Introduction
Hepatitis B virus (HBV) infection is one of the
major causes of liver inflammation. Epidemiological data indicate
that ~15–20% of the population in Asia and the Western Pacific are
affected by HBV (1). In
susceptible individuals, primary HBV infection can be either
symptomatic or asymptomatic and the majority of primary infections
are self-limiting, with clearance of the virus and development of
lasting immunity (2). However, an
estimated 3–5% of adults and up to 95% of children develop chronic
HBV infection, which can also be either symptomatic or asymptomatic
(3,4). Therefore, it is not uncommon for
hepatologists to encounter patients carrying an underlying,
unnoticed hepatitis B infection requiring glucocorticoid
treatment.
The reactivation of HBV is a common and
well-recognized complication in patients with chronic HBV infection
receiving cytotoxic or immunosuppressive therapy (5,6). The
risk of HBV reactivation is particularly high in areas where HBV is
endemic, including Asia and the Western Pacific. Glucocorticoids, a
component of the partial therapeutic procedure for chronic
hepatitis B (CHB), are implicated as an important predisposing
factor for HBV reactivation. In addition, long-term glucocorticoid
treatment in patients with CHB increases the levels of hepatitis B
virus surface antigen (HBsAg), hepatitis B virus core antigen and
HBV DNA in hepatocytes (7). These
observations of reactivation of latent infection and increasing
levels of HBV markers during glucocorticoid therapy in CHB patients
suggest that glucocorticoids affect HBV replication and gene
expression in vivo.
The glucocorticoid receptor (GR) is essential for
glucocorticoid action on various effector cells. In humans,
alternative splicing of GR pre-mRNA generates two highly homologous
isoforms, GRα and GRβ (8). GRα is
a ligand-activated transcription factor, which regulates the
expression of glucocorticoid-responsive genes by binding to a
specific glucocorticoid-responsive element (GRE) DNA sequences. By
contrast, GRβ is transcriptionally inactive, does not bind to
glucocorticoid and may be an endogenous inhibitor of glucocorticoid
action and an important negative regulator determining
glucocorticoid sensitivity (9,10).
The stimulatory effect of glucocorticoids on the production of HBV
markers is mediated through specific GRs (11). It has been demonstrated that GRα
binds to a restriction fragment of the HBV genome containing the
GRE consensus sequence and transmits a signal for augmenting the
glucocorticoid-dependent activity of the HBV enhancer (12).
To the best of our knowledge, the mechanisms
underlying the association between glucocorticoids and potentially
fatal HBV reactivation remain to be fully elucidated, as do the
expression profiles of the two GR isoforms in CHB patients. In the
present study, the mRNA expression levels of GRα and -β in
peripheral blood mononuclear cells (PBMC) from CHB patients and
healthy controls were examined. In addition, the correlation
between the mRNA expression of the GR isoforms and HBV serological
and virological characteristics in patients with CHB was
examined.
Materials and methods
Study subjects
A total of 29 patients were recruited from the
Department of Infectious Diseases at Jingzhou Hospital (Jingzhou,
China). The diagnosis of CHB was based on the Guidelines for the
Prevention and Treatment of CHB (13). No patients had evidence of
decompensated liver disease or hepatocellular carcinoma and no
markers for autoimmune liver disease, including antinuclear
antibody, smooth muscle antibody and liver-kidney microsome type 1
autoantibody, were detected. All patients also tested negative for
other viral infections, including hepatitis A virus, hepatitis C
virus, hepatitis E virus, human immunodeficiency virus,
cytomegalovirus and Epstein-Barr virus, had never received
antiviral treatment or immunotherapy and had not received
glucocorticoids for >six months prior to blood collection. The
control group consisted of 43 healthy individuals who were selected
based on medical history evaluation and physical and laboratory
examination from the Health Examination Center of the same
hospital. The characteristics of the CHB patients and healthy
controls are listed in Table I.
All individuals provided written consent prior to their inclusion
in the study and the study protocol was approved by the Medical
Ethics Committee of Jingzhou Hospital.
 | Table IClinical, serological and virological
markers in healthy controls and in CHB patients. |
Table I
Clinical, serological and virological
markers in healthy controls and in CHB patients.
| Markers | Controls | CHB patients |
|---|
| Number (n) | 43 | 29 |
| Gender
(male/female) | 26/17 | 18/11 |
| Age (years) | 30 (21–36) | 35 (25–42) |
| ALT (IU/l) | 22.7 (13–40) | 116.1 (92–157) |
| HBV eAg (+/−) | 0/43 | 13/16 |
| HBV pre-S1Ag
(+/−) | 0/43 | 12/17 |
| Viral load (log
copies/ml) | NT | 6.48 (4.04–8.73) |
Serological markers and HBV DNA
assay
The levels of HBsAg and HBeAg were assessed through
enzyme-linked immunoassays using diagnostic kits for hepatitis B
virus surface antigen and e antigen, respectively (Wantai
Biological Pharmacy Enterprise Co., Ltd., Beijing, China). Pre-S1Ag
was also detected using a diagnostic kit for hepatitis B virus
pre-S1 antigen (Alpha Biotechnology, Shanghai, China). The HBV DNA
was extracted from 100 μl patient serum using DNA extraction
reagents in the diagnostic kit for hepatitis B virus DNA (Daan
Gene, Guangzhou, China) according to the manufacturer’s
instructions. The viral load of the HBV DNA in the serum samples
was then quantified through a high-sensitivity fluorescent
quantitative polymerase chain reaction (qPCR) using a diagnostic
kit for hepatitis B virus DNA (Daan Gene) and amplified using an
ABI 7300 instrument (Applied Biosystems, Carlsbad, CA, USA). Each
50-μl reaction contained 2 μl sample extracts (template). Following
initial heating at 93°C for 2 min, the samples were subjected to 10
cycles of denaturation at 93°C for 45 sec followed by annealing and
synthesis for 1 min at 55°C and 30 cycles of denaturation at 93°C
for 30 sec followed by annealing and synthesis for 45 sec at
55°C.
Isolation of PBMCs
Blood (5 ml) was obtained by venipuncture into
heparinized tubes. The PBMCs were isolated via density gradient
centrifugation using Ficoll-Paque plus (Amersham Pharmacia
Biotechnology, Uppsala, Sweden) according to the manufacturer’s
instructions. Cells were resuspended in RBC lysis buffer (123
mmol/l NH4Cl, 8 mmol/l KHCO3, and 25 μmol/l
EDTA) to lyse the remaining red blood cells, which were then
collected by centrifugation at 300 × g for 10 min.
RNA isolation and reverse transcription
(RT)-qPCR
All procedures for RNA isolation were performed
immediately for all samples. The RNA extraction was performed using
the RNAiso Plus kit (Takara Biotechnology, Tokyo, Japan) according
to the manufacturer’s instructions. A cell suspension of
5×106 PBMCs was dissolved in 2 ml extraction reagent.
The concentration of RNA was measured using a UV-1750
spectrophotometer (Shimadzu, Tokyo, Japan). All samples had a
260/280 absorbance ratio of ~2.0. The RNA integrity was determined
by the presence of an 18S rRNA band when samples were analyzed by
agarose gel electrophoresis running for 30 min with constant
voltage (100 V) on Wide Mini-Sub® Cell GT
electrophoresis system (Bio-Rad Laboratories, Segrate, Italy).
Prior to RT, all RNA samples were treated with DNAse I (Invitrogen
Life Technologies, Carlsbad, CA, USA) to remove any contaminating
genomic DNA. cDNA was synthesized from 500 ng RNA and RT was
performed using oligo-dT primers and an PrimeScript RT reagent kit
(Takara Biotechnology, Dalian, China) according to the
manufacturer’s instructions. The RNA and cDNA samples were stored
at −80°C until use.
qPCR was performed for the GRα and GRβ splice
variants using the common upstream primer:
5′-AAACTCTTGGATTCTATGCATGAA-3′ and the specific downstream primers:
GRα, 5′-TATTAATTCGACTTTCTTTAAGGCAA-3′ and GRβ,
5′-CCACGTATCCTAAAAGGGCAC-3′, described by Boullu-Ciocca et
al (14). Human GAPDH, which
served as a normalization control to correct for loading
discrepancies, was amplified from the same cDNA samples using the
following primers: upstream primer, 5′-GAAGGTGAAGGTCGGAGTC-3′ and
downstream primer, 5′-GAAGATGGTGATGGGATTTC-3′. The Taq Man probes
used were 5′-ATTCCCCGAGATGTTAGCTGAAATCA-3′,
5′-TCTTGGCGCTCAAAAAATAGAACTCA-3′ and 5′-CAAGCTTCCCGTTCTCAGCC-3′ for
GRα, GRβ and GAPDH, respectively. All primers and probes were
synthesized by Takara Biotechnology (Dalian, China) The reaction
contained 3 μl cDNA template, 25 μl 2× Premix ExTM Taq
reagent, 1 μl 50× 5-carboxy-X-rhodamine reference dye (ROX), 0.2
pmol/μl forward and reverse primer and 0.4 pmol/μl probe. Water was
added to obtain a total volume of 50 μl. The reactions were
performed using an ABI 7300 instrument (Applied Biosystems). The
PCR cycling program consisted of a hot start activation step at
95°C for 30 sec followed by 40 amplification cycles at 95°C for 5
sec and 60°C for 31 sec. The relative quantification of target gene
expression was evaluated using the comparative CT method, as
previously described by Hettinger et al (15). The ΔCT value was calculated by
subtracting the target CT of each sample from its own GAPDH CT
value. The ΔΔCT values were determined by using the highest sample
ΔCT value as an arbitrary constant to subtract from the ΔCT values
of all other samples. Fold-changes in the target gene expression
were equivalent to 2−ΔΔCT.
Statistical analysis
The results are expressed as the mean ± standard
deviation. All data were tested for a normal distribution using the
Kolmogorov-Smirnov test. Statistical analysis of the data was
evaluated using the one-way analysis of variance (ANOVA) or
Student’s t-test, as appropriate. In cases where the data was not
normally distributed, a Mann-Whitney U test or a Kruskal-Wallis
ANOVA with Dunn’s method was used. The degree of association
between the variables was assessed using Pearson’s correlation. All
statistical analyses were performed using SPSS v12.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
mRNA expression of GR isoforms in the
PBMCs
The mRNA expression of the GRα and GRβ transcripts
in the PBMCs of 29 CHB patients and 43 healthy controls were
analyzed by RT-qPCR. The RT-PCR products of GRα were detected in
the PBMCs of all patients and healthy controls, whereas the
GRβ-specific products were only found in certain samples. Of the
subjects tested, GRβ mRNA was detected in the RNA from the PBMCs of
27 CHB patients (93.1%) and 37 healthy controls (86.0%). The
incidence of GRβ expression in patients with CHB was similar to
that in the healthy controls (P>0.05).
Quantitative analysis of the mRNA
expression of GR isoforms
The mean expression levels of GRα were significantly
different between the CHB patients and healthy controls
(P<0.001) and were significantly lower in the CHB patients
(60.51±23.73) compared with those in the healthy controls
(100.00±40.75; Table II).
However, no significant differences were observed in the mRNA
expression levels of GRβ between the CHB patients and healthy
controls (0.11±0.08, vs. 0.17±0.13; P=0.061). No significant
correlation was observed in the RNA levels between the two splice
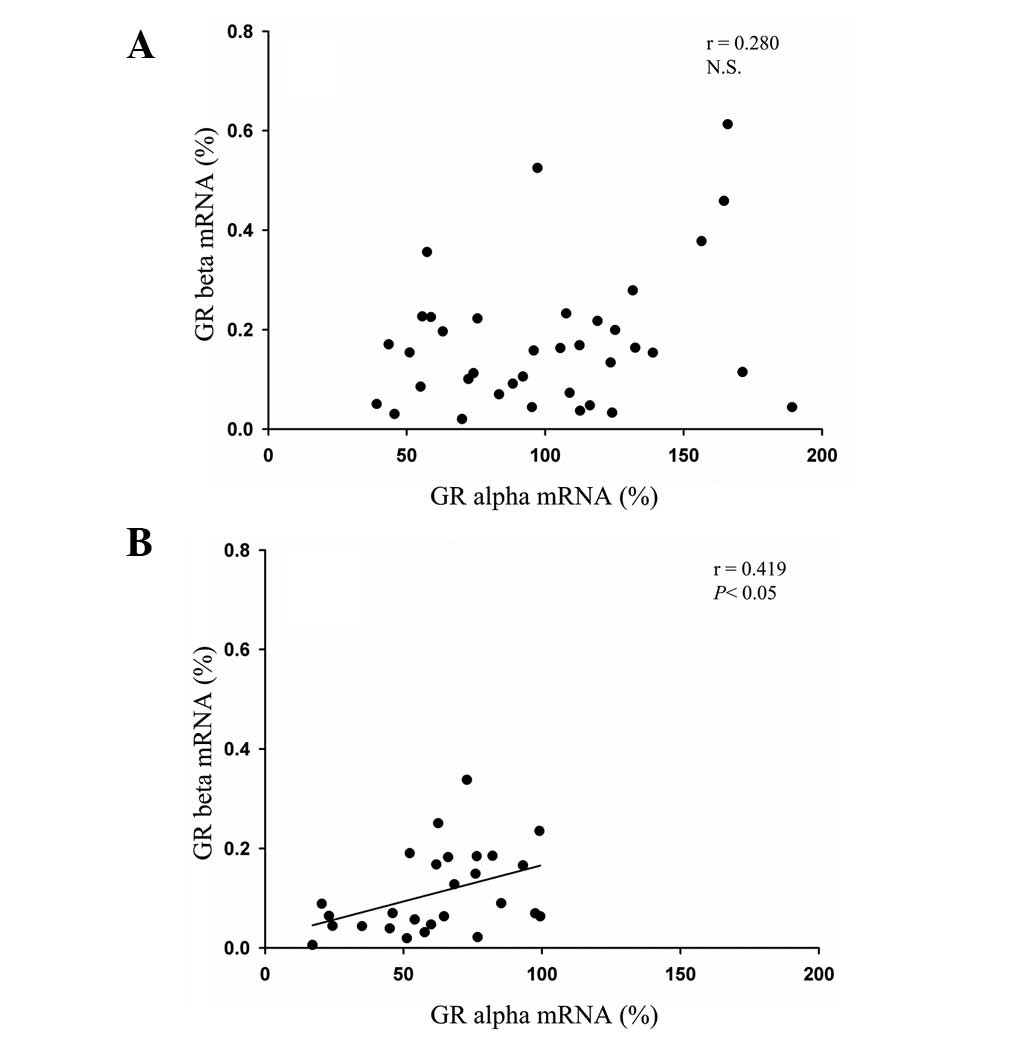
variants in healthy controls (P>0.05; Fig. 1A). However, a significant positive
correlation was observed between the GRα levels and the expression
of GRβ in the PBMCs of patients with CHB (r=0.419; P<0.05;
Fig. 1B).
 | Table IImRNA levels of GRα and GRβ in the
PBMCs of healthy controls and CHB patients. |
Table II
mRNA levels of GRα and GRβ in the
PBMCs of healthy controls and CHB patients.
| mRNA | Controls (n=43) | CHB patients
(n=29) | P-value |
|---|
| GRα | 100.00±40.75 | 60.51±23.73 | <0.001 |
| GRβ | 0.17±0.13 | 0.11±0.08 | 0.061 |
Association between the mRNA expression
levels of the GR isoforms and serum HBV markers
Compared with the healthy controls, lower mRNA
levels of GR were observed in the CHB patients, who were further
divided into subgroups according to their serum HBV marker status.
The PBMC expression of GRα was significantly reduced in the
HBeAg-positive patients (n=13) and HBeAg-negative patients (n=16)
compared with those in the healthy controls (P<0.05; Fig. 2A). Regarding the pre-S1Ag status,
however, only HBV pre-S1Ag-negative patients (n=17) exhibited a
significant decrease in GRα mRNA relative to the healthy controls
(P<0.05; Fig. 2B). The GRβ mRNA
values in the PBMCs were not associated with the serum HBV marker
status of the patients and no significant differences were observed
in the expression of GRβ between the patient subgroups and the
healthy controls (P>0.05; Fig. 2C
and D).
Association between the mRNA expression
levels of the GR isoforms and HBV viral loads
The CHB patients were divided into two sub-groups
according to their levels of serum HBV DNA of
1×104–105 copies/ml (n=10) and
>1×105 copies/ml (n=19). Compared with the healthy
controls, the mRNA expression of GRα was significantly decreased in
the patients of the two subgroups (P<0.05; Fig. 3A). However, no significant
difference was observed in the mRNA expression of GRβ between the
controls and either the 1×104–105 copies/ml
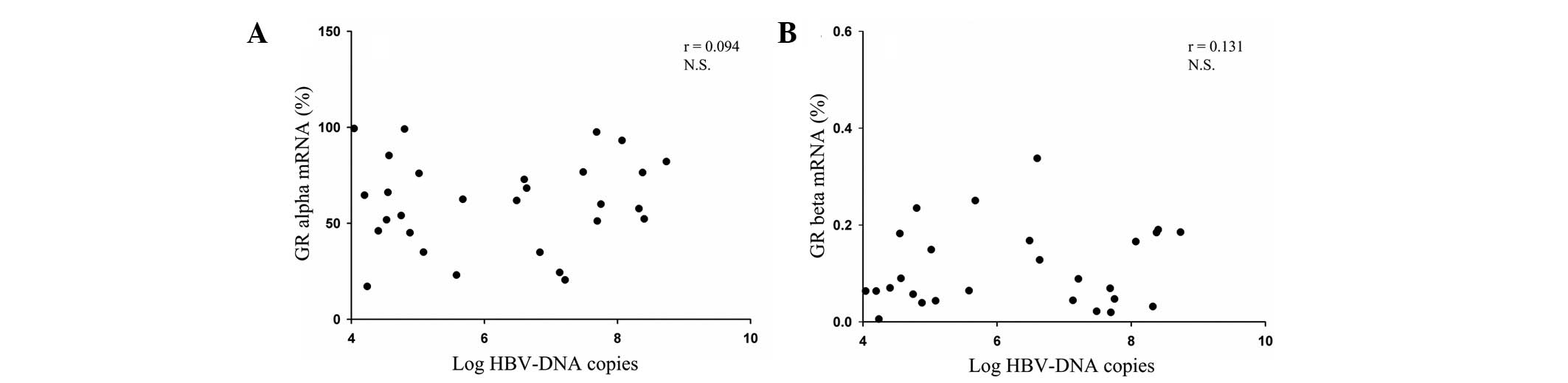
or the >1×105 copies/ml subgroup (P>0.05; Fig. 3B). In terms of the association
between serum HBV viral load and the GR mRNA levels, no correlation
was observed in the HBV DNA-positive patients, as demonstrated by
the HBV viral load and the mRNA expression levels in the CHB
patients (Fig. 4A and B).
Discussion
GRα and GRβ mRNA are generated by alternative
splicing of common GR gene transcripts, which contain exon 9a and
9b gene transcripts. GRα is a ligand-activated transcription
factor, whereas GRβ may be an endogenous inhibitor of
glucocorticoid action and transcriptionally inactive (16). In the present study, the mRNA
expression levels of GRα and GRβ in the PBMCs of CHB patients and
healthy individuals were quantified using RT-PCR and the
association between the mRNA expression of the GR isoforms and HBV
serological and virological markers was examined.
A previous study by Rai et al (17) indicated that GRα mRNA exists in the
PBMCs of all autoimmune hepatitis (AIH) patients, chronic viral
hepatitis (CVH) patients and healthy volunteers and that the
incidence of GRβ mRNA in AIH patients (57.6%) is significantly
higher compared with that in patients with CVH (28.6%) and healthy
volunteers (20.0%). In the present study, GRα mRNA was also present
in the PMBCs of all subjects, whereas GRβ mRNA was detected in the
PBMCs of 93.1% CHB patients and 86.0% healthy controls. In
addition, the expression of GRβ in the patients with CHB was not
significantly different from that in the healthy volunteers
(P>0.05) and the mRNA expression of GRβ was markedly higher. The
discrepancy between the incidences of GRβ may result from
differences in the population studied and the sensitivity of the
methods used.
The mRNA expression of GRβ has been demonstrated in
various human tissues by RT-PCR. Compared with GRα, the levels of
GRβ were observed to be relatively low and considered to be
0.2–0.3% of GRα mRNA levels (10).
In the present study, the mRNA levels of GRβ were 0.17±0.13% in the
healthy controls and 0.11±0.08% in the CHB patients relative to
those of GRα.
Gao et al (18) investigated the expression of GR in
T lymphocytes in patients with acute onset of chronic hepatitis B
liver failure and CHB. The results revealed that the mRNA
expression of GRα in CHB patients was significantly decreased
compared with that in healthy controls; however, the difference in
the mRNA expression of GRβ between CHB patients and healthy
controls was not significant. The findings of the present study
were consistent with this, as, compared with those of the healthy
controls, the mRNA levels of GRα in the CHB patients was
significantly lower (60.51±23.73, vs. 100.00±40.75; P<0.001).
The mRNA expression of GRβ in the CHB patients also declined;
however, the difference in these values was not significant between
the two groups (0.11±0.08, vs. 0.17±0.13;P=0.061). In a previous
study, a significant correlation was identified between the mRNA
expression levels of GRα and GRβ in the PBMCs of healthy controls
and patients with Cushing’s syndrome (19). In the present study, the GRα levels
were only significantly positively correlated with the expression
of GRβ in the PBMCs of CHB patients (r=0.419; P<0.05; Fig. 1B), while no such correlation was
observed in the PBMCs of the healthy controls.
At present, the mechanisms underlying the decreased
mRNA expression of GR in the PBMCs of CHB patients remain to be
elucidated. A possible mechanism underlying the downregulation of
GR mRNA may involve the inability of PBMCs to produce peptides and
protein following HBV infection. HBV is not strictly hepatotropic,
early observations have demonstrated that this virus can infect
PBMCs and viral DNA is detectable in the PBMCs of the majority of
CHB patients (20). The PBMC cell
compartment represents an extra-hepatic viral reservoir, not only
during infection, but for an extended time following resolution of
acute hepatitis B (21). Previous
studies have demonstrated a dysregulation of pro- and
anti-inflammatory cytokines in CHB patients and elevated levels of
proinflammatory cytokines may inhibit the expression and function
of GR, weakening its anti-inflammatory and immunoregulatory effects
during a systemic inflammatory response (22,23).
Furthermore, the mRNA levels of GRβ were low compared with total GR
levels and their effect on the action of GRα is limited, suggesting
that concomitant downregulation in the mRNA expression levels of
GRα and GRβ in the PBMCs of CHB patients may result in attenuation,
rather than enhancement, of glucocorticoid efficacy (24).
The relatively low mRNA expression levels of GRβ
compared with those of GRα may contribute to the lack of
significance between differences in the mRNA expression of GRβ in
CHB patients and healthy controls. Exogenously administered
glucocorticoid may downregulate mRNA levels of GR (24,25).
However, in the present study, this possibility can be excluded as
the CHB patients had not received glucocorticoid treatment for
>six months prior to blood sampling.
The HBV genome contains a specific DNA binding site
for the GR localized at HBV map positions 341–370, which serves as
a signal for augmenting the glucocorticoid-dependent activity of
the HBV enhancer (12). In
vivo, glucocorticoid therapy in CHB patients may result in
activation of latent infection, increasing levels of HBV markers
and increasing the severity of liver disease, implying that
glucocorticoids are involved in HBV replication and gene expression
(26). In vitro,
glucocorticoid can increase the production of HBsAg, HBeAg and
viral RNAs, mediated through specific GRs, in HBV-transfected human
hepatoma cells (11). In addition,
a previous study reported that serum levels of the endogenous
glucocorticoid cortisol were marginally higher in CHB patients
compared with those in healthy controls, although the difference
was not significant (18).
The present study further examined the association
between the mRNA expression of the GR isoforms and HBV serum
markers and viral status in CHB patients. To the best of our
knowledge, there is little reference to this area in previous
studies. Compared with the healthy controls, significant
differences were observed in the mRNA expression levels of GRα in
the HBeAg-positive and HBV DNA-positive CHB patients (P<0.05).
The mechanisms underlying these findings remain to be elucidated,
although it can be hypothesized that the endogenous glucocorticoid
stimulated the production of HBeAg and HBV DNA through the
mechanisms mentioned above and that, when the concentrations of HBV
protein antigens and HBV DNA reach a certain limit, feedback
mechanisms downregulate the production of GRα. The concentrations
of HBV antigen and HBV viral load were particularly high in CHB
patients.
Significant differences were also observed in the
mRNA expression of GRα in HBeAg-negative and pre-S1Ag-negative
patients with CHB compared with those in the healthy controls.
These CHB patients also had high concentrations of other HBV
serological and virological markers, including HBsAg and HBV DNA,
which were likely to have induced feedback mechanisms that
downregulate the expression of GRα. The details of these feedback
mechanisms, however, remain to be elucidated.
In conclusion, the present study demonstrated that
the mRNA expression profile of GRα was different between CHB
patients and healthy controls. Although the expression of GRα in
the CHB patient subgroups was found to significantly decrease
compared with that in the healthy controls, the HBV serological and
virological marker status was not associated with the mRNA levels
of the GR isoforms in the CHB patients.
Acknowledgements
This study was financially supported by the Hubei
Provincial Science & Technology Department Research Program of
China (no. 2007AA301C54).
References
|
1
|
Custer B, Sullivan SD, Hazlet TK, et al:
Global epidemiology of hepatitis B virus. J Clin Gastroenterol.
38:S158–S168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wright TL and Lau JY: Clinical aspects of
hepatitis B virus infection. Lancet. 342:1340–1344. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ganem D and Prince AM: Hepatitis B virus
infection - natural history and clinical consequences. N Engl J
Med. 350:1118–1129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan CQ and Zhang JX: Natural history and
clinical consequences of hepatitis B virus infection. Int J Med
Sci. 2:36–40. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belle A, Bronowicki JP and Peyrin-Biroulet
L: Reactivation of viral hepatitis in immunosuppressed patients: an
ounce of prevention is worth a pound of cure. Gastroenterology.
140:360–362. 2011. View Article : Google Scholar
|
|
6
|
Kim MK, Ahn JH, Kim SB, et al: Hepatitis B
reactivation during adjuvant anthracycline-based chemotherapy in
patients with breast cancer: a single institution’s experience.
Korean J Intern Med. 22:237–243. 2007. View Article : Google Scholar
|
|
7
|
Lee SD, Tong MJ, Wu JC, et al: A
randomised double-blind placebo-controlled trial of prednisolone
therapy in HBeAg and HBV DNA positive Chinese patients with chronic
active hepatitis B. J Hepatol. 12:246–250. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hollenberg SM, Weinberger C, Ong ES, et
al: Primary structure and expression of a functional human
glucocorticoid receptor cDNA. Nature. 318:635–641. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bamberger CM, Schulte HM and Chrousos GP:
Molecular determinants of glucocorticoid receptor function and
tissue sensitivity to glucocorticoids. Endocr Rev. 17:245–261.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oakley RH, Sar M and Cidlowski JA: The
human glucocorticoid receptor beta isoform. Expression, biochemical
properties, and putative function. J Biol Chem. 271:9550–9559.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chou CK, Wang LH, Lin HM and Chi CW:
Glucocorticoid stimulates hepatitis B viral gene expression in
cultured human hepatoma cells. Hepatology. 16:13–18. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tur-Kaspa R, Shaul Y, Moore DD, et al: The
glucocorticoid receptor recognizes a specific nucleotide sequence
in hepatitis B virus DNA causing increased activity of the HBV
enhancer. Virology. 167:630–633. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lok AS and McMahon BJ: Chronic hepatitis
B. Hepatology. 45:507–539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boullu-Ciocca S, Paulmyer-Lacroix O, Fina
F, et al: Expression of the mRNAs coding for the glucocorticoid
receptor isoforms in obesity. Obes Res. 11:925–929. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hettinger AM, Allen MR, Zhang BR, et al:
Presence of the acute phase protein, bikunin, in the endometrium of
gilts during estrous cycle and early pregnancy. Biol Reprod.
65:507–513. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okano M: Mechanisms and clinical
implications of glucocorticosteroids in the treatment of allergic
rhinitis. Clin Exp Immunol. 158:164–173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rai T, Ohira H, Tojo J, et al: Expression
of human glucocorticoid receptor in lymphocytes of patients with
autoimmune hepatitis. Hepatol Res. 29:148–152. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao L, Wang JF, Xiang M, et al: Expression
of human glucocorticoid receptor in T lymphocytes in
acute-on-chronic hepatitis B liver failure. Dig Dis Sci.
56:2605–2612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hagendorf A, Koper JW, de Jong FH, et al:
Expression of the human glucocorticoid receptor splice variants
alpha, beta, and P in peripheral blood mononuclear leukocytes in
healthy controls and in patients with hyper- and hypocortisolism. J
Clin Endocrinol Metab. 90:6237–6243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pontisso P, Vidalino L, Quarta S and Gatta
A: Biological and clinical implications of HBV infection in
peripheral blood mononuclear cells. Autoimmun Rev. 8:13–17. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Michalak TI, Pasquinelli C, Guilhot S and
Chisari FV: Hepatitis B virus persistence after recovery from acute
viral hepatitis. J Clin Invest. 93:230–239. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gustot T, Durand F, Lebrec D, Vincent JL
and Moreau R: Severe sepsis in cirrhosis. Hepatology. 50:2022–2033.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schlaak JF, Tully G, Löhr HF, Gerken G and
Meyer zum Büschenfelde KH: HBV-specific immune defect in chronic
hepatitis B (CHB) is correlated with a dysregulation of pro-and
anti-inflammatory cytokines. Clin Exp Immunol. 115:508–514. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hori T, Watanabe K, Miyaoka M, et al:
Expression of mRNA for glucocorticoid receptors in peripheral blood
mononuclear cells of patients with Crohn’s disease. J Gastroenterol
Hepatol. 17:1070–1077. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peeters RP, Hagendorf A, Vanhorebeek I, et
al: Tissue mRNA expression of the glucocorticoid receptor and its
splice variants in fatal critical illness. Clin Endocrinol (Oxf).
71:145–153. 2009. View Article : Google Scholar
|
|
26
|
Yang CH, Wu TS and Chiu CT: Chronic
hepatitis B reactivation: a word of caution regarding the use of
systemic glucocorticosteroid therapy. Br J Dermatol. 157:587–590.
2007. View Article : Google Scholar : PubMed/NCBI
|