO: Spontaneous
spiking and synaptic depression underlie noradrenergic control of
feed-forward inhibition. Neuron. 71:306–318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oertel D and Young ED: What’s a cerebellar
circuit doing in the auditory system? Trends Neurosci. 27:104–110.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaltenbach JA: The dorsal cochlear nucleus
as a participant in the auditory, attentional and emotional
components of tinnitus. Hear Res. 216–217:224–234. 2006. View Article : Google Scholar
|
|
4
|
Manis PB, Spirou GA, Wright DD, Paydar S
and Ryugo DK: Physiology and morphology of complex spiking neurons
in the guinea pig dorsal cochlear nucleus. J Comp Neurol.
348:261–276. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang S and Oertel D: Cartwheel and
superficial stellate cells of the dorsal cochlear nucleus of mice:
intracellular recordings in slices. J Neurophysiol. 69:1384–1397.
1993.PubMed/NCBI
|
|
6
|
Caspary DM, Hughes LF, Schatteman TA and
Turner JG: Age-related changes in the response properties of
cartwheel cells in rat dorsal cochlear nucleus. Hear Res.
216–217:207–215. 2006. View Article : Google Scholar
|
|
7
|
Smith PF: The Endocannabinoid System in
the Cochlear Nucleus and Its Implications for Tinnitus Treatment.
Textbook of Tinnitus. Springer; New York, NY: pp. 639–647. 2011,
View Article : Google Scholar
|
|
8
|
Dib-Hajj SD, Tyrrell L, Black JA and
Waxman SG: NaN, a novel voltage-gated Na channel, is expressed
preferentially in peripheral sensory neurons and down-regulated
after axotomy. Proc Natl Acad Sci USA. 95:8963–8968. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tate S, Benn S, Hick C, et al: Two sodium
channels contribute to the TTX-R sodium current in primary sensory
neurons. Nat Neurosci. 1:653–655. 1998. View Article : Google Scholar
|
|
10
|
Herzog RI, Cummins TR and Waxman SG:
Persistent TTX-resistant Na+ current affects resting
potential and response to depolarization in simulated spinal
sensory neurons. J Neurophysiol. 86:1351–1364. 2001.PubMed/NCBI
|
|
11
|
Amaya F, Decosterd I, Samad TA, et al:
Diversity of expression of the sensory neuron-specific
TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol
Cell Neurosci. 15:331–342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wouterlood FG and Mugnaini E: Cartwheel
neurons of the dorsal cochlear nucleus: a Golgi-electron
microscopic study in rat. J Comp Neurol. 227:136–157. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kononenko NI, Shao LR and Dudek FE:
Riluzole-sensitive slowly inactivating sodium current in rat
suprachiasmatic nucleus neurons. J Neurophysiol. 91:710–718. 2004.
View Article : Google Scholar
|
|
14
|
Shuai J, Bikson M, Hahn PJ, Lian J and
Durand DM: Ionic mechanisms underlying spontaneous CA1 neuronal
firing in Ca2+-free solution. Biophys J. 84:2099–2111.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taddese A and Bean BP: Subthreshold sodium
current from rapidly inactivating sodium channels drives
spontaneous firing of tuberomammillary neurons. Neuron. 33:587–600.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamada-Hanff J and Bean BP: Persistent
sodium current drives conditional pacemaking in CA1 pyramidal
neurons under muscarinic stimulation. J Neurosci. 33:15011–15021.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bouhadfane M, Tazerart S, Moqrich A, Vinay
L and Brocard F: Sodium-mediated plateau potentials in lumbar
motoneurons of neonatal rats. J Neurosci. 33:15626–15641. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yue C, Remy S, Su H, Beck H and Yaari Y:
Proximal persistent Na+ channels drive spike
afterdepolarizations and associated bursting in adult CA1 pyramidal
cells. J Neurosci. 25:9704–9720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
D’Ascenzo M, Podda MV, Fellin T, et al:
Activation of mGluR5 induces spike afterdepolarization and enhanced
excitability in medium spiny neurons of the nucleus accumbens by
modulating persistent Na+ currents. J Physiol.
587:3233–3250. 2009. View Article : Google Scholar
|
|
20
|
Xie RG, Zheng DW, Xing JL, et al: Blockade
of persistent sodium currents contributes to the riluzole-induced
inhibition of spontaneous activity and oscillations in injured DRG
neurons. PloS One. 6:e186812011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ziskind-Conhaim L, Wu L and Wiesner EP:
Persistent sodium current contributes to induced voltage
oscillations in locomotor-related hb9 interneurons in the mouse
spinal cord. J Neurophysiol. 100:2254–2264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong H, Fan YH, Wang YY, Wang WT and Hu
SJ: Lidocaine suppresses subthreshold oscillations by inhibiting
persistent Na(+) current in injured dorsal root ganglion
neurons. Physiol Res. 57:639–645. 2008.
|
|
23
|
Parham K and Kim DO: Spontaneous and
sound-evoked discharge characteristics of complex-spiking neurons
in the dorsal cochlear nucleus of the unanesthetized decerebrate
cat. J Neurophysiol. 73:550–561. 1995.PubMed/NCBI
|