Introduction
Cigarette smoke contains numerous harmful compounds,
including nicotine, heavy metals, free radicals, oxidants, and
reactive oxygen and nitrogen species. Smoking is known to be the
main risk factor for chronic obstructive pulmonary disease (COPD)
(1). Apoptosis of the structural
cells of the lung, is a pathogenetic event in the pathogenesis of
COPD (2). In cases of severe and
prolonged smoking, cigarette smoke is capable of inducing
endoplasmic-reticulum (ER) stress, which causes the unfolded
protein response and apoptosis in pulmonary cells (3–5).
Sirtuin 1 (SIRT1) is a well-known longevity gene,
that regulates senescence, stress resistance, inflammation and DNA
repair by deacetylation of intracellular signaling molecules and
histones (6). Previous studies
have shown that the therapeutic effects of SIRT1 can be achieved
following gene activation by resveratrol (RES) (7,8). The
vasoprotective effects of RES have mainly been observed against
apoptosis induced by insults including hypoxia and cigarette smoke
(CS). Evidence for the anti-apoptotic effects of RES has also come
from retinal and hepatic cells undergoing different insults
including ethanol, palmitate and a specific antibody (9–12).
Furthermore, in the lungs of patients and murine models of COPD,
SIRT1 has been shown to be downregulated (13,14),
indicating the importance of intact, or upregulated SIRT1 function
in the prevention of COPD.
Oxygen-regulated protein 150 (ORP150) is an
important member of the 70kDa heat shock protein family. Knockdown
of ORP150 has previously been shown to promote endoplasmic
reticulum (ER)-stress, induce apoptosis and increase the expression
of CCAAT-enhancer binding protein (CHOP), an ER-stress-induced
apoptosis marker (15,16). Jung et al (17) previously reported that through the
induction of ORP150, SIRT1 alleviated palmitate-induced ER-stress
in HepG2 human hepatocytes. Furthermore, studies have shown that
SIRT1 protects against CS-induced cellular senescence through the
SIRT1-forkhead box O3 (FOXO3) axis (13,18).
SIRT1 has also been demonstrated to be associated with the
regulation of ORP150 gene expression, by FOXO1 (19).
Numerous studies have determined the anti-apoptotic
effects of RES, in particular through its activation of SIRT1,
which may be associated with ORP150. Therefore it was hypothesized
in the present study that the anti-apoptotic effects of RES may be
observed in human bronchial epithelial cells (HBEpC), and that
ORP150 may be associated with the activation of SIRT1 by RES,
resulting in an anti-apoptotic effect.
To evaluate the anti-apoptotic effects of RES on
HBEpC, and to explore the role of ORP150 in the activation of SIRT1
by RES, the well-established cigarette smoke extract (CSE)
apoptosis cell model in HBEpC was used. Techniques including cell
culture, cell counting kit-8 assay, gene knockdown, quantitative
polymerase chain reaction (qPCR), western blotting, and Hoechst
33342 staining and AnnexinV-PI flow cytometry apoptosis analyses
were used throughout the study.
Materials and methods
CSE preparation and cell culture
CSE was prepared using a smoke machine as described
by previous methods (20,21). The direct and side-stream smoke
from a cigarette (Fu Rong Wang brand) was directed via a tube
through 3 ml phosphate-buffered saline, using a peristaltic pump
(Cole-Parmer, Vernon Hills, IL, USA). A spectrometer (Inifinite 200
PRO; Tecan, Männedorf, Switzerland) was used to determine the
optical density of the extract at a wavelength of 450 nm. The 3 ml
solution was determined as 100% CSE. In all of the experiments,
freshly prepared CSE was used.
HBEpC were obtained from Xiangya Central Laboratory
and were cultured in a humidified incubator containing 95% air and
5% CO2, at 37°C, in RPMI-1640 medium (Gibco-BRL,
Carlsbad, CA, USA), supplemented with 10% heat-inactivated fetal
bovine serum (FBS; Hyclone Laboratories, Inc., Logan, UT, USA). The
cells were detached for subculture using 1% trypsin (Beyotime
Institute of Biotechnology, Haimen, China). Once the cells had
reached 80% confluence, they were seeded into six-well plates at a
density of 1×105 cells/well, and grown to 80%
confluence, prior to being used for further experiments.
Preparation of RES solution
RES (purity >99%), was purchased from
Sigma-Aldrich (Shanghai, China). A stock solution of 105 μmol/l was
made by dissolving 22.8 mg RES in 1 ml dimethyl sulfoxide. The
final concentration of RES used in the present study was 20
μmol/l.
Cell Counting kit-8 (CCK-8) assay
To determine a suitable concentration and duration
of the CSE intervention, a CCK-8 assay was used to monitor cellular
viability. The cultured HBEpC, at 80% confluence, were treated with
CSE at various concentrations for 0, 6, 12, 18 and 24 h in 37°C in
an incubator containing 95% air and 5% CO2. The CCK-8
assay reagent (Dojindo Laboratories, Kumamoto, Japan) was added to
the culture media and incubated for 2 h. A micro-plate reader was
used to measure the absorbance at 450 nm. Each assay was performed
in triplicate.
Analyses of apoptosis
To visualize the morphological changes of HBEpC
during apoptosis, a Hoechst 33342 staining kit (Beyotime Institute
of Biotechnology) was used, as described by previous methods
(22). At the end of each
experiment, the cells were stained with Hoechst 33342 (1 mg/ml) for
15 min. The cells were then observed under an IX71 inverted
fluorescence microscope (Olympus, Tokyo, Japan).
To quantitatively analyze the rate of apoptosis of
the cells, Annexin V-propidium iodide (PI) flow cytometry was
performed using an Annexin V-fluorescein isothiocyanate (FITC)
apoptosis detection kit (BD Biosciences, Franklin Lakes, NJ, USA).
Following the treatment of the cells with RES, with or without CSE
for 24 h, ~1×105 cells were collected and resuspended in
500 μl Annexin V binding buffer (1X). Annexin V-FITC (5 μl) and PI
(5 μl) were then added to each sample, followed by an incubation
for 15 min at room temperature (25°C) in the dark. The cells were
then quickly subjected to fluorescence-activated cell sorting
analysis (FACSCalibur; BD Biosciences).
qPCR
Total RNA was isolated from HBEpCs using
TRIzol® (Invitrogen Life Technologies, Beijing, China),
according to the manufacturer’s instructions. cDNA was synthesized
from the RNA using the Superscript II Reverse Transcription system
(Invitrogen Life Technologies). qPCR was performed using a SYBR
Green PCR kit (Takara Biotechnology Co. Ltd., Dalian, China) using
an iCycler (ABI ViiATM7; Applied Biosystems, Carlsbad, CA, USA).
The thermocycler parameters were set as follows: Step one,
activation of the HotStartTaq DNA polymerase (Takara Biotechnology
Co. Ltd.) at 95°C/30 sec; step two, PCR was performed for 40
cycles, denaturation at 95°C/5 sec, annealing at 60°C/34 sec; step
three, fixed parameters set by the ABI ViiA 7 Fast Real-time PCR
system (Applied Biosystems). GAPDH was used as an internal
standard. The primer sequences were designed using the NCBI-Primer
Basic Local Alignment Search Tool (National Institutes of Health,
Bethesda, MD, USA) online tool and synthesized by Sangon Biotech,
Shanghai, Co., Ltd. (Shanghai, China). The oligonucleotides used
were as follows: SIRT1 forward, 5′-ATTCCAGCCATCTCTCTGTCAC-3′, and
reverse, 5′-GTCTTGTATCTGTGCGACCTTG-3′; ORP150 forward,
5′-CAGAGGGAGAGAAGAAGCAGAA-3′, and reverse
5′-CAAGACCTGGACGGACTGAA-3′; GAPDH forward,
5′-AGAAGGCTGGGGCTCATTTG-3′, and reverse,
5′-AGGGGCCATCCACAGTCTTC-3′.
Western blot analysis
Following cell culture, HBEpC were harvested and
RIPA lysis buffer and 100 mM phenylmethanesulfonyl fluroide
(Beyotime Institute of Biotechnology) were used for isolating the
total protein. The lysates were cleared by centrifugation at 12,000
× g for 10 min at 4°C. Equal amounts of protein from each sample
(30 μg) were separated by 8–12% SDS-PAGE and then transferred to
polyvinylidine fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with Tris-buffered saline (TBS; 10
mM Tris, pH 7.5, 100 mM NaCl), containing 5% non-fat dry milk,
followed by an overnight incubation at 4°C with the primary
antibodies. The primary antibodies used were against: SIRT1 (1:400
dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
ORP150 (1:1,000 dilution; Epitomics, Burlingame, CA, USA), caspase
12 (1:1,000 dilution; Abcam, Cambridge, MA, USA), CHOP (1:500
dilution; Cell Signaling Technology, Inc., Danvers, MA, USA),
caspase 4 (1:500 dilution; Protein Technologies, Manchester, UK),
active-caspase 3 (1:1,000 dilution; Abcam). Rabbit anti-GAPDH was
used as a control (1:1,000 dilution; Good HERE Biology, Hangzhou,
China). The following day, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies (1:1,000
dilution; Protein Technologies) in TBS with Tween®. The
blots were visualized using an enhanced chemiluminescence detection
system (Advansta, Menlo Park, CA, USA). Each assay was performed in
triplicate.
Lentivirus conduction and stable
transfection of human bronchial epithelial cells
Based on findings from the present study, an
ORP150-specific small hairpin (sh)RNA was selected from a total of
three candidate small interfering (si)RNAs targeting ORP150 mRNA.
The selected shRNA had the highest ORP150 gene inhibitory effect,
as determined by western blot analysis and qPCR. The sequence of
the ORP150-specific siRNA, and the negative control siRNA were as
follows: ORP150 siRNA, CATGGAAATTGTCTTGAAT; negative control siRNA,
TTCTCCGAACGTGTCACGT. The oligonucleotides were designed according
to the structure of the siRNA, the sequences were as follows:
ORP150 sense, 5′-CCCATGGAAATTGTCTTGAAT-3′ and antisense,
5′-ATTCAAGACAATTTCCATGGG-3′; negative control sense,
5′-TTCTCCGAACGTGTCACGT-3′ and antisense, 5′-ACGTGACACGTTCGGAGAA-3′.
The oligonucleotides containing both the ORP150 and control siRNA
sequences were cloned into the GV118-green fluorescent protein
(GFP) and GV112 vectors, to produce recombinant lentiviral vectors.
The recombinant vectors were then cotransduced into human embryonic
kidney 293T cells (Shanghai Institutes for Biological Sciences,
Shanghai, China) using Lipofectamine® 2000 (Invitrogen
Life Technologies, Carlsbad, CA, USA). The human embryonic kidney
293T cells were maintained in Dulbecco’s modified Eagle’s medium
supplemented with 10% heated FBS, 2 mM glutamine (Gibco-BRL), 100
U/ml penicillin and 100 μg/ml streptomycin (SV30010; Hyclone
Laboratories, Inc.) at 37°C in an atmosphere of 5% CO2.
Cells at 85–90% confluence were passaged by trypsinization. The
supernatants containing the lentiviruses that expressed either the
ORP150-specific shRNA or the control shRNA were harvested 48 h
after transfection. The lentivirus was then purified by
ultracentrifugation; the final titer of recombinant virus was 108
TU/ml.
Cultured normal HBEpC were infected with the ORP150
shRNA lentivirus, with a multiplicity of infection value of 100.
After co-incubation with ORP150 RNAi for 8 hours, the cell culture
media was refreshed. The transfection efficiency was confirmed
after three days, based on fluorescence expression levels.
Statistical analyses
The results are presented as the means ± standard
deviation. SPSS version .17.0 (SPSS, Inc, Chicago, IL, USA) was
used for statistical analyses. A one-way analysis of variance was
used to determine the statistical significance of the measurement
data, and a least significantly different t-test was used for
comparison between two groups. A P≤0.05 was considered to indicate
a statistically significant difference.
Results
Establishment of a CSE-induced apoptosis
cell model in HBEpC
Consistent with previous reports (21,23),
HBEpC underwent apoptosis after being exposed to 2% CSE (volume of
CSE/volume of medium) for 6–24 h, as detected by Hoechst 33342
staining (Fig. 1A). A CCK-8 assay
(Fig. 1B) confirmed that at the
end of the 24 h 2% CSE exposure, the survival rate of HBEpC was
≥50%, which was suitable for showing any significant cell
protective effects using this cell model.
Protective effects of RES against
CSE-induced apoptosis and the expression of SIRT1 and ORP150
genes
In the present study, HBEpC were divided into four
groups: 1) Control, HBEpC at 80% confluence cultured without CSE or
RES; 2) RES, 20 μmol/l RES added to the cell culture media; 3) CSE,
HBEpC underwent 2% CSE exposure, for 24 h; 4) RES+CSE, HBEpC were
pre-cultured with 20 μmol/l RES for 2 h, followed by a 24 h
exposure to 2% CSE. A 24 h 2% CSE exposure resulted in a typical
apoptotic change in the cells, as compared with the cells from the
other groups (Fig. 2A). RES
pre-culture markedly alleviated the severity of apoptosis, which
was confirmed by Hoechst 33342 staining and Annexin V-PI flow
cytometry (Fig. 2B). Following
Hoechst 33342 staining, fewer positive apoptotic cells were
observed in the RES pre-treated cells, and Annexin V-PI showed a
significant decrease in the apoptotic cell rate of the RES
pre-treated cells, as compared with the cells without RES treatment
(13.7 vs. 45.3%; P<0.05).
 | Figure 2The protective effects of resveratrol
(RES) against cigarette smoke extract (CSE)-induced apoptosis and
the activity of Sirtuin 1 (SIRT1) and oxygen-regulated protein 150
(ORP150) genes (A) Hoechst staining of human bronchial epithelial
cells (HBEpC). HBEpC pre-treated with RES, exhibited fewer
apoptotic cells, as compared with the non-RES treated group,
following a 24 h exposure to 2% CSE. Apoptotic cells are strongly
stained bright turquoise (magnification, ×400). (B) Annexin V-PI
flow cytometry confirmed that 24 h 2% CSE exposure caused severe
cell damage, with ~45.3% apoptotic cells. With RES pre-treatment,
the apoptotic cell count significantly decreased, indicating that
RES may attenuate CSE-induced apoptosis in HBEpC. (C) Western blot
analysis showed that RES induced the relative protein expression
levels of Sirtuin 1 (SIRT1) and oxygen-regulated protein 150
(ORP150) in the RES and RES + CSE groups. CSE induced an
upregulation in the protein expression levels of apoptosis markers
caspase 4, CCAAT-enhancer binding protein homologous protein (CHOP)
and caspase 3, and RES attenuated the overexpression of those
markers in the RES+CSE group, as compared with the CSE group. (D)
Quantitative polymerase chain reaction (qPCR) demonstrated a
simultaneous overexpression of SIRT1 and ORP150 at the mRNA level
following RES pre-treatment of HBEpC, indicating that RES may
activate both SIRT1 and ORP150. *P<0.05 vs. control
groups; #P<0.05 vs. CSE group. The data are presented
as the means ± standard deviation. kDa, kilodaltons. |
At the molecular level, the activation of both SIRT1
and ORP150 by RES, and the consequently downregulated expression
levels of the apoptotic markers caspase 3, caspase 4 and CHOP, were
observed in Fig. 2C and D. During
apoptosis of the cells of the CSE group, the relative protein
expression levels of the apoptotic markers were overexpressed, as
detected by western blotting analysis. However, when the cells were
pre-cultured with RES, there was a significant upregulation in the
relative mRNA and protein expression levels of SIRT1 and ORP150
(Fig. 2C and D), accompanied by a
significant downregulation of the expression levels of the
apoptotic markers. These results indicate that RES had protective
effects against CSE-induced apoptosis in HBEpC.
ORP150 gene knockdown attenuates the
protective effects of RES against CSE-induced apoptosis in
HBEpC
To determine whether ORP150 was associated with the
protective effects of SIRT1 against CSE-induced apoptosis in HBEpC,
an ORP150 gene knockdown was performed (Fig. 3). HBEpC were divided into six
groups: 1) Control, HBEpC at 80% confluence cultured without any
treatment for 24 h; 2) ORP150 RNAi+RES, ORP150 shRNA-transduced
HBEpC, pre-cultured with 20 μmol/l RES; 3) RNAi + CSE, negative
control shRNA-transduced HBEpC exposed to 2% CSE for 24 h; 4)
ORP150 RNAi + CSE, ORP150 shRNA-transduced HBEpC exposed to 2% CSE
for 24 h; 5)RNAi + RES + CSE, negative control shRNA-transduced
HBEpC, pre-cultured with 20 μmol/l RES for 2 h, followed by a 24 h
exposure to 2% CSE; 6) ORP150 RNAi + RES + CSE, ORP150
shRNA-transduced HBEpC, pre-cultured with 20 μmol/l RES for 2 h,
followed by a 24 h exposure to 2% CSE.
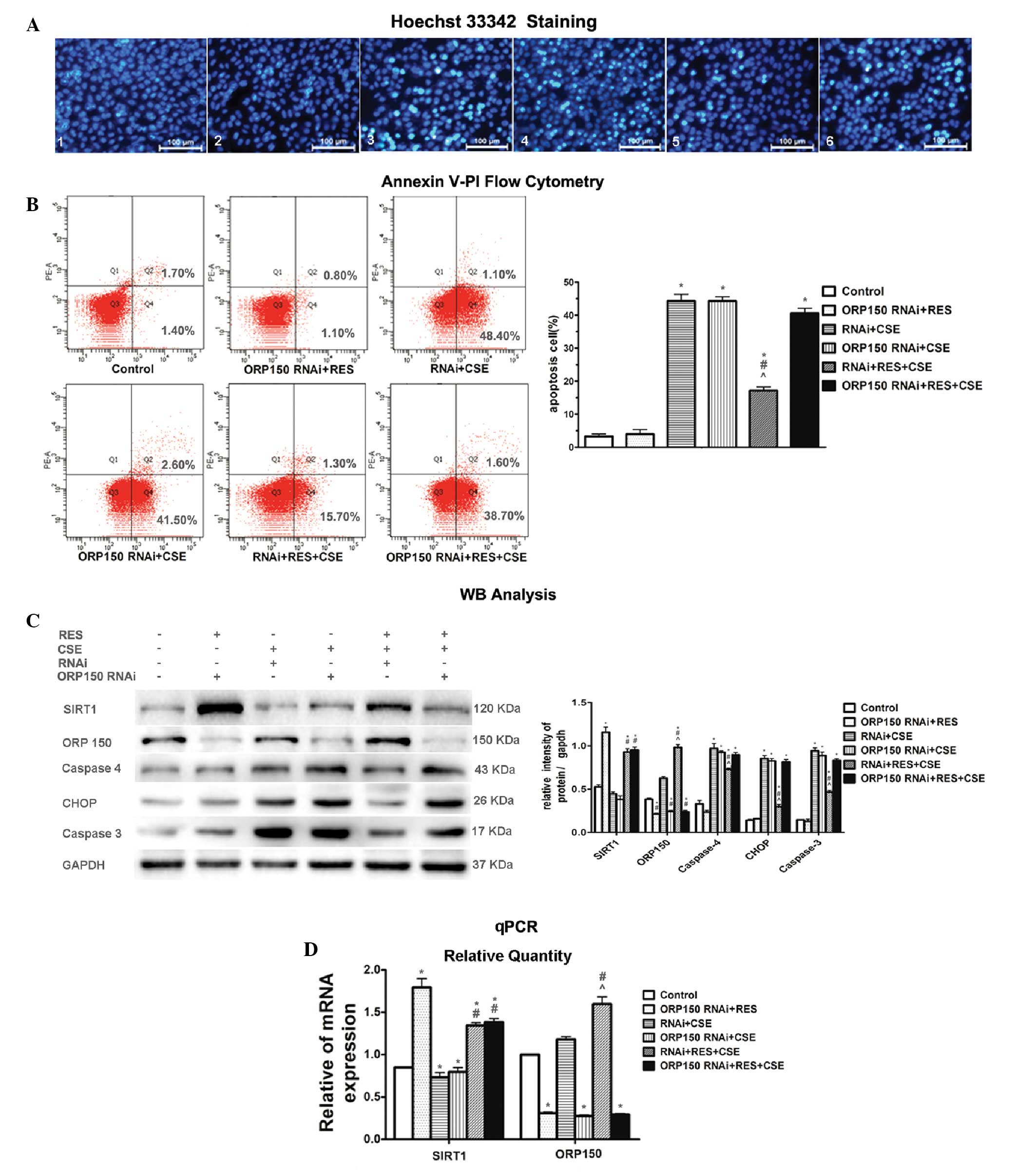 | Figure 3ORP150 gene knockdown attenuated the
protective effects of resveratrol (RES) against cigarette smoke
extract (CSE)-induced apoptosis in human bronchial epithelial cells
(HBEpC) (A1–6) Hoechst 33342 staining of the experimental groups.
(A3) The 24 h 2% CSE exposure lead to significant apoptosis (bright
turquoise stained cells, whereas (A5) RES markedly prevented the
cells from undergoing apoptosis. (A6) The protective effects of RES
were attenuated by the knockdown of ORP150 by RNA interference
(RNAi), as compared with (A5) the cells transfected with control
RNAi (magnification, ×400). (B) The apoptotic cell count detected
by flow cytometric analysis of Annexin V-PI clearly confirmed the
findings obtained from Hoechst 33342 staining. The CSE-induced
apoptosis was markedly attenuated by RES pre-treatment (RNAi + RES
+ CSE); however, this protective effect was abolished by ORP150
gene knockdown (ORP150 RNAi + RES + CSE). (C) Western blot analysis
showed the relative protein expression levels of Sirtuin 1 (SIRT1),
oxygen-regulated protein 150 (ORP150), and the apoptosis marker
molecules in all of the groups. CSE exposure caused upregulation of
the apoptosis marker molecules caspase 4, CCAAT-enhancer binding
protein homologous protein (CHOP) and caspase 3. SIRT1 and ORP150
expression were increased following RES pre-treatment. The
knockdown of ORP150 eliminated the protective effects of RES as
shown in the ORP150 RNAi + RES + CSE group, as compared with the
RNAi + RES + CSE group. (D) Quantitative polymerase chain reaction
(qPCR) showed relative SIRT1 and ORP150 mRNA expression levels.
ORP150 shRNA significantly decreased the expression of ORP150 mRNA
in HBEpCs transfected with OPR150 RNAi, as shown in groups 2, 4 and
6, whereas control shRNA was not effective at all. SIRT1 gene
expression was not affected by the knockdown of ORP150
gene.*P<0.05 vs control group. #P<0.05
vs RNAi + CSE group. ^P<0.05 vs ORP150 RNAi + RES +
CSE. The data are presented as the means ± standard deviation. kDa,
kilodaltons. |
Western blot analysis demonstrated that following
HBEpC exposure to CSE (groups 3, 4, 5 and 6), there was a marked
upregulation of the apoptotic markers CHOP, caspase 4 and caspase
3. Both Hoechst 33342 staining and Annexin V-PI flow cytometry
(Fig. 3A and B) demonstrated
similar changes in the severity of the apoptosis among the
differently treated cell groups. Following exposure to CSE, HBEpC
in groups 3, 4 and 6 suffered obvious damage, with a significant
portion of the cells positively stained by Hoechst 33342 staining
(Fig. 3A), and a high apoptotic
cell rate (Fig. 3B). The
protective effects of RES were still present in the cells that were
transfected with the negative control shRNA, undergoing CSE
exposure (group 5). However, following transfection of HBEpC with
the specific ORP150 shRNA (group 6), the protective effects of RES
were markedly attenuated (Fig.
3C). The cells pre-incubated with control shRNA showed no
effect on ORP150 expression, implying that a successful knockdown
of the targeted ORP150 gene was constructed in HBEpC (Fig. 3D). As compared with group 6, the
cells in group 5 showed marked durability against CSE-induced
apoptosis when the cells were transfected with negative control
siRNA, and downregulation of the apoptotic markers CHOP, caspase 4
and caspase 3 (Fig. 3C), thus
implying that the protective effects of RES on the cells may be
achieved by expression of ORP150. The apoptotic cell rate in group
5 was 17.0%, as compared with 49.5, 44.1 and 40.3% in groups 3, 4
and 6. However, the protective effects of RES were markedly reduced
following the shRNA knockdown of ORP150, as shown in group 6. An
increased number of apoptotic cells were detected, together with a
much higher apoptotic rate and a marked upregulation of the
apoptotic marker molecules (Fig.
3C), as compared with group 5.
These results indicate that ORP150 is necessary for
RES to exert its anti-apoptotic effects in the CSE apoptosis cell
model. However, the mechanisms by which SIRT1 interacts with
ORP150, remains unclear.
Discussion
CS typically causes ER-stress, which may eventually
lead to apoptosis. Our previous study demonstrated that cigarette
smoke may induce GRP78 expression in A549 cells, and the
upregulated GRP78 expression in the cells may have an
anti-apoptotic effect (24). Human
bronchial epithelial cells are the first line of defense against
external pathogens in the respiratory tract. Excessive apoptosis of
the epithelial cells of the airways and defective repair processes
are hallmarks of COPD (25). In
the CS-induced apoptotic HBEpC model used in the present study, the
apoptosis of HBEpC was confirmed to be due to ER-stress, as shown
by the upregulation of the apoptotic markers caspase 4 and CHOP,
which was consistent with the findings of Tagawa et al
(26). SIRT1 exerts
anti-inflammatory and anti-aging effects in the pathogenesis of
COPD. RES, which is a natural activator of SIRT1, is currently
under investigation as an alternative in COPD therapy, which may
focus on the anti-oxidative and anti-ageing effects of RES.
However, whether RES regulates endoplasmic reticulum stress-induced
apoptosis, particularly in the development of COPD remains unclear.
In 2012, Yao et al (27)
revealed that the level of SIRT1 is significantly decreased in the
lungs of COPD patients, as well as the lungs of rodents exposed to
cigarette smoke. In agreement with these findings, in the present
study, using the HBEpC apoptosis model, the levels of SIRT1 were
decreased in the HBEpC following exposure to CSE, as compared with
the normal HBEpC (control group). When HBEpC were pre-cultured with
RES, there was an upregulation of the relative mRNA and protein
expression levels of both SIRT1 and ORP150. The parameters that
measured the occurrence and severity of apoptosis also showed that
following RES pre-culture, the cells had a lower apoptotic cell
rate and fewer apoptotic cells were detected. These findings were
accompanied by a down-regulation of the relative protein expression
levels of apoptosis marker molecules caspase 3, caspase 4, and
CHOP.
The anti-apoptotic effects of RES observed in the
present study are of particular relevance, since ER-stress and
ER-stress-induced apoptosis are the primary triggers for the
development of COPD (15,28,29).
Treatments that alleviate the severity of ER-stress or ER-stress
induced apoptosis would be highly useful in the development of a
new therapy for COPD.
To elucidate the mechanism behind the anti-apoptotic
effects of RES in the CSE-HBEpC model is beyond the realm of the
present study. However, the findings of the present study clearly
indicate that RES is capable of protecting HBEpC against
CSE-induced apoptosis through a pathway involving both SIRT1 and
ORP150. RES was shown to induce upregulation of both SIRT1 and
ORP150 mRNA and protein expression levels in HBEpC, which was
strongly associated with the decrease in apoptosis. Furthermore,
the ORP150 gene knockdown abolished the protective effects of RES
on CSE-induced apoptosis of HBEpC.
In conclusion, the results of the present study
demonstrate that RES has a protective effect against CSE-induced
apoptosis in HBEpC. This anti-apoptotic effect may be exerted
through the activation of a pathway involving SIRT1 and ORP150.
Considering that apoptosis and senescence have a crucial role in
the pathogenesis of COPD, the therapeutic effects of RES shows
significant potential for the prevention and treatment of COPD.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 30971324) and the National
Key Scientific & Technology Support Program: Collaborative
Innovation of Clinical Research for Chronic Obstructive Pulmonary
Disease and Lung Cancer (no. 2013BAI09B09).
References
|
1
|
Pauwels RA and Rabe KF: Burden and
clinical featuRES of chronic obstructive pulmonary disease (COPD).
Lancet. 364:613–620. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Demedts IK, Demoor T, Bracke KR, Joos GF
and Brusselle GG: Role of apoptosis in the pathogenesis of COPD and
pulmonary emphysema. RESpir RES. 7:532006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuwano K: Epithelial cell apoptosis and
lung remodeling. Cell Mol Immuno. 4:419–429. 2007.
|
|
4
|
Kelsen SG, Duan X, Ji R, Perez O, Liu C
and Merali S: Cigarette smoke induces an unfolded protein RESponse
in the human lung: a proteomic approach. Am J RESpir Cell Mol Biol.
38:541–550. 2008. View Article : Google Scholar
|
|
5
|
Kim I, Xu W and Reed JC: Cell death and
endoplasmic reticulum stRESs: disease relevance and therapeutic
opportunities. Nat Rev Drug Discov. 7:1013–1030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahman I, Kinnula VL, Gorbunova V and Yao
H: SIRT1 as a therapeutic target in inflammaging of the pulmonary
disease. Prev Med. 54:S20–S28. 2012. View Article : Google Scholar :
|
|
7
|
Lagouge M, Argmann C, Gerhart-Hines Z, et
al: Resveratrol improves mitochondrial function and protects
against metabolic disease by activating SIRT1 and PGC-1alpha. Cell.
127:1109–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wood JG, Rogina B, Lavu S, et al: Sirtuin
activators mimic caloric REStriction and delay ageing in metazoans.
Nature. 430:686–689. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen CJ, Yu W, Fu YC, Wang X, Li JL and
Wang W: Resveratrol protects cardiomyocytes from hypoxia-induced
apoptosis through the SIRT1-FoxO1 pathway. Biochem Biophys RES
Commun. 378:389–393. 2009. View Article : Google Scholar
|
|
10
|
Csiszar A, Labinskyy N, Podlutsky A, et
al: Vasoprotective effects of Resveratrol and SIRT1: attenuation of
cigarette smoke-induced oxidative stRESs and proinflammatory
phenotypic alterations. Am J Physiol Heart Circ Physiol.
294:H2721–H2735. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anekonda TS and Adamus G: Resveratrol
prevents antibody-induced apoptotic death of retinal cells through
upregulation of Sirt1 and Ku70. BMC RES Notes. 1:1222008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu LQ, Fan ZQ, Tang YF and Ke ZJ: The
Resveratrol attenuates ethanol-induced hepatocyte apoptosis via
inhibiting ER-related caspase-12 activation and PDE activity in
vitro. Alcohol Clin Exp RES. 38:683–693. 2014. View Article : Google Scholar
|
|
13
|
Yao H, Chung S, Hwang JW, et al: SIRT1
protects against emphysema via FOXO3-mediated reduction of
premature senescence in mice. J Clin Invest. 122:2032–2045. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rajendrasozhan S, Yang SR, Kinnula VL and
Rahman I: SIRT1, an antiinflammatory and antiaging protein, is
decreased in lungs of patients with chronic obstructive pulmonary
disease. Am J Crit Care Med. 177:861–870. 2008. View Article : Google Scholar
|
|
15
|
Tagawa Y, Hiramatsu N, Kasai A, et al:
Induction of apoptosis by cigarette smoke via ROS-dependent
endoplasmic reticulum stRESs and CCAAT/enhancer-binding
protein-homologous protein (CHOP). Free Radic Biol Med. 45:50–59.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu YB, Li HQ, Ren MS, Li WT, Lv XY and
Wang L: CHOP/ORP150 ratio in endoplasmic reticulum stress: a new
mechanism for diabetic peripheral neuropathy. Cell Physiol Biochem.
32:367–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung TW, Lee KT, Lee MW and Ka KH: SIRT1
attenuates palmitate-induced endoplasmic reticulum stRESs and
insulin RESistance in HepG2 cells via induction of oxygen-regulated
protein 150. Biochem Biophys RES Commun. 422:229–232. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kusaczuk M and Cechowska-Pasko M:
Molecular chaperone ORP150 in ER stRESs-related diseases. Curr
Pharm Des. 19:2807–2818. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Wu Z, Li D, et al: Involvement of
oxygen-regulated protein 150 in AMP-activated protein
kinase-mediated alleviation of lipid-induced endoplasmic reticulum
stRESs. J Biol Chem. 286:11119–11131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Braber S, Koelink PJ, Henricks PA, et al:
Cigarette smoke-induced lung emphysema in mice is associated with
prolyl endopeptidase, an enzyme involved in collagen breakdown. Am
J Physiol Lung Cell Mol Physiol. 300:L255–L265. 2011. View Article : Google Scholar :
|
|
21
|
Yuan T, Luo BL, Wei TH, Zhang L, He BM and
Niu RC: Salubrinal protects against cigarette smoke extract-induced
HBEpC apoptosis likely via regulating the activity of PERK-eIF2α
signaling pathway. Arch Med RES. 43:522–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiong XL, Jia RH, Yang DP and Ding GH:
Irbesartan attenuates contrast media-induced NRK-52E cells
apoptosis. Pharmacol RES. 54:253–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tagawa Y, Hiramatsu N, Kato H, et al:
Induction of CCAAT/enhancer-binding protein-homologous protein by
cigarette smoke through the superoxide anion-triggered PERK-eIF2α
pathway. Toxicology. 287:105–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He B, Luo B, Chen Q and Zhang L: Cigarette
smoke extract induces the expression of GRP78 in A549 cells via the
p38/MAPK pathway. Mol Med Rep. 8:1683–1688. 2013.PubMed/NCBI
|
|
25
|
Hodge S, Hodge G, Holmes M and Reynolds
PN: Increased airway epithelial and T-cell apoptosis in COPD
remains despite smoking cessation. Eur Respir J. 25:447–454. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tagawa Y, Hiramatsu N, Kasai A, Hayakawa
K, Okamura M, Yao J and Kitamura M: Induction of apoptosis by
cigarette smoke via ROS-dependent endoplasmic reticulum stress and
CCAAT/enhancer-binding protein-homologous protein (CHOP). Free
Radic Biol Med. 45:50–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao H, Chung S, Hwang JW, Rajendrasozhan
S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Rönty M, et
al: SIRT1 protects against emphysema via FOXO3-mediated reduction
of premature senescence in mice. J Clin Invest. 122:2032–2045.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Malhotra D, Thimmulappa R, Vij N, et al:
Heightened endoplasmic reticulum stRESs in the lungs of patients
with chronic obstructive pulmonary disease: the role of
Nrf2-regulated proteasomal activity. Am J RESpir Crit Care Med.
180:1196–1207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanel A, Pallepati P, Bettaieb A, Morin P
and Averill-Bates DA: Acrolein activates cell survival and
apoptotic death RESponses involving the endoplasmic reticulum in
A549 lung cells. Biochim Biophys Acta. 1843.827–835. 2014.
|

















